Thick exchange layer evaporation model with natural convection effect and evaporation experimental study for multicomponent droplet
Fang WANG, Xiang GAO, Yangchun XIAO, Zhaoyang WU, Jie JIN
Aero-Engine Numerical Simulation Research Center, School of Energy and Power Engineering, Beihang University, Beijing 100083, China
KEYWORDS Droplet;Evaporation rate;Multicomponent fuel evaporation model;Natural convection;Suspended droplet experiment;Thick exchange layer model
Abstract In order to investigate the high-temperature evaporation characteristics of multicomponent liquid fuel, three kinds of blended fuel: n-heptane/n-decane/RP-3 aviation kerosene-ethanol were experimentally studied with and without forced convection. Further, based on zerodiffusion and infinite diffusion concept, this study expanded Thick Exchange Layer evaporation model with Natural Convection effect(NC-TEL)to multicomponent liquid fuels.The experimental results show that the droplet evaporation rate increases significantly with the increase of ambient temperature. Higher temperature leads to more significant relationships between the composition ratio and the evaporation rate. The effect of forced convection is not obviously under the circumstance in this paper.Then,the evaporation models were validated by experimental data.In general,the new NC-TEL model behaves better than the Ranz-Marshall (R-M) model, and the prediction accuracy at high temperature is improved by 8%to 35%.In lower temperature conditions,the prediction of zero-diffusion NC-TEL model is better than the infinite diffusion NC-TEL model. In high-temperature conditions, for n-heptane-ethanol droplet, the predictions of NC-TEL model are accurate, but for n-decane/RP-3 aviation kerosene-ethanol, the predictions are lower than experimental results. This may be caused by the micro-explosion phenomenon and the Marangoni phenomenon.
1. Introduction
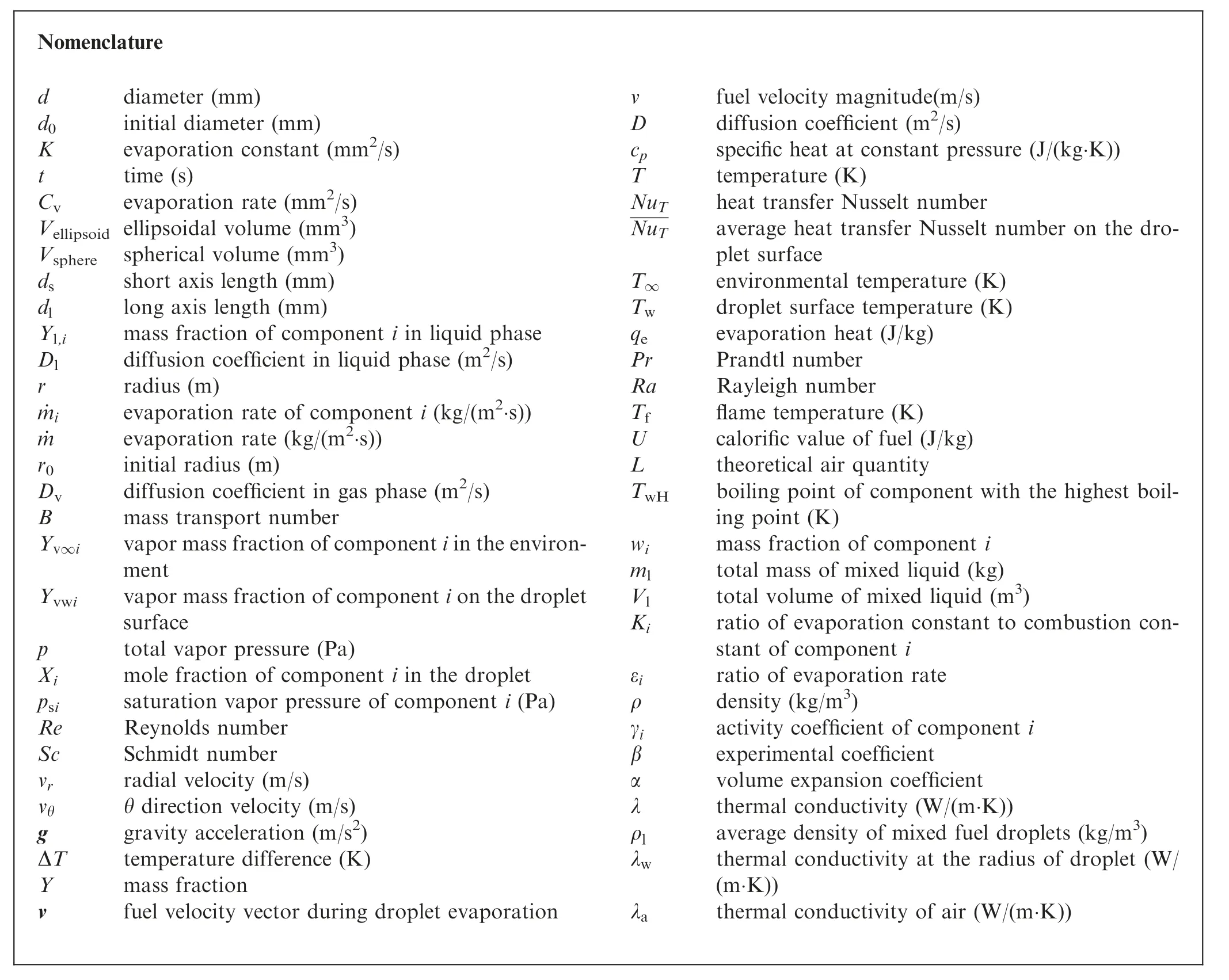
Nomenclature d diameter (mm)d0 initial diameter (mm)K evaporation constant (mm2/s)t time (s)Cv evaporation rate (mm2/s)Vellipsoid ellipsoidal volume (mm3)Vsphere spherical volume (mm3)ds short axis length (mm)dl long axis length (mm)Yl,i mass fraction of component i in liquid phase Dl diffusion coefficient in liquid phase (m2/s)r radius (m)˙mi evaporation rate of component i (kg/(m2·s))˙m evaporation rate (kg/(m2·s))r0 initial radius (m)Dv diffusion coefficient in gas phase (m2/s)B mass transport number Yv∞i vapor mass fraction of component i in the environment Yvwi vapor mass fraction of component i on the droplet surface p total vapor pressure (Pa)Xi mole fraction of component i in the droplet psi saturation vapor pressure of component i (Pa)Re Reynolds number Sc Schmidt number vr radial velocity (m/s)vθ θ direction velocity (m/s)g gravity acceleration (m/s2)ΔT temperature difference (K)Y mass fraction v fuel velocity vector during droplet evaporation v fuel velocity magnitude(m/s)D diffusion coefficient (m2/s)cp specific heat at constant pressure (J/(kg·K))T temperature (K)NuT heat transfer Nusselt number NuT average heat transfer Nusselt number on the droplet surface T∞ environmental temperature (K)Tw droplet surface temperature (K)qe evaporation heat (J/kg)Pr Prandtl number Ra Rayleigh number Tf flame temperature (K)U calorific value of fuel (J/kg)L theoretical air quantity TwH boiling point of component with the highest boiling point (K)wi mass fraction of component i ml total mass of mixed liquid (kg)Vl total volume of mixed liquid (m3)Ki ratio of evaporation constant to combustion constant of component i εi ratio of evaporation rate ρ density (kg/m3)γi activity coefficient of component i β experimental coefficient α volume expansion coefficient λ thermal conductivity (W/(m·K))ρl average density of mixed fuel droplets (kg/m3)λw thermal conductivity at the radius of droplet (W/(m·K))λa thermal conductivity of air (W/(m·K))
Liquid fuel needs to be atomized into droplets to make it combust fully,which is important in improving the performance of engine and reducing pollution emission. With the increasing attention to environmental protection and energy conservation, the research and development of hybrid fuels and other new fuels have become a hot topic in the field of energy research. Studies have shown that adding ethanol to gasoline and diesel fuel to form a hybrid fuel can greatly reduce the emissions1. Adding methanol, ethanol and other oxygencontaining fuels to aviation fuel can improve its combustion characteristics2. In Refs.3-6, the distillation range and saturated vapor pressure of ethanol-gasoline with different mixing ratios were tested. However, there are few relevant researches on the evaporation characteristics test of single droplet of ethanol blended fuel.
The single component droplet evaporation model has been studied earlier with abundant results. Based on the earliest d2model7,8, infinite9,10/finite conduction model11,12, Ranz-Marshall (R-M) model13, effective heat conduction model14(Abramzon-Sirignano model) and non-equilibrium model15have been proposed.The main difference among these models is the description of the thermal conductivity inside the droplet, but most of them adopt one-dimensional hypothesis in gas phase transport, or suppose that the mass and heat exchange layer on the droplet surface has the characteristics of boundary layer.
There are few studies on the multicomponent droplet evaporation model, and the following two models are representative in the liquid phase transport model. Zero-diffusion model assumes that the fuel diffusion rate inside the droplet is zero, and the concentration distribution of each component within the droplet remains constant with time and is consistent with the initial value of the droplet. Infinite diffusion model assumes that the internal diffusion rate of the droplet is infinite,and the internal components and temperature of the droplet are uniform but change continuously with time.The whole evaporation process presents series stages depending on the ease of evaporation for each component16.
Daı¨f et al.17studied the evaporation characteristics of nheptane-n-decane fuel droplet and compared it with Abramson-Sirignano model, and found that the experimental results were consistent with the model under natural convection and forced convection conditions.Ravindran and Davis18studied the evaporation mechanism of two-component droplet through the experiment of evaporation of single droplet in inert gas, and compared the theoretical changes of twocomponent droplet area with the experimental results of single-component droplet. The above two cases were in good agreement. Ghamari and Ratner19studied the evaporation combustion characteristics of diesel and Jet-A droplet added different content of polymer additives. It was found that, in polymer added fuel, combustion characteristic was controlled by the more volatile component, and adding polymer to fuel led to slightly inhibition in droplet evaporation and combustion rate. And the main factor that affects the evaporation rate was viscidity. Gavhane et al.20studied the evaporation characteristics of n-heptane-dodecane and methanol-ethanol fuel droplets at both high and low temperatures,and the results showed that the fuel evaporation in alkane fuel mixture was controlled by the component with heavier molecular weight, while the fuel evaporation in alcohol fuel mixture was controlled by the component with lighter molecular weight. Megaridis21studied the evaporation characteristics of multicomponent droplet in laminar convection environment,and studied the influence of laminar flow on the eddy inside droplet.Liu and Avedisian22studied the combustion characteristics of iso-octane-n-heptane fuel droplet. The experimental results showed that the combustion rates of iso-octane and n-heptane and their mixture droplets were all higher than those of gasoline droplet. Stengele et al.23studied the evaporation characteristics of pentane-nonane fuel droplet by droplet drop method.The experimental results were compared with the highpressure model based on finite heat conduction and finite diffusion.The Abramson-Sirignano model and R-M model were in the best agreement. Ghassemi et al.24studied the evaporation characteristics of n-heptane-hexadecane blended fuel by hanging droplet method. The experimental results showed that two-component droplet evaporation presented three-stage characteristics, and bubble formation and droplet distortion could not occur in high-pressure environment.
In addition, some researchers studied the interfacial phenomena of multicomponent droplet evaporation. Aharon and Shaw25carried out a theoretical study on Marangoni instability in two-component droplet evaporation. The results showed that changes of composition in droplet surface would cause changes of temperature and boiling point in droplet surface,and thermal capillary effect and diffusion capillary effect might resist each other. Based on Aharon’s studies, Ha and Lai26found that droplet surface tension decreased monotonously with the increase of temperature and volatile component concentration, and the thermal capillary effect and diffusion capillary effect were mutually enhanced.Zhao27used LES-VOF (Large Eddy Simulation-Volume Of Fluid) method considering Marangoni effect to conduct numerical simulation of droplet evaporation process. The results showed that Marangoni effect affected droplet internal temperature and streamline distribution, and promoted heat and mass transfer, which was manifested as the increase of droplet evaporation rate.
Mercier’s28study indicates that the mass and heat transfer layer around the droplet is thicker (without boundary layer characteristics) and is not spherically symmetric when it evaporates in high-temperature environment. Recent studies have shown that the thick exchange layer model considering natural convection is superior to the evaporation model integrated in FLUENT and some other models in predicting the hightemperature static and convection evaporation rate of singlecomponent droplets29,30.
In this paper, based on liquid phase zero-diffusion model and infinite diffusion model, thick exchange layer model with natural convection is applied in multicomponent droplet evaporation in high-temperature air. n-heptane-ethanol, n-decaneethanol, and RP-3 aviation kerosene-ethanol fuel droplets’evaporation characteristics in high-temperature static and convection air were experimentally studied. And the new models were validated by experimental data. The research in this paper is important to the accumulation of basic experimental data of evaporation rate of ethanol blended fuels,the development and application of new mixed aviation fuels,and the simulation and design of advanced combustion chambers.
2. Experimental system
2.1. Experimental facility and content
The schematic diagram of the experimental facility is shown in Fig.1.The method of hanging drop on thermocouple was used in experiment.The thermocouple is K-type and the wire diameter is 0.15 mm. In the experiment, the wire of thermocouple with liquid drop is pushed into the heating furnace rapidly by guide rail. The size of the main heating furnace is 400 mm×220 mm×220 mm, which is electrically heated and filled with nano-fiber materials for heat insulation. The air passage in the furnace is cylindrical, and 4 heating resistance wires are distributed along the direction of the air passage. In addition, another K-type thermocouple probe is extended into the furnace to measure the air temperature in the furnace. Temperature control and display cabinet can adjust the furnace temperature from room temperature to 1200 ℃continuously.The main heating furnace has a window in front and with a transparent quartz glass for insulation.During the experiment, the high-speed camera with a macro lens is placed at an appropriate position in front of the window and the whole process of droplet evaporation in the furnace is recorded by high-speed camera. A quartz glass window is opened directly behind the furnace, and a LED light is placed behind it as a background light source.
The high-speed camera is MONO SN1400183 produced by Olympus Company in UK.The maximum shooting frequency is 10000 Hz,which is set as 100 Hz in experiment.The air compressor can supply air sustainably and stably,which is used to provide the forced convection environment required by experiment conditions. Air flow of different speeds can be obtained by adjusting the outlet flow rate from 0 to 40 L/min. The air flowmeter is D07-9E produced by Qixing Huachuang.Its measurement range is from 0 to 200 L/min, and the measurement error range is±2%. The control and display instrument is D08-2F produced by Qixing Huachuang.The rectifier contains two sets of ceramic rectifying gratings with square holes to ensure the smooth laminar flow of air into the main furnace passage.
The proportion of components in multicomponent droplet should be precisely configured.The liquid transfer device used in the experiment is single-channel liquid transfer gun produced by Lichen Technology whose maximum measuring range is 1 mL and transfer precision is 1 μL. Different liquid fuels are measured by different nozzles in the pipette,and then fully mixed in a sealed centrifugal tube with the volume of 2 mL.Finally,part of the mixture is extracted with a microliter syringe and then suspended on the wire of the thermocouple.The thermocouple has been accurately calibrated with a measurement accuracy of 1 K.The initial diameter of the droplet is obtained by comparing the droplet with the reference object,and the size of the reference object is measured by micrometer with a precision of 0.001 mm.The airflow velocity is calculated by the ratio of the airflow rate to the cross-sectional area of the flow passage in the main heating furnace.The blending ratio is the volume fraction of ethanol in the mixed fuel droplet.
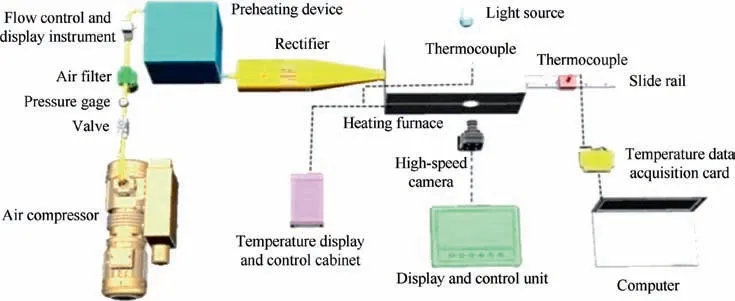
Fig. 1 Schematic diagram of experimental system.
The experimental mixed fuels are n-heptane-ethanol, ndecane-ethanol and RP-3 aviation kerosene-ethanol droplets.Test variables are environment temperature,convection velocity and mixing proportion. The environment temperature is from 200 to 800 ℃. The air velocity range is from 0 to1.0 m/s and the mixing ratio range is from 0 to 40%.The initial diameter of droplets is from 0.9 to 1.5 mm.The initial droplet temperature is room temperature,about 298 K.The experimental environment pressure is atmospheric pressure,about 0.1 MPa.RP-3 aviation kerosene itself is a complex mixture. The key parameters of kerosene are as follows:specific heat at constant pressure cp=2090 J/(kg·K), latent heat of evaporation E=226000 J/kg, and thermal conductivity λ=395 W/(m·K), 200 ℃;435 W/(m·K), 400 ℃;471 W/(m·K), 600℃;504 W/(m·K),800 ℃.
2.2. Experimental data processing
According to the classical droplet evaporation theory, when droplet evaporates in high temperature environment,its diameter d and time t satisfy the equation:

Series of droplet evaporation experimental photos taken by high-speed camera were imported into Photoshop, and the outline of the droplet was cut off and exported by magnetic lasso tool. The folder containing droplet contour figures was imported into the MATLAB code. The code could automatically identify the number of pixels occupied by each axis of the droplet in the pictures. According to the actual hanging wire size and the pixels occupied by wire in the picture, the actual axis length of the droplet could be worked out and the relevant size data were output. Fig. 2 is the determination of droplet’s size.Due to the influence of gravity,the droplet is regarded as an ellipsoid, and the actual diameter of the equivalent spherical droplet needs to be obtained by the method of converting the equivalent diameter. In other words, the ellipsoid is transformed into a sphere with the same volume, and the diameter of the sphere is exactly the equivalent diameter.The ellipsoidal volume and the spherical volume are
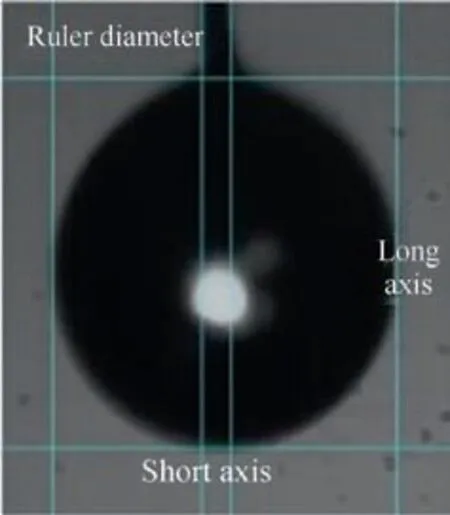
Fig. 2 Determination of droplet’s size.

Finally, according to Eqs. (1) and (2), the least quadratic regression is carried out in the stable evaporation stage of droplet evaporation curve(d2-t)under different experimental conditions, and the slope of regression line is obtained, which is the average evaporation rate of droplet under this experimental condition.
In order to verify the reliability of the experimental system and data processing method,part of the experimental results in this paper are compared with the corresponding experimental results in the literature. Fig. 3 is the comparison between the experimental results of n-heptane droplet and the corresponding experimental results in Ref.31(both experimental pressure is 0.1 MPa; both environmental temperatures are 500°C and 600°C respectively). It can be seen from the comparison results that the experimental data of n-heptane droplet in this paper are in good agreement with the experimental data in Ref.31. The evaporation rate error is within 1% at both temperatures. It is considered that the experimental system and data processing method in this paper are reliable and can obtain accurate and believable experimental results.
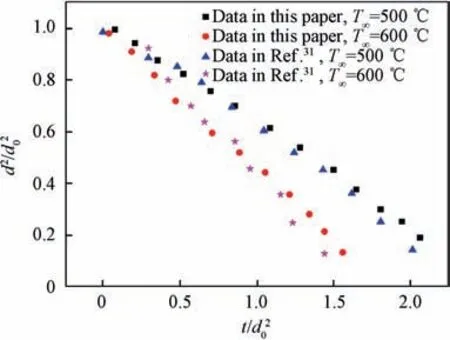
Fig. 3 Comparison between experimental results in this paper and those in Ref.31 for n-heptane.
3. Evaporation model
Different from single-component droplet evaporation, when multicomponent droplet evaporates, different components have different evaporation rates, resulting in mass diffusion in the liquid phase. If only radial diffusion is considered, the mass diffusion of components in the droplet can be expressed as16,32
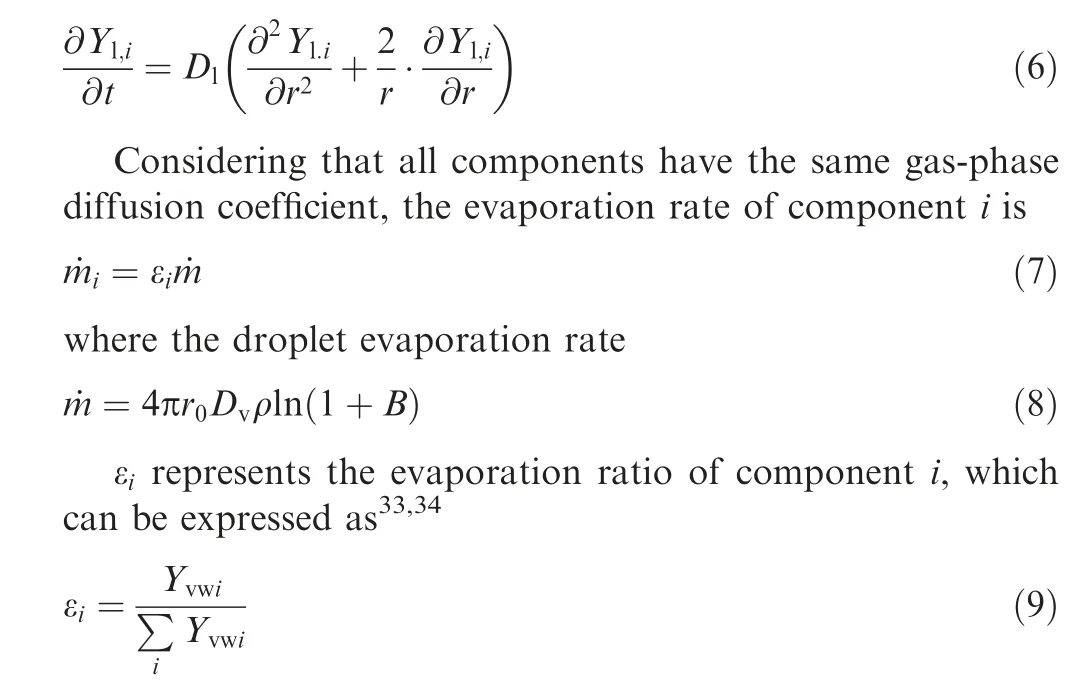
If there is no supplemental process in the droplet evaporation process, the mass fraction of components on the droplet surface is the same as the evaporation rate of components,that is Yl,si=εi. B is the mass transport number, and the expressions in the infinite diffusion model and in the zero-diffusion model are different because they assume different diffusion rates inside the droplet35.
In infinite diffusion model,the fuel diffusion rate inside the droplet is infinite, and the internal circulation within the droplet will reduce the difference in the spatial distribution of the components inside the droplet, resulting in uniform composition and temperature within the droplet but changing with time. The evaporation process is staged, that is, each component evaporates successively according to the degree of difficulty of evaporation.

In contrast, the zero-diffusion model, also known as the freezing model, assumes that the fuel diffusion rate inside the droplet is zero, that is, the concentration distribution of each component in the liquid phase remains constant with time and is consistent with the initial value. It can be understood as the co-evaporation of ‘‘mutual assistance” among different components:

where β is the experimental coefficient, and usually set to 0.6.
Eq. (13) is actually the R-M expansion model under the‘‘stagnant film” theory, which is R-M model for multicomponent droplets evaporation considering liquid phase diffusion.The classical model considers that the mass and heat transfer process of droplet evaporation is similar,and with the increase of transport number,the ratio of heat transfer Nusselt number to convection heat transfer Nusselt number of imaginary solid sphere without evaporation and combustion decreases significantly. However, many experimental studies show that the influence of temperature on droplet evaporation is much stronger than that predicted by stagnant film theory. Some droplet evaporation photos show that when evaporating at high temperature, especially when evaporating with combustion, the thickness of the mass and heat transfer layer around the droplet is often much larger than the droplet radius, which is not much smaller than droplet radius. That is to say, when the droplet is under the condition of forced convection at high temperature,the flow field and temperature field around it are not spherically symmetric and do not have the characteristics of boundary layer. On this basis, Zhou proposed a thick exchange layer evaporation model36. The model has two important assumptions: (A) the flow field around the droplet does not have the characteristics of boundary layer, and the thickness of heat and mass transfer layer is larger than or much larger than the droplet radius. (B) radial diffusion flow and heat flow are much larger than those in the circumferential direction. At the same time, considering the influence of natural convection at high temperature, the floating lift force is introduced into the momentum equation30.
The basic equations describing two-dimensional axisymmetric laminar flow of droplet evaporation in spherical coordinates are as follows:
Continuous equation



For Eqs. (53) and (55), the natural convection term (Ra term)is ignored in forced convection of high Reynolds number environment. Natural convection term will be taken into account when Reynolds number is small or medium. In static environment, the forced convection term (Reynolds number term) is neglected and the natural convection term is considered.
The following is the calculation of physical property parameters of mixed fuel.
Droplet surface temperature:
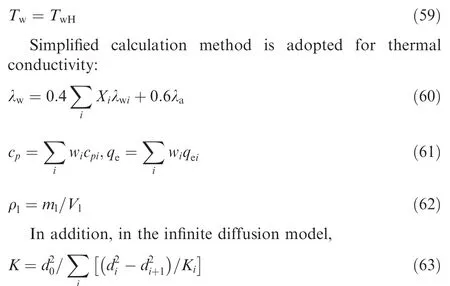
where diis the droplet diameter after the ith component has completely evaporated, and i starts from 0. Eq. (63) reflects the series stages of mixed fuel droplet evaporation process depending on the degree of difficulty (boiling point) of evaporation of the components.
The calculation formulas of evaporation constants and transport numbers of the above multicomponent droplet evaporation models are shown in Table 1.
4. Experimental results and model validation
4.1. Comparison between NC-TEL model and R-M model
The gas-phase transport model is the basis and core of the single droplet evaporation model, which describes the mass and heat transfer process between the droplet and the surrounding environment, that is, the energy transfers from the environmental gas to the fuel droplet and the mass diffuses from the fuel droplet to the environmental gas. Fig. 4 and Fig. 5 show the comparison among NC-TEL model, R-M model and the experimental data. In NC-TEL model, for n-heptane-ethanol,experimental constant δ=3×103and ζ=5×103. For ndecane-ethanol, experimental constant δ=2×103and ζ=1×103.Verepresents the volume fraction of ethanol in the mixture droplet, and vais the convection volecity.
As shown in Fig. 4 and Fig. 5, no matter using infinite diffusion assumption or zero-diffusion assumption,R-M model’s prediction of average evaporation rate under relatively high temperature (above 300°C) is obviously low. In other words,R-M model’s description of droplet evaporation at high temperature is deficient. The experimental results show that the influence of ambient temperature on droplet evaporation rate is greater than that predicted by R-M model. It is considered in NC-TEL model that when multicomponent droplets evaporate, the surface is not a thin boundary layer, but is a thick mass and energy exchange layer which is controlled by threedimensional Navier-Stokes equation. In addition, NC-TEL model believes that, in the mass transfer process through the droplet surface,the buoyancy force caused by temperature difference plays a positive role at the interface. Therefore, the evaporation characteristics of droplet can be better described at high temperature and the evaporation rate of droplet can be accurately predicted.In conclusion,the physical significance of the thick exchange layer model is clear,considering the driving effect of natural convection on evaporation is closer to the actual situation. Therefore, NC-TEL model performs well in all temperature test sections, especially in high-temperature test section in which NC-TEL model is significantly better than R-M model.
4.2. Comparison between zero-diffusion model and infinite diffusion model
For multicomponent droplet, the droplet internal diffusion model (liquid phase transport model) is also important,because it reflects the mixing of components within the droplet.Figs. 6-8 show the comparison among zero-diffusion model,infinite diffusion model and the experimental data. In NCTEL model, for n-heptane-ethanol, experimental constant δ=3×103, ζ=5×103. For n-decane-ethanol, experimental constant δ=2×103, ζ=1×103. For the RP-3 -ethanol,experimental constant δ=1×103, ζ=2×103.
It can be seen from Fig. 6 that for n-heptane-ethanol fuel droplet, both the zero-diffusion NC-TEL model and the infinite diffusion NC-TEL model are in good agreement with the experimental data.The reason may be that the evaporation rate of n-heptane and ethanol within the ambient temperature from 200°C to 450°C are close,and the mixture of n-heptane and ethanol compared with their pure substances respectively has little difference in overall physical property (the boiling points of the two are also similar). Thus whether usingzero-diffusion assumption or infinite diffusion assumption, the results are similar and consistent with the experimental data.
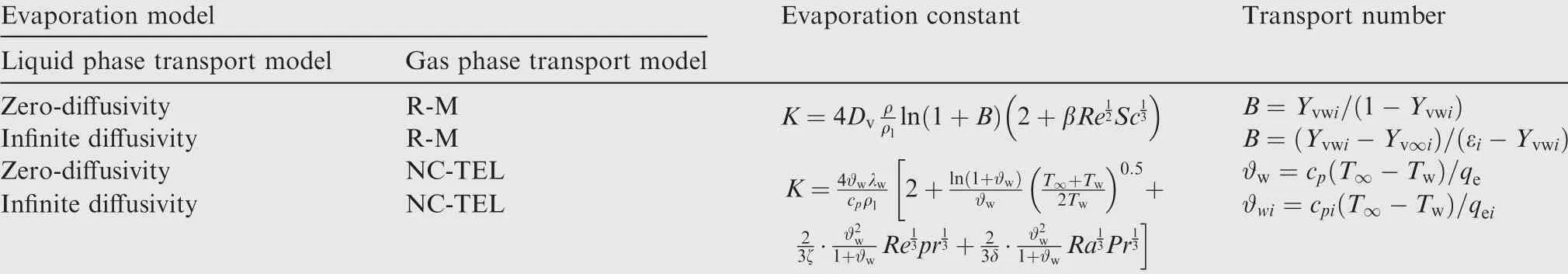
Table 1 Evaporation models for multicomponent droplet and calculation of evaporation constants.
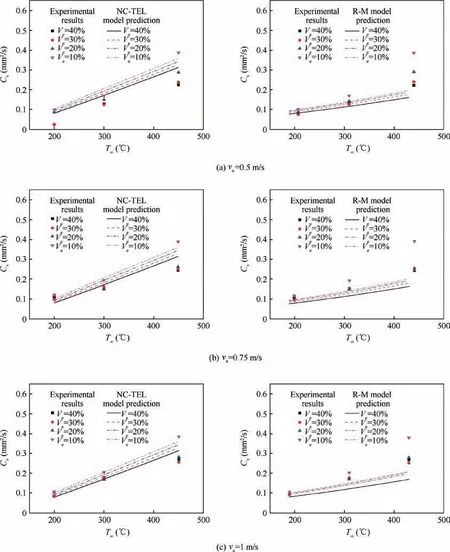
Fig.4 Comparison between infinite diffusion NC-TEL model and infinite diffusion R-M model(n-heptane-ethanol droplet,δ=3×103,ζ=5×103).
As can be seen from Fig. 7, for the droplet of n-decaneethanol blended fuel, zero-diffusion model agrees better with the experimental data,while the prediction of infinite diffusion model is higher. The results are consistent with the actual situation. Due to the large mass and high boiling point of ndecane molecules, the circulation inside the mixture droplet at medium and low temperature is weak. The diffusion rate is very limited, so the evaporation of the droplet mainly depends on the mass exchange between the surrounding air and the surface of droplet.
As can be seen from Fig.8,for the droplet of RP-3 aviation kerosene-ethanol mixture fuel,the situation is similar to that of n-decane-ethanol. The zero-diffusion model is in good agreement with the experimental data, while the infinite diffusion model predicts a larger value; in the zero-diffusion model,the component composition has a great influence on the evaporation rate, while in the infinite diffusion model, the component composition has little influence on the evaporation rate.
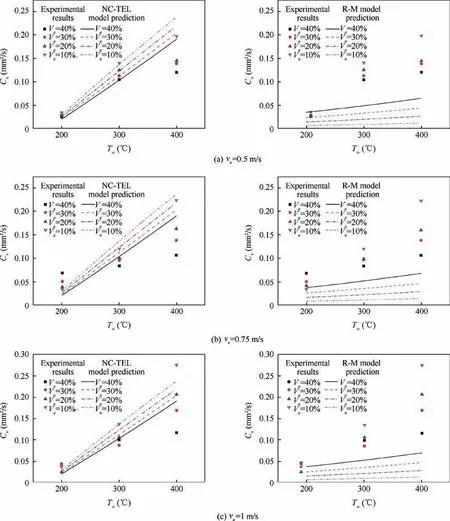
Fig. 5 Comparison between zero-diffusion NC-TEL model and zero-diffusion R-M model (n-decane-ethanol droplet, δ=2×103,ζ=1×103).
Further observation finds that for zero-diffusion model,the proportion of components has a great influence on the evaporation rate while the infinite diffusion model shows that the proportion of components has a small influence on the evaporation rate. This is because the infinite diffusion model believes that the internal diffusion rate of droplet is very large,and the mixed droplet with different proportion of components can be quickly mixed in the evaporation process. Therefore, the composition proportion has little influence on the evaporation rate of the whole mixed fuel droplet.
4.3. Evaporation characteristics comparison of different mixed fuels
As shown in Section 4.2,the evaporation characteristics of the three kinds of fuel droplets at middle and low temperature are similar. With the increase of environmental temperature, the evaporation rate of the fuel droplet increases significantly.With the increase of convection velocity, the evaporation rate of fuel droplet does not increase obviously, so the forced convection environment has little influence on droplet evaporation. Fig. 9 is the comparison between experimental results of three kinds of blended fuel droplet and zero-diffusion/infinite diffusion NC-TEL model. For n-heptane-ethanol and n-decane-ethanol droplet,with the increase of ambient temperature, the difference of the evaporation rate among different mixing proportion is more significant. The higher ambient temperature leads to more obvious influence of the composition proportion of fuel droplet on droplet evaporation. In addition,under the same test condition,the higher the ethanol content is,the lower the evaporation rate of the mixed droplets is, that is, the addition of ethanol has an inhibitory effect on the overall evaporation of the mixed fuel. However, for RP-3 aviation kerosene-ethanol fuel droplet, there is no significant difference in the evaporation rate among the fuel droplets with different composition proportion under the same test condition, that is, the addition of ethanol has little impact on the overall evaporation rate of the mixture droplet (see Fig. 10,the numbers in the figure represent volume fractions).
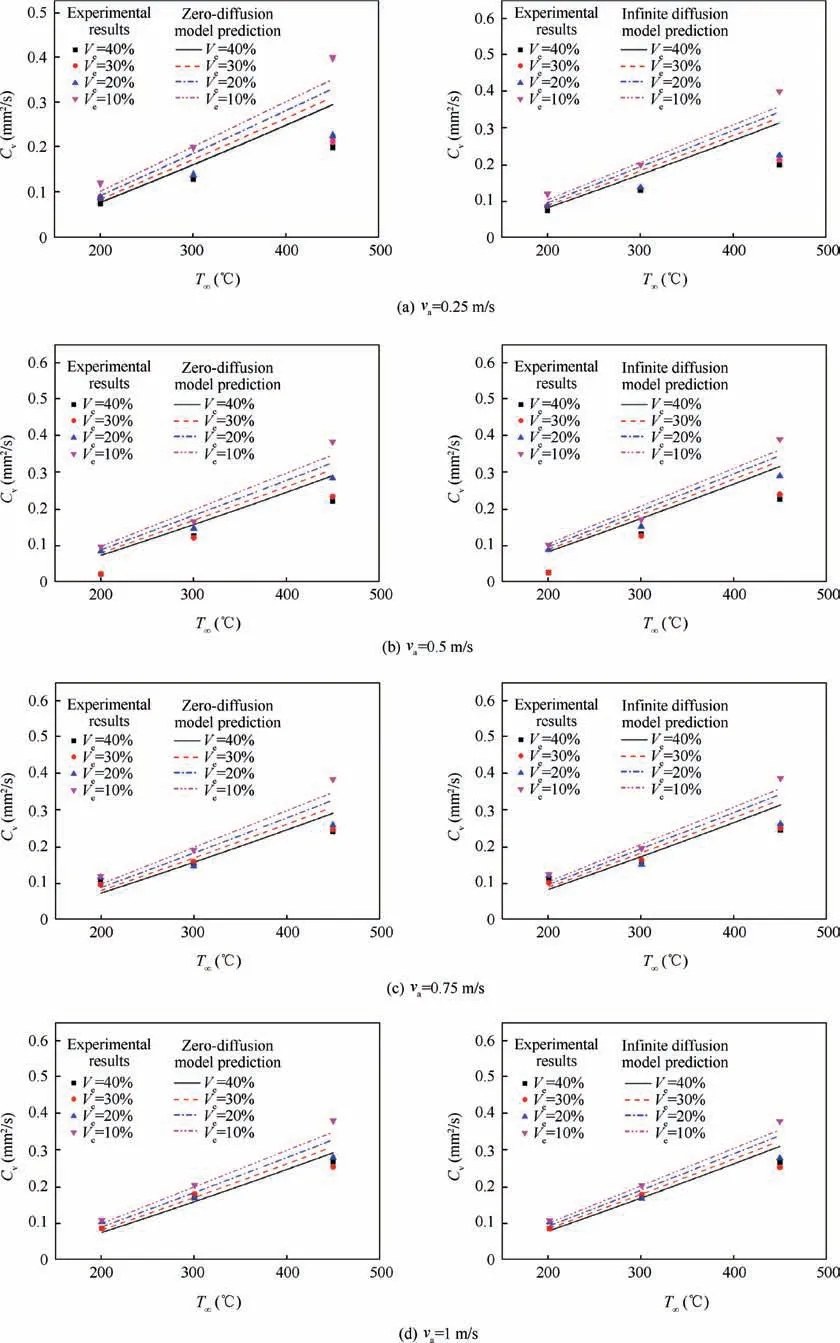
Fig. 6 Comparison between zero-diffusion NC-TEL model and infinite diffusion NC-TEL model (n-heptane-ethanol droplet,δ=3×103, ζ=5×103).
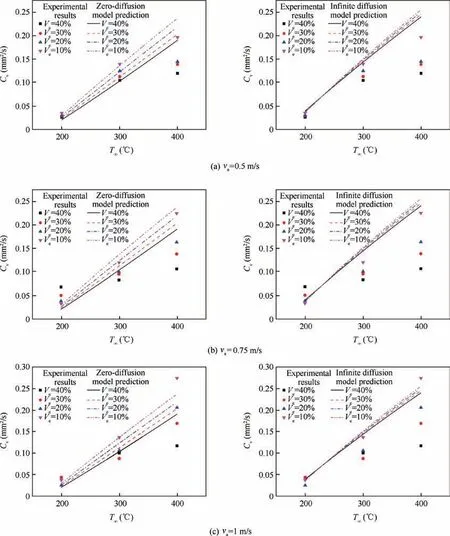
Fig. 7 Comparison between zero-diffusion NC-TEL model and infinite diffusion NC-TEL model (n-decane-ethanol droplet,δ=2×103, ζ=1×103).
At high temperature (600°C and 800°C), the blended fuel droplet evaporation process is more complex. Firstly, in static conditions, droplet evaporation at high temperature is accompanied by combustion,resulting in a significant increase in the average evaporation rate compared with that at medium and low temperature. For n-heptane-ethanol and n-decaneethanol fuel droplet, in low temperature static environment,these two kinds of droplets’evaporation rates are close to each other. But at high temperature, the evaporation rate of ndecane-ethanol is significantly greater than that of n-heptaneethanol. This may be due to the fact that the boiling point of n-decane is much higher than that of ethanol.And at high temperatures, there will be bubbles (ethanol gas) inside the mixed fuel droplet. The bubbles will continue to expand and release gas with evaporation process, which is the so-called microexplosion phenomenon.Micro-explosion takes a small amount of liquid away from the droplet surface and accelerates the evaporation process of droplet macroscopically. The boiling point difference of n-decane-ethanol is much larger than that of n-heptane-ethanol, so the high-temperature microexplosion phenomenon of n-decane-ethanol droplet is more obvious, resulting in a significantly higher actual evaporation rate.
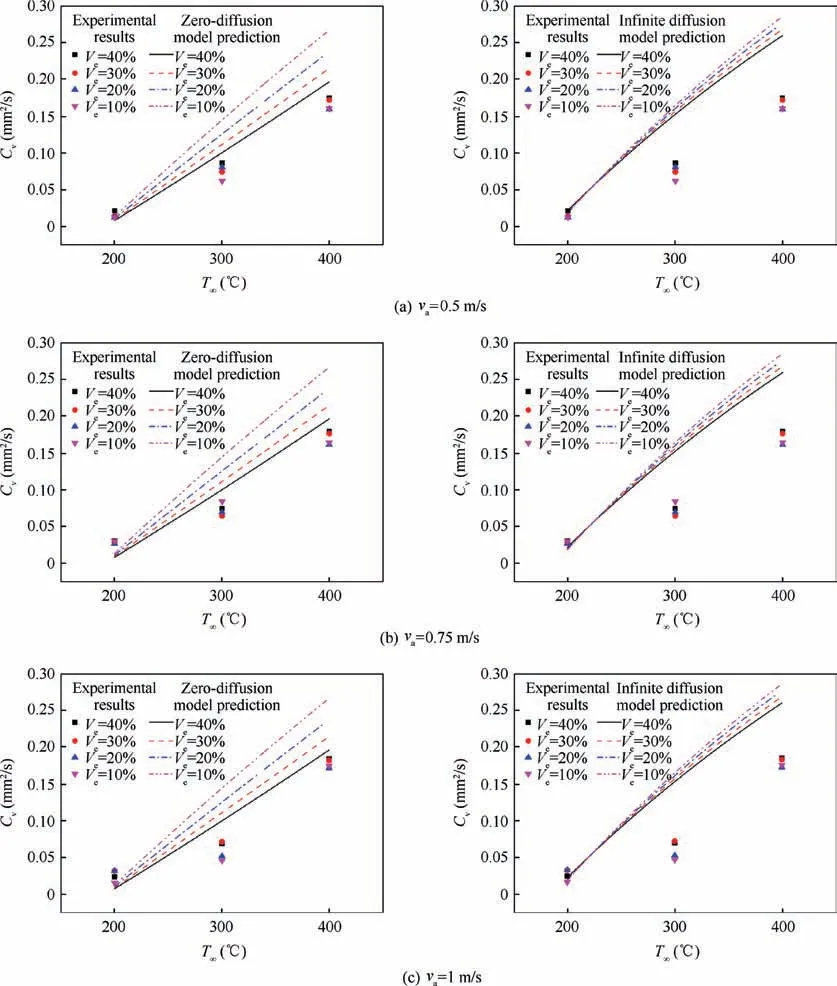
Fig. 8 Comparison between zero-diffusion NC-TEL model and infinite diffusion NC-TEL model (RP3 aviation kerosene-ethanol droplet, δ=1×103, ζ=2×103).
For the n-decane-ethanol fuel droplet, as a result of the existence of micro-explosion phenomenon, at high temperature (600°C and 800°C), both the zero-diffusion NC-TEL model and the infinite diffusion NC-TEL model’s forecasts are significantly lower than the experimental values. The reason may be that the model considers that there is only liquid phase but no gas phase inside the droplet during the whole evaporation process, so the influence of high-temperature micro-explosion cannot be considered, resulting in the significantly lower predicted value of the model. At 800°C, this is also the reason why the actual evaporation rate of RP-3 aviation kerosene-ethanol fuel droplet is higher than the model prediction. In addition, aviation kerosene itself is a complex mixture and its boiling range is wide. When evaporating at high temperature, bubbles exist for a long time and many times to vent gas resulting in the whole evaporation process appearing more complex. For the droplet of n-heptaneethanol mixture fuel, because the micro-explosion phenomenon is very weak or even does not exist, the prediction of the model at high temperature is consistent with the test value, and the model performs well.
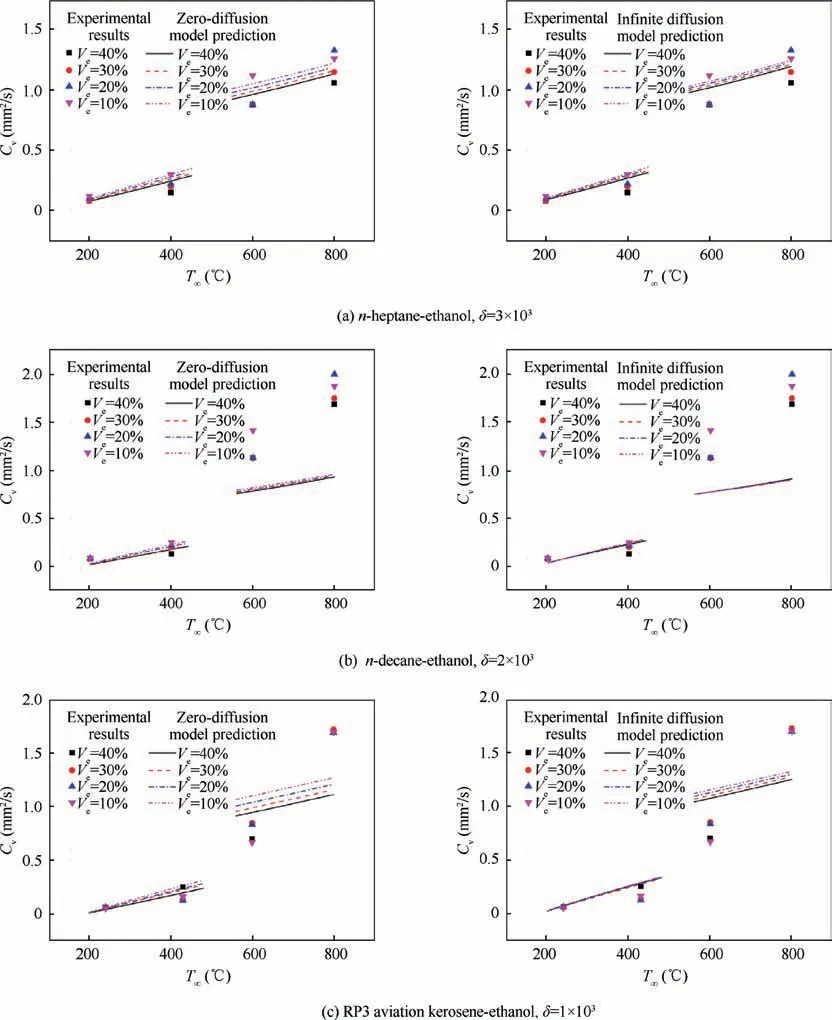
Fig. 9 Comparison between experimental results of three kinds of blended fuel droplet and zero-diffusion/infinite diffusion NC-TEL model.
In addition, different from the evaporation of single component droplet, the evaporation rate of different components is different when multicomponent droplet evaporates. During the evaporation process, the concentration of various components at the droplet surface (phase interface) changes continuously (volatile component concentration decreases rapidly),which results in surface tension gradient and thus Marangoni effect.This effect is a kind of interface instability phenomenon,which is manifested as spontaneous flow at the phase interface and has an impact on the two phases mass and heat transfer processes. Since the evaporation rate of n-decane and RP-3 aviation kerosene is quite different from that of ethanol(especially at high temperature), the Marangoni effect at the phase interface of the two mixed droplets is obvious in the evaporation process. As mentioned above, the Marangoni effect can enhance the mass and heat transfer processes of multicomponent droplets and increase the actual evaporation rate. Therefore, besides the micro-explosion phenomenon, Marangoni phenomenon may be another important reason why the actual evaporation rate of n-decane-ethanol and RP-3 aviation kerosene-ethanol mixed droplet at high temperature is significantly higher than that predicted by the model.
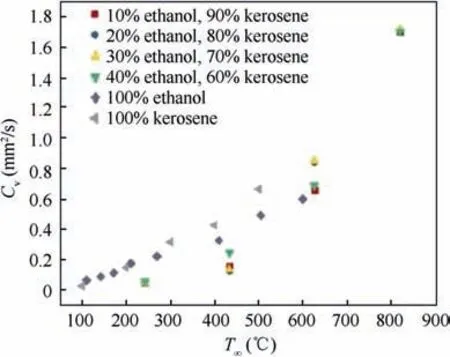
Fig.10 Evaporation rate of ethanol,kerosene and their mixtures vs ambient temperature.
5. Conclusions
(1) The evaporation rate of mixed fuel droplet increases significantly with the increase of ambient temperature.And the higher the temperature is, the greater the difference in evaporation rate between droplets with different components’ proportion is. In the experimental conditions,the increase of convection velocity does not have obvious effect on the evaporation of the three kinds of fuel droplets. Under the same experimental condition, the evaporation rate of the two kinds of alkane-ethanol droplets decrease with the increase of the ethanol volume fraction while the evaporation rate of RP-3-ethanol droplet has no obvious relationship with the ethanol volume fraction.
(2) The predicted value of R-M model for droplet hightemperature evaporation rate is significantly lower than the experimental value, that is, there are defects in the description of droplet high-temperature evaporation characteristics. In contrast, the thick exchange layer model considering natural convection not only performs well at middle and low temperature,but also can predict the evaporation rate of droplets more accurately at high temperature. In the high-temperature experimental condition (450°C), compared with the R-M model, the prediction accuracy of NC-TEL model for n-heptaneethanol droplet improves by 8% on average. And for n-decane-ethanol droplets, the increase is 35%.
(3) In general, compared with the infinite diffusion-thick exchange layer model,the zero-diffusion-thick exchange layer model is in better agreement with the experimental data at middle and low temperature(below 400°C).The main reason is that the zero-diffusion hypothesis at lower ambient temperature is more consistent with the actual situation, that is, the internal circulation of droplet is very weak at low temperature. For hightemperature conditions (above 500°C), the predicted evaporation rates of n-heptane-ethanol droplet by the two models are consistent with the experimental values,while the predicted evaporation rates of n-decaneethanol and RP-3-ethanol droplet are significantly lower than the experimental values. The reason may be that micro-explosion phenomenon exists in the latter two evaporation processes, which causes that the actual evaporation rate of the mixture droplet is very high,and micro-explosion phenomenon is not considered in the model calculation. In addition, according to the existing research results and theoretical analysis,Marangoni effect may also be an important reason why the actual evaporation rate of droplets at high temperature is higher than the predicted value of the model, but further experiments are needed.
Acknowledgements
This study was co-supported by the National Key R&D Program of China (Nos. 2017YFB0202400 and 2017YFB0202402) and the National Natural Science Foundation of China (No. 91741125).
 CHINESE JOURNAL OF AERONAUTICS2020年7期
CHINESE JOURNAL OF AERONAUTICS2020年7期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- An experimental method for squealer tip flow field considering relative casing motion
- A novel none once per revolution blade tip timing based blade vibration parameters identification method
- Highly efficient computation method for hazard quantification of uncontained rotor failure
- Optimal motion cueing algorithm for accelerating phase of manned spacecraft in human centrifuge
- Effective control allocation using hierarchical multi-objective optimization for multi-phase flight
- Fault-tolerant control and vibration suppression of flexible spacecraft: An interconnected system approach
