The role of transporters in cancer redox homeostasis and cross-talk with nanomedicines
∗Ruijie Chen∗∗
a Department of Pharmacy, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou 325027,China
b School of Pharmaceutical Sciences, Wenzhou Medical University, Chashan, Wenzhou 325035, China
Keywords: Cancer metabolism ROS,Redox homeostasis Membrane transporter xCT/SLC7A11 Nanomedicine
ABSTRACT Tumor cell usually exhibits high levels of reactive oxygen species and adaptive antioxidant system due to the metabolic,genetic,and microenvironment-associated alterations.The altered redox homeostasis can promote tumor progression,development,and treatment resistance.Several membrane transporters are involved in the resetting redox homeostasis and play important roles in tumor progression.Therefore,targeting the involved transporters to disrupt the altered redox balance emerges as a viable strategy for cancer therapy.In addition,nanomedicines have drawn much attention in the past decades.Using nanomedicines to target or reset the redox homeostasis alone or combined with other therapies has brought convincing data in cancer treatment.In this review,we will introduce the altered redox balance in cancer metabolism and involved transporters,and highlight the recent advancements of redox-modulating nanomedicines for cancer treatment.
1.Introduction
Cancer is remaining as one leading cause of death in the world.Even though a considerable number of investigations and great efforts have been made in cancer therapy during the past decades,patients are still suffering from therapy failure and high mortality.Therefore,new strategies to enhance therapeutic outcomes in patients with aggressive or resistant cancers are still very much needed.Recent investigations showed that the redox homeostasis in cancer cells was highly associated with the altered cancer metabolism,and was a hallmark of tumors with malignant progression and therapy resistance [1–5].Many pieces of evidence suggested that cancer cells exhibit higher levels of intracellular reactive oxygen species (ROS) than that in normal cells,as a combined result from metabolic,genetic,and microenvironmentassociated alterations [6,7].The elevated ROS levels in cancer contributed to cancer cell proliferation,invasiveness,and metastasis [8].For example,H2O2could oxidize and inactive the prolylhydroxylase 2 (PHD2) to stabilize hypoxia inducible factor 1α(HIF-1α),and the resultant increased HIF-1αlevel in tumor cells could further promote angiogenesis,metabolic reprogramming,and metastasis [9].The transcription factor,nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB),was also activated by ROS via epidermal growth factor receptor (EGFR) associated signaling to promote tumor development [10].However,the increased ROS could also induce genetic mutations and damages to proteins and lipids.To avoid that,the cancer cells displayed their increased antioxidant ability (including increased antioxidants and upregulated antioxidant enzymes) to against the increased ROS and adaptive to higher oxidative levels [2,11].
Plasma membrane transporters are critical for the absorption of nutrients,including glucose,vitamins,amino acids,and ions,for all mammalian cells [12].So far,over 400 transporters have been identified and characterized,which could be classified into two families:ATP-binding cassette(ABC) transporters and solute carriers (SLC) transporters.In cancer cells,the metabolic pathways have been changed,and the requirement for nutrients was markedly increased to support the malignant growth of tumors.Many transporters have been upregulated in cancers to satisfy the increased demand for nutrition [13].Several transporters have been confirmed to be involved in the intracellular redox homeostasis of cancers,e.g.,Cystine/glutamate transporter(xCT,SLC7A11),P-glycoprotein (P-gp,ABCB1),and breast cancer resistance protein (BCRP,ABCG2) [1,5].Taking xCT as an example,it transports extracellular cystine into cancer cells in particular for the maintenance of glutathione (GSH)level to protect cells from oxidative stress,and the enhanced GSH directly contributes to the redox balance that running at a high level [14].Due to the key function in redox homeostasis,xCT has been confirmed as a target for cancer therapy by generating cystine/cysteine starvation and thereby disrupting the redox balance in the tumor [15].In addition,xCT-mediated redox balance in cancer cells was supposed to be critically involved in chemo-,radio-,and photo-resistance,which also confirmed that xCT could be a viable therapeutic target for cancer therapy [1].
With the development of nanotechnology and biomaterials in the past decades,nanomedicine,the biomedical application of biomaterials using nanotechnology in pharmaceutical science,is emerging as one of the promising approaches in cancer therapy and so much evidence confirmed the enhanced efficacy of nanomedicine against cancer [12,16–21].Some of them have been approved to be used in clinic,including Doxil,Abraxane,and Genexol-PM.Unlike traditional medicine,nanomedicines were tending to be trapped in tumors from blood circulation with leaky blood vasculatures,which called enhanced permeability and retention (EPR) effect [20].Additionally,tumor specific ligands/antibodies were employed to modify the nanoparticles for active tumor targeting [12,22–26].Furthermore,nanomedicine could be designed to be responsive to the specific stimuli,including endogenous stimuli (e.g.,lower pH,certain types of enzymes,high content of GSH/ROS,ATPs and hypoxia) and external stimuli (e.g.,magnetic field,temperature,light,and ultrasound) for tumor specific treatment [27–32].The feasible modification to impart designed functionalities on the nanomedicine makes them more suitable for cancer drug delivery and cancer therapy.In recent years,nanomedicines targeted to modulate the redox balance of tumor cells emerge in the anti-cancer drugs[33–37].Many studies have elevated the intracellular ROS production to enhance treatment outcomes by chemotherapy,phototherapy,and radiotherapy.Different from those,some studies also showed evidence that an ambidextrous approach by simultaneously enhancing ROS production and inhibiting the reductive system to disrupt the intracellular redox balance could also achieve enhanced anti-tumor outcomes.
In this review article,we would like to briefly introduce the altered cancer metabolism and list involved transporters in redox homeostasis.Afterward,we summarize the reported redox-modulating nanomedicines for the cancer treatment and highlight the cross-talks that happens between nanomedicine and the specific membrane transporters.
2.Cancer metabolism and redox homeostasis
2.1.Altered metabolism in cancers
Many studies indicated that multiple metabolic alterations occurred in tumors with malignant progression,and these alterations fostered the uncontrolled growth of tumors by linking the altered metabolic pathways with the synthesis of essential components (like nucleotides,amino acids,and lipids) [6,38].ROS was mainly generated in the metabolic organelle,mitochondria,as a byproduct of mitochondrial electron transport chain (mETC) activity.In addition,it was well recognized that some antioxidative molecules,including GSH and nicotinamide adenine dinucleotide phosphate (NADPH),were often generated in the altered metabolic pathways to maintain the redox homeostasis.The intimate connection between cancer metabolism and redox balance has drawn many research interests,especially on glycolysis (Warburg effect),glutaminolysis,fatty acid oxidation (FAO),the pentose phosphate pathway (PPP) and one carbon metabolism(Fig.1).
2.1.1.Glycolysis(Warburgeffect)
Otto Warburg firstly observed the tumor cells employed glycolytic pathway in metabolism regardless of the presence of oxygen,and therefore this phenomenon was named as Warburg effect.Glycolysis is a critical pathway that glucose is metabolized to pyruvate in the cytosol.Due to the high demand for energy,Glucose transporter 1 (GLUT1,SLC2A1)was highly expressed in cancer cells to meet the requirement[13].Glucose was imported from the extracellular space via GLUT1,and then converted into glucose-6-phosphate (G6P) via hexokinase enzymes before finally metabolized to pyruvate,nicotinamide adenine dinucleotide (NADH),and adenosine tri-phosphate (ATP) [6].Pyruvate was further diverted into lactate,which was exported out of cells via monocarboxylate transporter 4 (MCT4,SLC16A3).The increased lactate production created an acidic tumor microenvironment,which promoted angiogenesis,extracellular matrix remodeling,immune evasion,and tumor metastasis.Several studies proved that the oncogene activation (i.e.,Ras and Myc)and the loss of tumor suppressors (i.e.,p53) facilitated the glycolytic shit [39,40].It should be mentioned that the glycolytic intermediates could be redirected to the other metabolic pathways,e.g.,contributing to the generation of NADPH and GSH by PPP and glutaminolysis,respectively.Therefore,glucose metabolism is of critical importance in the redox homeostasis in tumors,and the modulation of glucose metabolism and/or uptake could be an effective anticancer strategy.For example,Li et al.found that the inhibition of glycolysis,PPP,and thioredoxin (TRX) system was effective in suppressing the growth of pancreatic and breast cancer cells and more importantly,proved to be safe in normal cells,indicating the advantage of the glucose metabolism targeting approach in cancer treatment [41].
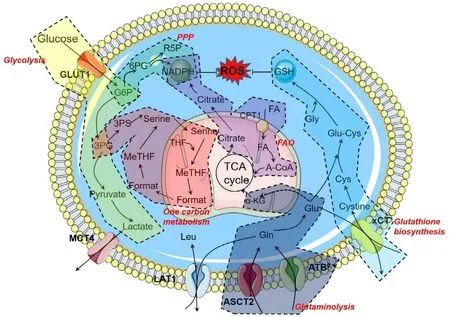
Fig.1–Altered metabolic pathways in the cancer cells.Schematic illustration of specific metabolic pathways involved in the redox homeostasis,including glycolysis,glutaminolysis,pentose phosphate pathway (PPP),fatty acid oxidation (FAO),and one carbon metabolism.In addition,GSH biosynthesis is also displayed.Abbreviations:G6P,glucose-6-phosphate;3PG,3-phosphoglycetare;3PS,3-phospho-serine;THF,tetrahydrofolate;MeTHF,5,10-methylene-tetrahydrofolate;6PG,6-phosphoglucono-1,5-lactone;R5P,ribulose-5-phosphate;FA,fatty acid;A-CoA,acetyl coenzyme A;CPT1,carnitine palmitoyltransferase-1;α-KG,alpha ketoglutarate.
2.1.2.Glutaminolysis
The increased demand for nutrients also fostered the increased expression of amino acid transporters in cancer cells [42].The accelerated influx of amino acids via these transporters drove upstream of related tumor-specific metabolic pathways.Accelerated glutamine metabolism has become a hallmark of tumor metabolism,called glutaminolysis [43].In the cancer cells,the tricarboxylic acid cycle (TCA cycle) is primarily driven by glutaminolysis,because pyruvate is converted into lactic acid in the cells.As a non-essential amino acid in normal cells,glutamine plays a crucial role in tumor metabolism,and the tumor cells cannot grow without excessive exogenous glutamine supply.Besides acting as a source of nitrogen and carbon for protein and nucleotide synthesis,glutamine is also critical in other metabolic pathways to support tumor growth.Glutamine is exchanging leucine by large neutral amino acid transporter 1 (LAT1,SLC7A5) to activate the mammalian target of rapamycin (mTOR) signaling,which is essential to cell growth and cell cycle progression [44,45].The metabolic intermediates of glutamine could sneak out from the TCA cycle and reach the cytosol,then generating ATPs and NADPH [46].This fact helps tumors keep GSH in a reduced state.In addition,glutamine also participates the GSH synthesis by directly converting to glutamate and also facilitating the xCT/SLC7A11-mediated cystine uptake [47].Besides that,Jin et al.found that glutamate dehydrogenase 1 in mitochondrial could positively regulate the activity of glutathione peroxidase (GPX) by manipulating the intracellar fumarate concentration [48].All these facts suggest that glutaminolysis represents a potential target for cancer therapy.Scientists have demonstrated that inhibiting glutaminolysis caused the intracellular GSH depletion and subsequent ROS production in human leukemia cells [49].A similar result was also found in neuroblastoma cells,and increased chemosensitivity was observed after glutamine deprivation [50].Collectively,these studies confirmed that glutaminolysis inhibition was rational to be a therapeutic approach to block cancer malignancy by disrupting the altered redox balance and adaptive resistance.
2.1.3.Pentosephosphatepathway(PPP)
As the major glucose metabolism pathway in cancer cells,PPP uses glucose-6-phosphate (G6P) catalyzed by hexokinase to generate NADPH and ribonucleotides (a precursor of nucleotide synthesis) [38].The PPP is also a critical hallmark of many cancers.As the rate-limiting enzymes of PPP,glucose-6-phosphate dehydrogenase (G6PD) catalyze G6P to 6-phosphoglucono-1,5-lactone (6PG) [51].And also,6-phosphogluconate dehydrogenase mediates the conversion of 6PG to ribulose-5-phosphate [51].Besides the precursor of nucleotide synthesis,NADPH is another important product during this process.NADPH could scavenge the harmful ROS for the maintenance of redox homeostasis and drive the anabolic processes.The latest evidence indicated that PPP also plays an essential role in cancer resistance.Polimeni et al.found that overexpression of G6PD in HT29 colon cancer cells enhanced doxorubicin resistance due to the increased NADPH content [52].By using the therapeutic method to prevent glucose entry into the PPP,oxidative stress was significantly increased,and the excessive ROS sensitizes multiple myeloma cells to bortezomib [53].In another study,2-deoxy-d -glucose and 6-aminonicotinamide were combined to inhibit glycolysis and PPP simultaneously,and this approach amplifies ROS production,which further sensitive the cancer cell to radiotherapy [54].These results suggest that the inhibition of PPP could be a promising approach for cancer therapy,or as a supplementary therapy for chemo/radio/phototherapy to reverse intrinsic or acquired resistance.
2.1.4.Fattyacidoxidation(FAO)
The FAO is a group of series oxidations occurring inside the mitochondria,in which NADH,flavin adenine dinucleotide(FADH2),and acetyl-CoA are produced to make ATP and support biosynthesis pathways [55].In cancer cells,NADH and FADH2 can be oxidized in the mETC to produce ATP,and acetyl-CoA enters the TCA cycle and converted into citrate[55].The citrate is exported to the cytosol to produce NADPH under the catalysis of isocitrate dehydrogenase 1 and malic enzyme,which plays a critical role in NADPH homeostasis and redox balance inside tumor [3].It has been found that carnitine palmitoyltransferase-1,a key regulator of FAO,was overexpressed in both leukemia and solid tumors [56,57].Pike et al.used etomoxir to inhibit the FAO in human glioblastoma cells and found that the production of NADPH and ATP was impaired,and oxidative stress-induced cell death was significantly increased [58].In a further study,a strengthened pro-apoptotic effect of cytotoxic agents was observed when the therapeutic inhibition on the FAO was applied in human leukemia cells [59].With the importance of FAO in the maintenance of redox homeostasis,targeting FAO could not only disrupt the redox balance to sensitize anticancer drugs but also interfere with the biosynthesis process,representing a novel and promising strategy for cancer therapy.
2.1.5.Onecarbonmetabolism
The one carbon metabolism is a complex metabolite network that is based on multiple chemical reactions that redistributes carbon units from glucose derivatives(mainly glycine and serine) or amino acids to the other cellular pathways (including the methionine cycle,the transsulfuration pathway and the folate cycle) [2,60].Folate was first reduced to tetrahydrofolate,and then further converted into methylenetetrahydrofolate under the catalysis of serine hydroxymethyl transferase.Afterward,the product can be converted to 10-formyltetrahydrofolate with the generation of NADPH.In the methionine cycle,S-adenosylmethionine is generated and further converted into homocysteine via S-adenosyl homocysteine hydrolase.The homocysteine was directed to the trans-sulfuration pathway and condensed with serine to cystathione by cystathionine synthase,and further metabolized to cysteine andα-Ketoglutaric acid via cystathione lyase.The metabolite cysteine could also fuel the GSH biosynthesis.One carbon metabolism is of great importance for cancer cell proliferation,and metastasis since it not only contributes to the synthesis of nucleic acid,lipids,and proteins but also helps to maintain the redox balance[61].It was also confirmed that cancer cells would overexpress one carbon metabolism related enzymes,including serine hydroxymethyl transferase 2,methylenetetrahydrofolate dehydrogenase 2,phosphoserine aminotransferase 1,and 3-phosphoglycerate dehydrogenase.Additionally,these enzymes were also regulated by antioxidant transcription factor nuclear factor-erythroid 2 related factor 2 (NRF2)[62–66].The compounds that could inhibit these enzymes provides an alternative option for cancer therapy.Antifolate(such as methotrexate and pemetrexed),fluorouracil,and gemcitabine have displayed significant anti-cancer effects by interfering with the altered one carbon metabolism in cancer cells [60,67].One carbon metabolism related redox modulation provides a valid therapeutic target for cancer treatment,which is of clinical interest.
2.2.Improved level of redox balance
It has been long acknowledged that tumor cells display higher ROS levels compared to that in normal cells due to the metabolic and genetic alternations.ROS is a kind of molecules containing oxygen with unpaired electrons,which could be generated in multiple cellular processes,including proliferation,differentiation,metabolism,and immune regulation.ROS could be divided into mainly two types,free radicals (like superoxide anion and hydroxyl radical) and non-radical molecules (like hydrogen peroxide and hypochlorous acid).These ROS were mostly produced as a byproduct of the mETC or induced by an exogenous stimulus,including chemotherapy,radiotherapy,and phototherapy[68,69].The mentioned factors jointly elevated the oxidative stress up to a high level.As compensation,the cancer cells will reinforce their antioxidative system to maintain the intracellular redox balance.For example,the cancer cells usually highly express antioxidant enzymes and result in the increased accumulation of antioxidant agents.As one of the major antioxidant enzymes,superoxide dismutases (SODs)can catalyze the conversion from highly reactive superoxide to less reactive H2O2.After that,H2O2is reduced to water and molecular oxygen by catalase.GPX,another antioxidant enzyme,is also responsible for the elimination of H2O2and lipid peroxide accumulation via reduced glutathione.In addition,the peroxiredoxin,TRX,and glutaredoxin are also jointly participated in the antioxidant defensive systems[3,70,71].Furthermore,the increased production of GSH and NADPH in cancer metabolism pathways compose the main antioxidant agents against the increased oxidative pressure.In sum,cancer cells usually run at a high level of redox balance with the enhanced antioxidative systems related adaptive protection.The ROS could interfere with biological macromolecules,like proteins,DNA,and lipids,and also involved in the regulation of redox signaling pathways.Furthermore,the high level of oxidative stress was associated with the malignant progression by inducing gene mutation,activating the inflammatory response,and stabilizing the HIF-1.Additionally,excessive oxidative stress also enhanced the antioxidative/reductive ability to protect cancer cells from ROS-induced cell death [7,9,10].However,studies showed that a high level of oxidative stress also leaves tumors cells more susceptible to additional elevated ROS.Therefore,the strategy that is aiming at distorying the intrinsic antioxidant system and enhancing ROS generation with a consequence of disrupted redox balance seems to be promising.Several research groups have confirmed the feasibility of targeting intracellular altered redox balance,including the antioxidant enzymes,as well as antioxidant agents for cancer therapy[36,72–74].More importantly,this strategy could be applied as a complementary treatment alongside traditional cancer therapy,e.g.,chemotherapy,radiotherapy,and phototherapy,to overcome the treatment resistance with the ability to destroy the adaptive protection in cancer cells.
3.Redox-associated drug transporter system and redox-regulated chemo/radio/photo resistance
3.1.xCT (SLC7A11)
As a consequence of metabolic reprogramming in cancer cells,oxidative stress was significantly increased.To maintain the intracellular redox balance,cancer cells would promote their antioxidant capability via increasing the expression of antioxidant enzymes and the production of endogenous antioxidants,like glutathione.xCT (also named as SLC7A11)is a transporter responsible for the transport of extracellular cystine into cells coupled with the release of glutamate from cells with a stoichiometry of 1:1 [75].xCT is overexpressed in many types of cancers to promote GSH synthesis,and thereby decreasing oxidative damage and mitochondrial apoptosis[47].Cysteine is a non-essential amino acid that can be biosynthesized in the human body from L -methionine.In cancer cells,the deficiency ofγ-cystathionase,one enzyme involved in the biosynthesis of cysteine,makes the pathway unable to proceed [14].Therefore,cysteine or cystine is essential for cancer cell growth and viability,and most cancer cells require xCT to help cysteine/cystine from the extracellular environment move inside.It has been demonstrated that xCT was upregulated in numerous cancers,including gliomas,pancreatic cancer,kaposi’s sarcoma,etc.[15,76,77].The overexpressed xCT in cancer cells could satisfy the increased requirement of cysteine/cystine.
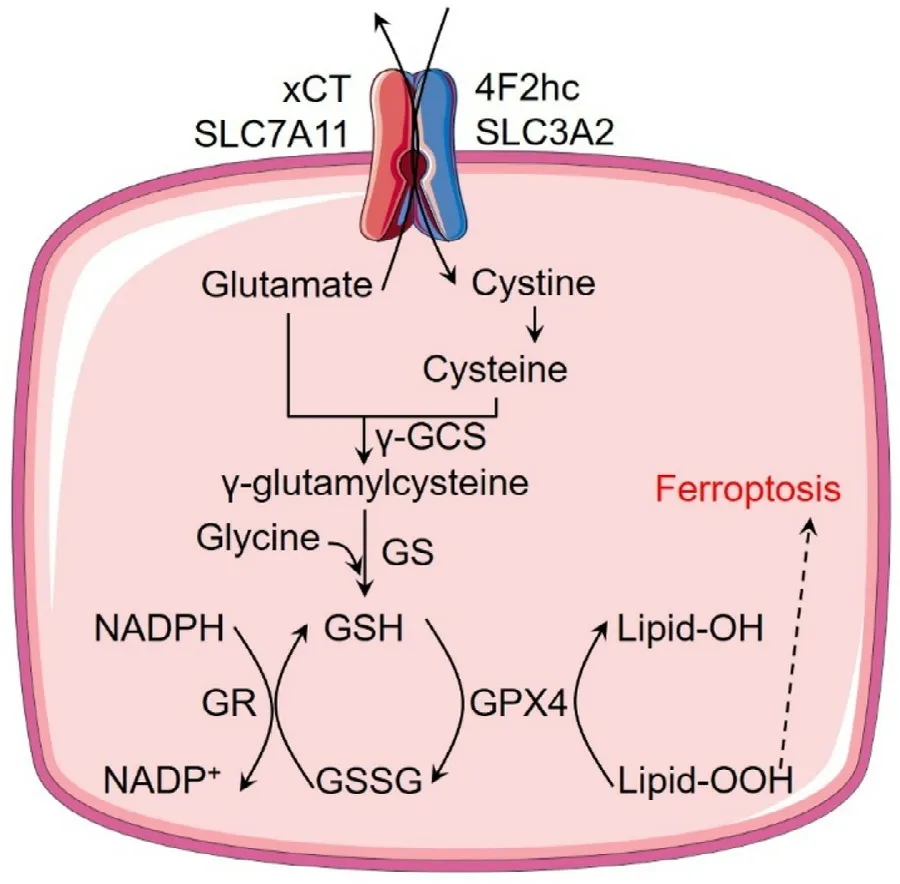
Fig.2–The role of xCT in cancer cells.xCT imports cystine into cells with the aid of 4F2hc and then cystine is converted into cysteine for GSH biosynthesis.GSH can be used to detoxify lipid peroxides by GPX4,and oxidative GSSG can be reduced back to GSH with the conversion of NADPH to NADP + by GR.Abbreviations:γ-GCS,γ-glutamylcysteine synthetase;GS,glutathione synthetase;GR,glutathione reductase;GPX4,glutathione peroxidase 4.
4F2hc (also named as CD98hc or SLC3A2),a type II membrane glycoprotein,is necessary for xCT to work (Fig.2)[14].While 4F2hc involves in the movement of the heterodimer to the plasma membrane,xCT determine the substrate specificity and link to 4F2hc with a disulfide linker to play the role.After imported into cells,cystine can be rapidly reduced to cysteine and then participate in the GSH biosynthetic pathway,providing the rate-limiting amino acid for the synthesis of the antioxidant peptide.xCT and the produced GSH are essential for the maintenance of the intracellular redox balance.What should be noted is that cysteine could also be imported into cells via alanine–serinecysteine transporter 2 (ASCT2,SLC1A5) [1].However,due to the extracellular oxidizing environment,the concentration of cysteine in blood plasma is only 10–20 μM,while the cystine concentration could be up to 100–200 μM (half cystine) [14].Therefore,it is cystine rather than cysteine that contributed to the GSH synthesis to a greater extent in the cancer cells.It is reasonable to conclude that xCT is the main contributor to translocate extracellular cystine into cancer cells and could be potentially exploited as a target to modulate the redox signaling.
Glutathione (GSH) is a tripeptide composed of glutamate,cysteine,and glycine.And among them,cysteine is the critical precursor.The biosynthesis of GSH contains two steps[11].Firstly,γ-glutamylcysteine was produced from cysteine and glutamate under the catalysis ofγ-glutamylcysteine synthetase (γ-GCS),which is the rate-limiting step due to the short supply of cysteine.Following that,glycine was conjugated toγ-glutamylcysteine by glutathione synthetase to generate the final product,GSH.GSH then serves as a cofactor alongside ROS detoxifying enzymes (like GPX) to scavenge peroxide-related components and protect cells from ROS-induced damage or death.Research data demonstrated that compounds like sulfasalazine and erastin,that could inhibit xCT,could significantly decrease the intracellular GSH concentration,and increase the ROS level,resulting in cell death [36,78,79].In recent years,this kind of cell death with cysteine depletion or xCT-inhibition mediated cysteine depletion was regarded as a new form of cell death,called ferroptosis [78,79].Ferroptosis describes a type of cell death that resulted from the accumulated lipid hydroperoxides and exhibit an iron-dependent manner.Usually,the lipid hydroperoxides could be converted to nontoxic lipid alcohol by GPX4 with the presence of glutathione.Therefore,pharmacological inhibition of xCT could result in GSH depletion and then induce ferroptosis.Besides its role in protecting cells from ferroptosis,xCT mediated GSH production could also protect cells from other stresses induced cell death,like apoptosis and necrosis [1,36].Overexpression of xCT in cancer cells could guarantee enough GSH production against oxidative stress or other stresses for redox balance and therapy resistance [80].As expected,pharmacological inhibition or siRNA silence of xCT could sensitize cancer treatment [81].In sum,xCT could remodel redox homeostasis and plays a pro-survival role in cancer cells.
The xCT transporter works in a Na+-independent method,and the driving force is mainly the intracellular glutamate,which is supposed to be converted from glutamine by glutaminase [1].Therefore,xCT transporter is usually coupled with glutamine transporter,ASCT2.ASCT2 is also highly expressed in cancer cells and responsible for the uptake of glutamine with a Na+-dependent manner [82].It should be noted that the oncogene,c-Myc,that induces the upregulation of both xCT and ASCT2 in cancer cells,could also induce the overexpression of glutaminase I,which makes xCT/ASCT2 coupling more effective [47].In addition,amino acid transporter B0+(ATB0,+,SLC6A14) was involved in xCT function since that it also can translocate glutamine into cells [83,84].The released glutamate could potentiate oncogenic signaling to further promote cancer development[85].Moreover,xCT was also found to be stabilized in the membrane by specific cancer associated variant of CD44 [86].Taken together,xCT was confirmed to be a promoter of cancer growth,and multiple transporters also involved in its function for redox balance.Cystine starvation,as well as interfering with the oncogenic signaling pathways,could be a potential strategy to inhibit xCT,and thereby destroy the intracellular redox balance in cancer cells.
3.2.Overexpression of ABC transporters
The resetting redox homeostasis was also implicated in drug efflux [87].ABC transporter family is mainly responsible for exporting drugs out of cells to protect cells from drug-induced damage/death,which is the primary mechanism for drug resistance.Therefore,the redox signals usually affect the drug efflux by interfering with the overexpression of ABC transporters.Liu et al.reviewed the imparts of the resetting redox homeostasis on drug resistance and highlight the role of redox reactions and redox signals on the expression of ABC transporters [5].The conformations of transporters were maintained by multiple chemical interactions,like the disulfide bond formed between cysteine residues of these proteins.The resetting redox homeostasis running at an elevated level could have an essential impact on the reversible disulfide bond formation,then influence the protein conformation and the transporter functions.In addition to the conformation change,the redox status could also induce the overexpression of ABC transporters in multiple levels,including transcriptional,translational,post-translational,and epigenetic regulations.For example,Glasauer et al.found that the upregulatedγ-GCS,a rate-limiting enzyme for GSH biosynthesis to maintain redox balance,could activate the transport function of several multidrug resistance-associated proteins (MRPs) [88].The intracellular stress induced by anticancer drugs could also contribute to the overexpressed ABC transporters.Clinical data have confirmed that recurrent cancers showed much higher levels of P-gp expression in patients that previously received chemotherapy.Additionally,P-gp and MRP2 were found to be activated by forkhead box protein O1 (FoxO1),the main regulator of redox balance and function in metabolic disease,in drug resistant breast cancer cells [89,90].Ke et al.used cisplatin and camptothecin to treat NCI-H446 cells,and they found the drug treatment upregulated the gene expression of BCRP and MRP2 by activating Ataxia telangiectasia mutated [91].In addition,Ge et al.found that the enhanced ROS exposure by xCT inhibition could significantly increase P-gp expression in MCF7 cells [92].All these results confirmed that the redox status in cancer cells could elicit the overexpression of ABC transporters.
It should be noted that NRF2,a redox-sensing transcription factor,is also involved in the GSH synthesis,redox homeostasis,and drug detoxification [93].The increased ROS can dissociate Kelch-like ECH-associated protein 1 from NRF2,and then NRF2 translocated into the nucleus and transactivated the target gene expression,e.g.,drug metabolism enzymes,cytoprotective genes,and ABC transporters.Bai et al.reported that the ABC transporter regulated by NRF2 included MRP1-5 and BCRP [93].The increased expression of NRF2 in cancer cells could promote tumor malignancy and chemoresistance,and even cancer stem cell resistance [4].
Moreover,ABC transporters are also critical in maintaining redox homeostasis.Nie et al.reported that BCRP/ABCG2 could relieve oxidative stress and inflammatory response by inhibiting the NF-κB signaling pathway in colorectal cancer cells.A lot of times,chemotherapeutics would be conjugated to the over-expressed GSH by glutathione S-transferases due to the self-protection mechanisms of cancer cells [94].The formed GSH-conjugated drugs could be easily exported out of the cell by MRP1 and MRP2,therefore decreasing drug concentration in cells and protecting cells from them-induced damage/death.Therefore,ABC transporters could not only be upregulated by the redox homeostasis of cancer cells but also contribute to resetting homeostasis.
3.3.Redox-regulated resistance
Drug resistance is a key factor contributing to the failure of chemotherapeutic treatments.ABC transporters mediated increased drug efflux plays a critical role in chemoresistance.As we mentioned above,redox homeostasis has been confirmed to have the capability to induce ABC transporter overexpression,therefore involved in the drug resistance of cancer cells.Besides the efflux transporter-mediated chemoresistance,redox homeostasis could also directly contributed to treatment resistance,including chemo-,radio-,and photo-resistance.
Firstly,antioxidant systems could directly hinder the therapeutic effect by scavenging ROS and abolished the drug effect.For example,platinum drugs could generate ultra-high ROS levels to exert anticancer effect [95].However,this effect could be inactivated by the elevated GSH level.This phenomenon was also observed in phototherapy and radiotherapy [96,97].While,buthionine sulphoximine,an irreversible inhibitor of gamma-glutamylcysteine synthase,could deplete intracellular GSH and destroy the antioxidant system,therefore enhancing paclitaxel cytotoxicity [98].In addition,the altered redox state could regulate the expression of certain enzymes,and potentially affect the drug activation,leading to failed outcome.For example,capecitabine is a prodrug which converted to 5 fluorouracil by thymidinephosphorylase.The increased H2O2from altered redox balance in cancer cells could make DNAmethyltransferase 1 (DNMT1) bind to chromatin resulting in increased DNA methylation,and this effect could inactivate the gene of thymidinephosphorylase,therefore hindering the conversion of capecitabine to 5 fluorouracil and causing drug resistance [99].Additionally,ROS caused DNA methylation of drug targets could also directly lead to resistance to anticancer drugs,like platinum drugs.
Redox-regulated autophagy was also involved in the acquired resistance.Autophagy was considered to have the ability to maintain cancer cell survival in a stress environment or with anticancer drug treatment.The altered redox balance has been shown to regulate autophagy,and more importantly,the increased ROS could induce autophagy[5].Mahoney et al.found that enhanced ROS induced by flavopiridol could lead to autophagy and drug resistance in chronic lymphocytic leukemia cells [100].When combining with the autophagy inhibitor,chemotherapy could achieve an increased anticancer effect.Shi et al.loaded anticancer drug docetaxel and autophagy inhibitor chloroquine into nano-sized micelles for enhanced anticancer therapy,and results showed that co-delivery micelles had combined therapeutic effect against cancer [101].Enhanced antioxidant system adaptive to the increased oxidative stress was also contributing to autophagy-mediated drug resistance.For example,the overexpression of catalase could facilitate the formation of LC3-II positive autophagosomes [102].It should be noted that autophagy plays a paradoxical role in cancers.Mei et al.delivered autophagy inducer rapamycin using polymer micelles to induce autophagic cell death first,followed with paclitaxel loaded micelles administration for cancer therapy [103].This strategy displayed excellent autophagy induction and synergistic antitumor efficacy,indicating that excess autophagy could also enhance the anticancer effects of chemotherapeutics.
Based on these results,targeting redox alterations with further increased ROS production and/or deregulated antioxidant capacity to disturb the redox balance provides a promising direction for resistant cancer therapy.
4.Nanomedicine strategies remolding redox balance for cancer therapy
As mentioned,the altered redox homeostasis plays a critical role in tumor progression,development,and treatment resistance.Targeting the redox balance for excess ROS production has approved to be a viable alternative for cancer therapy.In particular,nanomedicines drew much attention due to their outstanding properties,including increased drug solubility and stability,prolonged circulation,enhanced EPR effect,targeted drug delivery,and so on.In this section,we would like to introduce the nanomedicine strategies that target redox balance in cancer cells to exert anticancer effects.
4.1.Boosting free radical production
Current anticancer treatments,including chemotherapy,radiotherapy,and emerged phototherapy,in most cases,generate a great deal of ROS stress as one of the mechanisms against cancers.But the lack of selectivity could also lead to serious side effects since the normal cells were more sensitive to the ROS damage.The nanomedicine could make these traditional treatments smarter through precise targetability.It is worth noting that the Fenton reaction in which iron-or copper-catalyzed the formation of dangerous free radicals from comparable safe hydrogen peroxide is an effective method,and has been used in so many cases.An et al.prepared a lipid-coated calcium phosphate hybrid nanocarrier loading Fe3+and RSL3 (a GPX4 inhibitor for ferroptosis) as presented in Fig.3,and they firstly used ascorbate to increase the H2O2level in tumors and then used the hybrid nanoparticle for cancer therapy [104].The hybrid nanoparticles displayed accelerated drug release in tumors due to the pH-responsible property of calcium phosphate core.The released Fe3+converted H2O2to hydroxyl radical via Fenton reaction,therefore facilitating lipid peroxide accumulation.In addition,the released RSL3 restricted the detoxication by GPX4,further enhancing ferroptosis,resulting in a synergistic anticancer effect.Liu et al.developed an iron oxide nanoparticle-based RNAi strategy for cancer therapy[105].The nanoparticle could silience MCT4 to modulate cancer glycolysis,which resulting in tumor cell acidosis and H2O2accumulation.The increased H2O2further enhanced the treatment outcome via Fenton reaction.In order for further enhanced anticancer effects,the Fenton reaction could also be combined with other treatment options,including chemotherapy [106],phototherapy [107],immunotherapy [34],and even newly emerged tumor starving therapy [35].Yao et al.constructed a site-specific nanoreactor by encapsulating glucose oxidase and ferrocene in hyaluronic acid (HA)enveloped mesoporous silica nanoparticles (Fig.4) [35].After intravenous administration,this nanoreactor underwent a prolonged circulation and accumulated at the tumor site,and then endocytosed by tumor cells via CD44 receptor.In tumor cells,the HA shell was degraded by hyaluronidase,and the glucose oxidase and ferrocene were released for cascade catalytic reaction.The glucose oxidase converted glucose to gluconic acid along with the generation of H2O2,and ferrocene catalyzed upstream H2O2to hydroxyl radicals via the Fenton reaction.As a consequence,glucose exhaustion and generated free radicals could effectively kill cancer cells and suppress tumor growth,indicating a novel and effective strategy for cancer therapy.
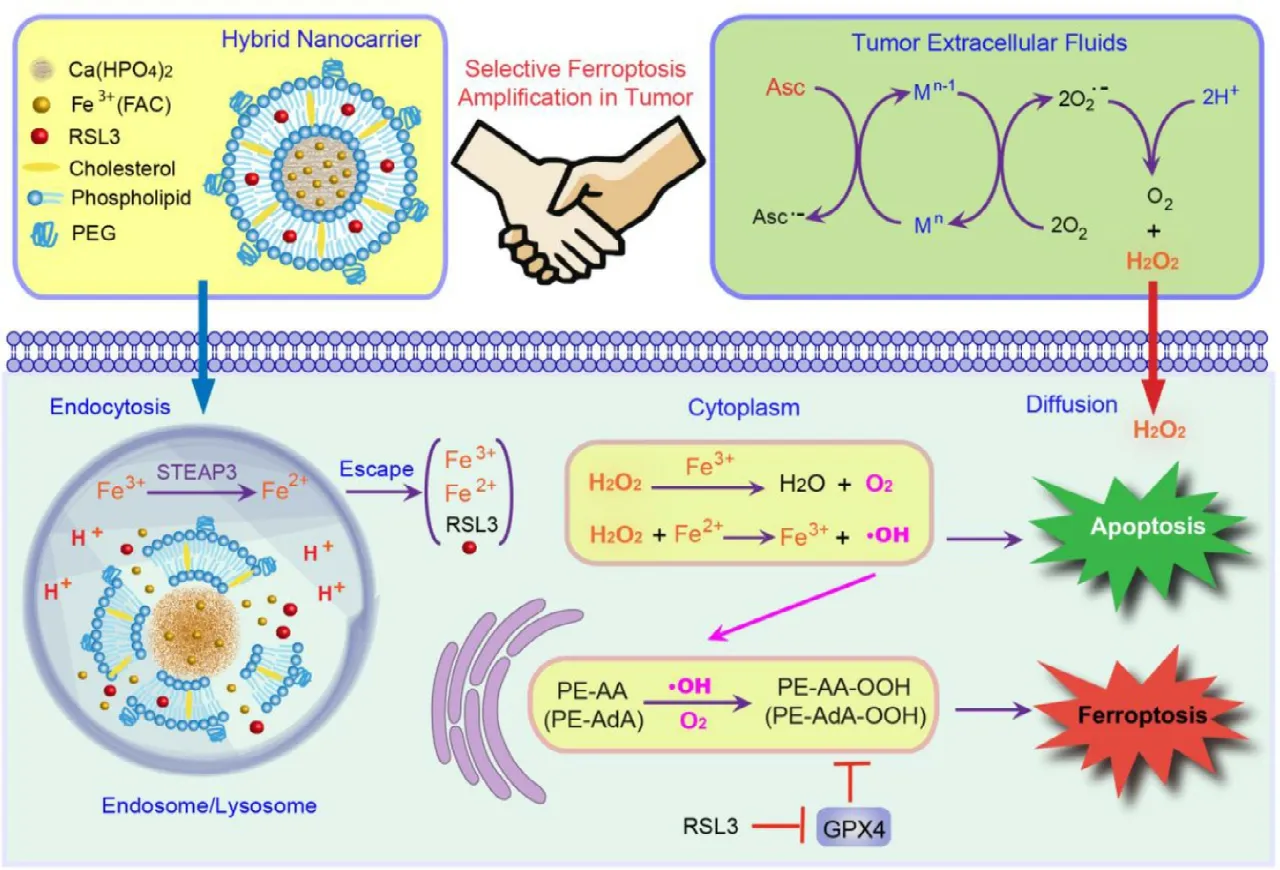
Fig.3–The ferroptosis inducing effect of the hybrid nanoparticles in the tumor.The hybrid nanocarrier was constructed by coating calcium phosphate core with the lipid bilayer with ferric ammonium citrate (FAC) loaded in core and RSL3 loaded in lipid layer.A high dose of ascorbate was used to induce H2O2accumulation in cancer cells.The nanocarrier could release Fe 3+ to induce Fenton reaction and RSL3 to inhibit GPX4,and the combined effect could induce and amplify ferroptosis.Reproduced with permission from [104],Copyright 2019 American Chemical Society.
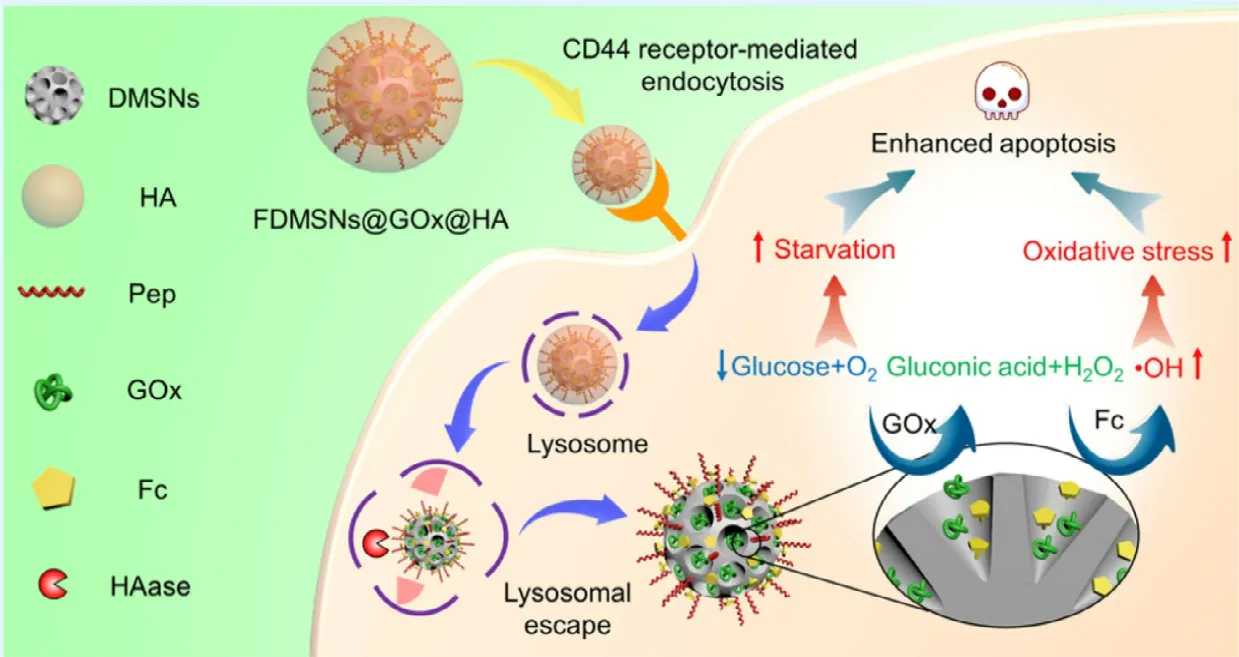
Fig.4–Schematic illustration of hyaluronic acid (HA) enveloped dendritic mesoporous silica nanoparticles co-loaded with glucose oxidase and ferrocene.After endocytosed into cancer cells via CD44 receptors,the nanoparticles were digested by the HAase and exposed the cargos.The glucose oxidase could accelerate glucose oxidation and H2O2generation.Then,the ferrocene converted H2O2to toxic hydroxyl radicals via Fenton reaction.The combined effect of glucose starvation and increased oxidative stress could effectively kill cancer cells and suppress tumor growth.Reproduced with permission from[35],Copyright 2019 American Chemical Society.
4.2.xCT-targeted approach for dual regulation
As mentioned above,xCT plays a critical role in the biosynthesis of GSH,and acts as a good therapeutic target to exhaust intracellular GSH for enhanced cancer therapy.Based on that,we designed an ultra-small nanoplatform using ZnO nanoparticles (ROS producer) as a carrier and loaded with salicylazosulfapyridine (an inhibitor of xCT),and this nanoparticle could disrupt the intracellular redox balance by increasing generation of ROS as well as decreasing synthesis of GSH [36].Theinvivodata showed that this nanoplatform could significantly suppress tumor growth even without any chemotherapeutics.In the following experiments,we found that phosphorylated p38 MAPK was significantly increased,indicating nanoplatform activating p38 MAPK signaling pathway.And the impaired redox balance stimulated the mitochondrial-dependent cell apoptosis pathway in this study.These results demonstrated that this ambidextrous approach has the potential for cancer therapy.It was believed that the anticancer effect would be further improved when combined with chemotherapeutics or other treatments.Zhu et al.developed a novel supramolecular nanodrug using chlorin e6 (a photosensitizer) and erastin (a specific xCT suppressor) by hydrogen bonding andπ−πstacking mechanisms [33].They combined phototherapy with xCT inhibition strategy for the anti-tumor application.It showed that the nanodrugs significantly suppressed the tumor growth with the decreased expression of xCT and Ki67,and increased cellular apoptosis as well as ferroptosis,indicating nanotechnology-based combination therapy provides an effective and more selective drug therapy for cancer treatment.
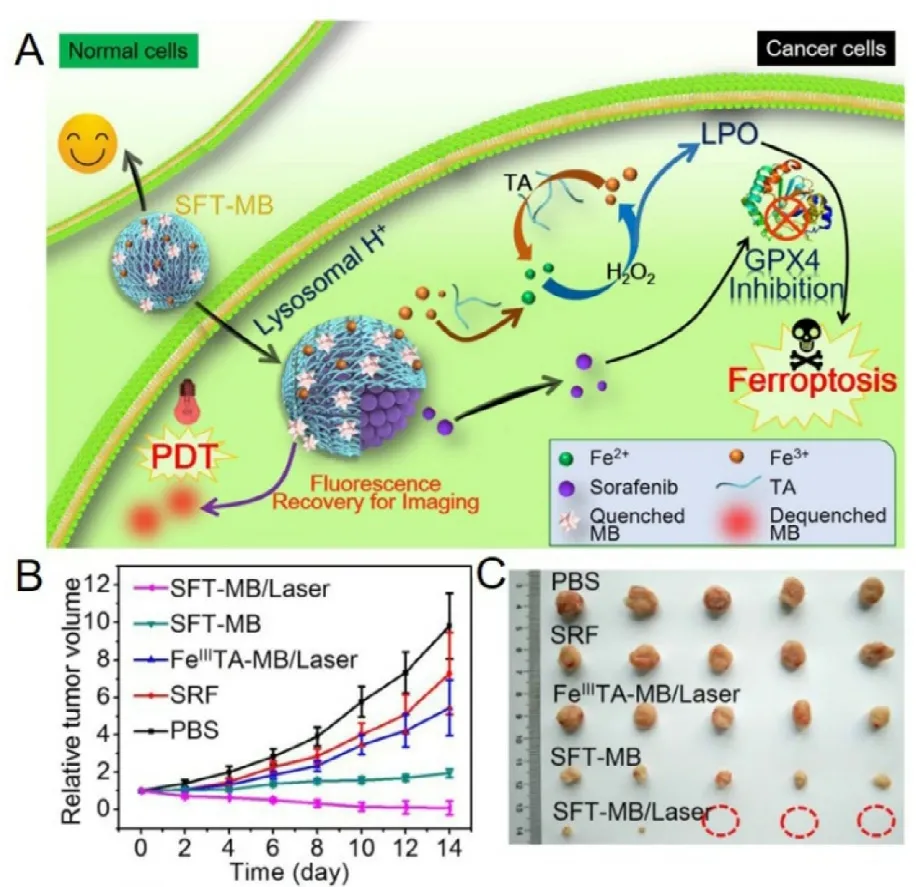
Fig.5–Schematic illustration of a sorafenib and methylene blue co-loaded nanoparticle with Fe 3+ ion and tannic acid formed network-like corona for antitumor application.A,The nanoparticles could respond to the acid environment with corona dissociation and then release sorafenib to inhibit xCT and then initiate ferroptosis,and meanwhile,tannic acid could reduce the conversion from Fe 3+ toward Fe 2+ to offer iron redox cycling for long term anticancer effect.B,The tumor volume change during treatment.C,the photos of tumors after treatments.Reproduced with permission from [108],Copyright 2018 American Chemical Society.
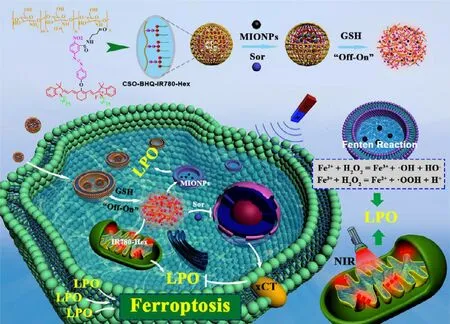
Fig.6–Schematic illustration of the functionalized delivery system promoting sorafenib-mediated xCT inhibition and iron-mediated Fenton reaction for cancer ferroptosis treatment.Reproduced with permission from [109],Copyright 2019 American Chemical Society.
Excessive lipid peroxidation leads to ferroptosis and cell death mainly through two pathways.First,xCT inhibition followed by GSH deprivation and GPX4 attenuation.Secondly,modulation of transition metal element mediated Fenton reaction along with increased free radical production.In some instances,ferroptosis could be enhanced with these two pathways combined.As presented in Fig.5,Liu et al.constructed a nanoparticle that could integrate the Fenton reaction and xCT inhibition for enhanced ferroptosis,and further combined with photodynamic therapy [108].They used Fe3+ion and tannic acid to form network-like corona onto sorafenib nanocrystal to construct the nanoparticles,and methylene blue (a photodynamic agent) was further loaded into the nanoparticles.After endocytosed into the cancer cell,the nanoparticles responded to the acid environment in lysosomes with corona dissociation and released the loaded cargos.The released Fe3+could be reduced to Fe2+with the presence of tannic acid,providing the ion redox cycle for Fenton reaction and following lipid peroxide accumulation;meantime,the released sorafenib could inhibit xCT to exhaust GSH,therefore inhibiting GPX4;these two effects jointly induce and amplify the ferroptosis.In addition,the absorbed methylene blue was used for fluorescence imaging and imaging-guided photodynamic therapy.The most exciting result was that the ferroptosis inducing nanoparticles combined with photodynamic therapy could achieve significant tumor suppression,and 60% of the tumors were fully eliminated.In a similar study,Sang et al.developed a concise functionalized delivery system loaded with IR780(photosensitizer),magnetic iron oxide nanoparticles (ion feeder for Fenton reaction),and sorafenib (xCT inhibitor)(Fig.6) [109].In addition,this system was magnetic targeting and GSH-responsive.Once endocytosed into cancer cells,the nanoparticles could release the cargos in response to the high level GSH.IR780 could generate a good deal of ROS under NIR irradiation,which could further promote iron release from the magnetic iron oxide nanoparticles.Sorafenib also attenuated xCT/GSH/GPX4 system,together with Fenton reaction,further enhanced lipid peroxidation and ferroptosis.Combined with photodynamic therapy,the complex nanoparticles effectively inhibited the tumor growth in a breast tumor mouse model.Similar results were also achieved in another independent study [110].These results suggested that nanomedicines could amplify xCT inhibition as well as the anticancer effect.
4.3.Other dual regulation approaches
Adapted to the increased ROS level,GSH level was also improved in cancer cells,which was closely related to the tumor progression and chemoresistance.Besides the xCT inhibition,other strategies were also proposed to exhaust GSH to reset redox balance for enhanced anticancer therapy.Manganese dioxide nanoparticle (MnO2) is a good case to reduce the GSH level,which could combine with chemotherapy or phototherapy for enhanced therapeutic outcomes.Wang et al.developed a type of arginine-rich manganese silicate nanobubbles that could effectively deplete GSH and thus induce ferroptosis [37].The results showed a remarkable tumor suppression by the designed redoxtargeting nanobubbles.Also,the nanobubble could further enhance T 1 -weighted magnetic resonance imaging and chemotherapy for synergistic cancer therapy.In a further study,Wang et al.designed a redox-and light-responsive nanoparticles composed of a core with ultrafine Fe3O4nanoparticle engineered hollow carbon framework and a shell with nanoflower-like MnO2[111].Under the high level of GSH in tumor cells,the nanoparticles could be degraded and activated by both elevated ROS levels from Fenton reaction and chemotherapy,and GSH depletion from MnO2function.It was showed that the nanoparticle achieved near-complete killing effects in cancer cells bothinvitroandinvivo.Moreover,nanomedicine loading thioredoxin reductase inhibitor orγ-GCS inhibitor could also disrupt intracellular redox balance and achieve enhanced treatment efficacy [112,113].These results suggested that this type of nanoparticles could advance current chemotherapy-based cancer treatment for the enhanced outcomes.
5.Conclusions
Due to the genetic,metabolic,and microenvironmentassociated alterations,cancer cells displayed adaptive redox homeostasis that running at a high level.The altered redox homeostasis promotes tumor progression,development,and even treatment resistance via activating multiple redox signaling pathways.Even though significant advances have been achieved in the last few decades,much more effort is still required to reveal and understand the underlying molecular mechanisms.What should be mentioned is that membrane transporter also participates in or responds to the redox balance,especially xCT (SLC7A11) and some ABC transporters.These transporters are critically important in tumor progression and resistance.Moreover,nanomedicine has drawn much more attention in these years due to the nanoparticulate related unique properties.What exciting is that these nano drug delivery systems have been tried to be used to target or reset the redox homeostasis for cancer treatment,which has been confirmed bothinvitroandinvivo.In addition to the traditional treatments induced redox alteration,specifically targeting the redox homeostasis for further free radical production or GSH depletion also brings in new opportunities for redox targeting.When this strategy combined with other treatments,like chemotherapy,phototherapy,immunotherapy,the treatment outcome was further amplified.However,what should be noted is that the toxicity and side effects are required for further evaluation since a considerable mass of metallic elements was composed in the designed complex nanomedicine.How to push the nanomedicine based redox targeting into clinic is another challenge,as a supplement treatment or as a new drug?In conclusion,remodeling redox balance is of clinic interest for cancer therapy and could be further developed with the advantages of nanotechnology.The redox targeting nanomedicine could be either used alone to kill cancer cells or combined with current clinic treatments for improved anticancer effects.
Declaration of interest
The authors report no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81803443,81903551),the Natural Science Foundation of Zhejiang Province (L Q19H300001),the Wenzhou Science and Technology Bureau (Z Y2019007,Y20180180,Y20180208,Y20190177),and the start-up funds from the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University.
 Asian Journal of Pharmacentical Sciences2020年2期
Asian Journal of Pharmacentical Sciences2020年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- The solute carrier transporters and the brain:Physiological and pharmacological implications
- Intestinal OCTN2-and MCT1-targeted drug delivery to improve oral bioavailability
- Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis
- Stimulatory effect on the transport mediated by organic anion transporting polypeptide 2B1
- Amino acid transporters:Emerging roles in drug delivery for tumor-targeting therapy
- Glutamine transporters as pharmacological targets:From function to drug design
