The solute carrier transporters and the brain:Physiological and pharmacological implications
a School of Pharmaceutical Sciences, Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education),Tsinghua University, Beijing 100084, China
b Advanced Innovation Center for Human Brain Protection, Beijing Anding Hospital, Capital Medical University, Beijing 100088, China
c Collaborative Innovation Center for Biotherapy, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, West China Medical School, Sichuan University, Chengdu 610041, China
Keywords: Solute carrier transporter Brain disorder Blood-brain barrier Drug
ABSTRACT Solute carriers (SLCs) are the largest family of transmembrane transporters that determine the exchange of various substances,including nutrients,ions,metabolites,and drugs across biological membranes.To date,the presence of about 287 SLC genes have been identified in the brain,among which mutations or the resultant dysfunctions of 71 SLC genes have been reported to be correlated with human brain disorders.Although increasing interest in SLCs have focused on drug development,SLCs are currently still under-explored as drug targets,especially in the brain.We summarize the main substrates and functions of SLCs that are expressed in the brain,with an emphasis on selected SLCs that are important physiologically,pathologically,and pharmacologically in the blood-brain barrier,astrocytes,and neurons.Evidence suggests that a fraction of SLCs are regulated along with the occurrences of brain disorders,among which epilepsy,neurodegenerative diseases,and autism are representative.Given the review of SLCs involved in the onset and procession of brain disorders,we hope these SLCs will be screened as promising drug targets to improve drug delivery to the brain.
1.Introduction
Solute carriers (SLCs) are a major superfamily of membrane transporters with 439 members (excluding pseudogenes)classified into 65 families,and they mediate the exchange of substances,such as ions,nutrients,signaling molecules and drugs across biological membranes ([1–4]and http://slc.bioparadigms.org).The brain is the body’s most delicate and complicated organ and maintains its internal homeostasis with the assistance of the blood–brain barrier(BBB) and blood–cerebrospinal fluid barrier (BCSFB).The brainparenchymal cells mainly consist of neurons,astrocytes,microglia,and oligodendrocytes.A total of 287 SLC genes have been identified in brain,especially in the cells that comprise the barriers and parenchymal cells,through which a variety of substrates,including sugars,amino acids,vitamins,neurotransmitters,and inorganic/metal ions are transported(Table 1,slc.bioparadigms.org and ascot.cs.jhu.edu).SLCs expressed in the endothelial cells of the BBB contribute to keeping the brain isolated from toxic substances and are necessary for absorbing essential components from the blood,while SLCs expressed in the choroid plexus of BCSFB regulate secretion and re-absorption of the cerebrospinal fluid (CSF).SLCs expressed in neurons and glial cells play irreplaceable roles in brain homeostasis maintenance and drug response regulation.Therefore,SLCs participate in cell type-specific drug delivery,and they are considered direct drug targets for treatment of various conditions [5–7].The basic characteristics of brain-expressed SLCs are summarized in Table 1 .

Table 1–The basic characteristic of the SLCs in human brain.60 out of 65 SLC families have members identified in the brain;these SLCs transport a variety of substrates and perform multiply functions.Information on SLC families discovered to date and their substrates were retrieved from slc.bioparadigms.org,ascot.cs.jhu.edu,and genecards.org.
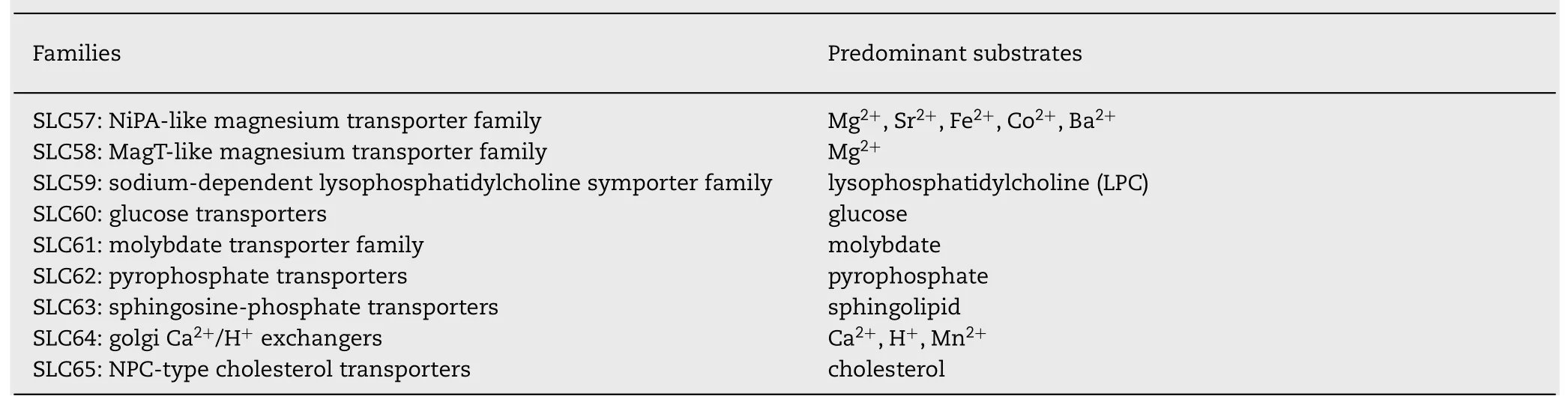
Table 1 (continued)
Membrane transport can be divided into two types:passive and active [8–10].Passive transport can be divided into diffusion and facilitated diffusion,while active transport includes primary active transport (transport protein contains ATPase,including ABC (ATP-binding cassette) transporters and P-type ATPases) and secondary active transport [11,12].SLCs function by facilitative diffusion and secondary active transport.Passive transport facilitates the movement of solutes and ions across the membrane and is usually accompanied by spontaneous and stochastic conformational changes.For example,SLC2A1 transports glucose across the BBB in a passive transport profile.Secondary active transporters transport the substrates and another solute or solutes (most typically ions) in the same or opposite directions utilizing preexisting gradients of ions as a source of energy [13].For instance,SLC1A2,which is mainly localized in astrocytes and responsible for over 90% of total glutamate uptake,co-transports Na+and H+and antiports K+to achieve glutamate transport [14].Symporters and antiporters are two types of secondary active transporters that utilize downhill ion gradients as the driving force in the same (symporter)or opposite (antiporter) direction of substrate transport[15,16].
SLCs have been frequently reviewed as drug targets,and developing new drugs with SLCs in mind can help improve bioavailability of the drugs due to SLCs involvement in drug disposition,drug–drug interactions,and corresponding toxicity [17–22].We aim to summarize the physiological characteristics of SLCs in the brain and the potential of SLCs to be efficient drug targets for the treatment of brain disorders.
2.The characteristics of SLCs in the brain
2.1.Localization of SLCs in the brain
2.1.1.TheBBBandBCSFB
The BBB is formed by endothelial cells,astrocyte end-feet,and pericytes.Disruption of the BBB is often the result of increased permeability,leading to various brain disorders,such as multiple sclerosis [23],hypoxia and ischemia [24],Alzheimer’s disease (AD) [25],and epilepsy [26].Passive diffusion,ABC and SLC mediated transport,transcytosis,and mononuclear cell migration are all available routes across the BBB.SLCs expressed in the BBB,especially members of the SLC7A,SLCO,and SLC22A families,have been compared to SLCs in other regions within the brain [27–30].SLCs in the BBB mediate the transport of various substrates and play crucial roles in brain homeostasis and drug delivery.SLC22A5,a well studied SLC in the BBB that act upon carnitine,can stimulate the synthesis of acetylcholine,decrease oxidative stress,and prevent neurodegeneration [31].SLC22A5 possesses great potential to facilitate drug delivery across the BBB through the following two strategies:(1) a dual prodrug strategy;Lcarnitine conjugated with nipecotic acid as a ‘double prodrug’passes through the BBB via SLC22A5 [32];(2) SLC22A5-targeted nanoparticles;SLC22A5 is expressed in the glioma cells,and L-carnitine-conjugated nanoparticles have been developed to enhance the permeability of nanoparticles across the BBB and uptake by glioma [30,33].Another representative example of SLCs in the BBB is the SLCO family.Members of SLCO can trigger the blood-to-brain transport of opioid analgesic,such as deltorphin II and DPDPE ([D-penicillamine(2,5)]-enkephalin),and are potential targets for the treatment of pain and cerebral hypoxia [34,35].
The turnover rate of human CSF is about 4 times per day,which requires a tightly-regulated ion and water transport system.Horace et al.(2012) usedinsituhybridization data of SLC genes in mice to show that 80% of SLCs were expressed in the BCSFB and 28 were expressed at the highest levels[36].These SLCs were involved in CSF production,drugclearance from the CSF,and transports of inorganic/metal ions,amino acids,carbohydrates,fatty acids,monoamines,and other substrates.Specific inhibitors or gene-knockout mouse models are in urgent need for further studies in the roles of SLCs in the BCSFB.

Table 2–Substrates of the brain SLCs.Seven kinds of endogenous substrates and the examples are shown for SLC families.Data were retrieved from genecards.org.
2.1.2.Astrocytesandneurons
Astrocytes play essential roles in various brain activities,in part by manipulating SLC functions.Despite the fact that most SLC families have members expressed in astrocytes,the characterization of their functions remains at initial stage and require further investigation.The functional expressions of SLCs in astrocytes have been summarized with an emphasis on several well-established families,including SLCO,SLC22A,and SLC29A,which are an organic anion transporter family,an organic cation/anion/zwitterion transporter family,and a facilitative nucleoside transporter family,respectively[37,38].These SLCs in astrocytes have the potential to be targeted by drugs against various CNS disorders.For example,SLC22A3 is expressed in astrocytes in several brain regions,including striatum,hippocampus,and hypothalamic nuclei,and is capable of transporting histamine,norepinephrine,and epinephrine.The inhibition of SLC22A3 is expected to improve the efficacy of anti-depressant drugs [37,39].SLC1A2 and SLC1A3 are Na+-dependent glutamate transporters mainly expressed in astrocytes and function in modulating glutamatergic activity in the brain.Dysfunction or mutation of SLC1A2 and SLC1A3 have been extensively studied in many brain disorders,including epilepsy,AD,Parkinson’s disease(PD),amyotrophic lateral sclerosis,major depressive disorder,and addiction [14,40–42].
All of the 60 SLC families in the brain contain members expressed in neurons.The presence of either SLC17A6/A7 or SLC32A1 defines excitatory and inhibitory neurons,respectively.SLC17A6 and SLC17A7 are mainly expressed in glutamatergic neurons mediating glutamate reuptake into synaptic vesicles at excitatory presynaptic nerve terminals [43];SLC32A1 is expressed in GABAergic neurons and mediates the uptake of GABA and glycine into the synaptic vesicles [44].SLC17A6/A7 and SLC32A1 may also be present in the same nerve terminals in subsets of neurons,indicating that both glutamate and GABA can be released from a single nerve terminal [45,46].SLC17A6/A7 and SLC32A1 play important roles in normal glutamatergic and GABAergic neurotransmission [47].Reduced expression of SLC17A7 leads to enhanced anxiety,depressive-like behavior and impaired recognition memory in mice [48].The SLC18A family is responsible for the transport of other small molecule neurotransmitters besides glutamate and GABA into synaptic vesicles.Vesicular acetylcholine transporter SLC18A3 overexpression induces major modifications of cholinergic interneuron morphology and function [49].Reduced SLC18A3 favors antidepressant behaviors in female mouse brain[50].The SLC6A family transports amino acids or amino acid-like substrates into cells in a Na+-dependent manner.SLC6A1 and SLC6A11 transport GABA into neurons and glial cells,respectively;SLC6A2 transports norepinephrine;SLC6A3 transports dopamine and SLC6A4 transports serotonin [51,52].SLC6A3,one of the most investigated neurotransmitter transporters,is involved in the pathogenesis of a number of brain disorders [53,54].
SLCs can also be detected in other brain cell types,including oligodendrocytes and microglial cells.For example,SLC44A1,localized to oligodendrocytes,is involved in membrane synthesis for cell growth and remyelination[55].SLC2A1 dominates glucose uptake in microglia under inflammatory conditions and targeting SLC2A1 could be effective for ameliorating neuroinflammation [56].Nevertheless,given the lack of functional studies of SLCs expressed in oligodendrocytes and microglial cells,these are not summarized in this topic.
2.2.Substrates and functions of the SLCs
Various substrates are transported by 60 families of SLCs expressed in the brain (Table 1) and include energetic substrates,amino acids,neurotransmitters,inorganic ions,metals,organic anions,and vitamins,as summarized in Table 2 .The multiple functions of SLCs in the brain are fulfilled by transport of these substrates.Specific functions of SLCs are summarized in the following sections.
2.2.1.SLCsfunctioninenergymetabolism
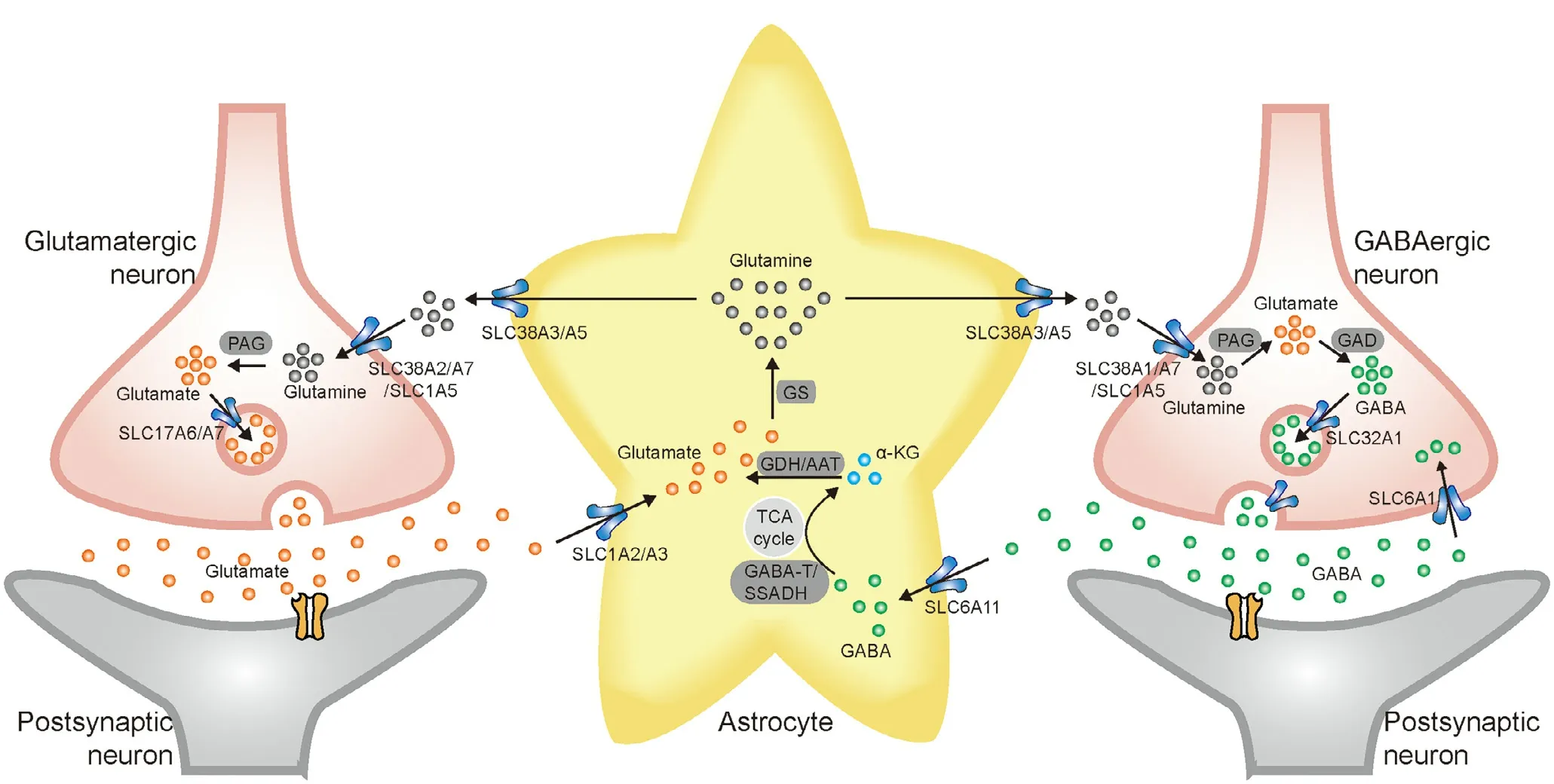
Fig.1–The main solute carrier transporters involved in glutamate/GABA-glutamine cycle.Glutamate is packaged into synaptic vesicles in pre-synaptic neurons by SLC17A6/A7.After release into the synaptic cleft and stimulating post-synaptic neurons,the signal is terminated by astroglial uptake of glutamate via SLC1A2/A3.Glutamate is converted to glutamine via glutamine synthase (GS).Glutamine is transported out of astrocytes through SLC38A3/A5,shuttled to neurons via SLC38A2/A7/SLC1A5,and converted to glutamate by phosphate-activated glutaminase (PAG) to complete the glutamate–glutamine cycle.GABA is packaged into synaptic vesicles by SLC32A1 and released into the synaptic cleft.SLC6A11 and SLC6A1 are responsible for GABA uptake in astrocytes and neurons,respectively.GABA is metabolized to α-ketoglutarate (α-KG) via TCA cycle and then glutamate.The following steps are similar to the glutamatergic synapses until glutamate is converted to GABA by glutamate decarboxylase (GAD) in GABA-ergic neurons.GABA is repackaged in vesicles for further synaptic release.
The energy demands of the brain are very high and glucose is the obligatory energy substrate of the adult brain [57,58].Glucose is a polar,hydrophilic molecule that is transported largely via the glucose transporter SLC2A [59].Ten SLC2A members are expressed in the brain,among which SLC2A1 and SLC2A3 play a major role in brain glucose uptake [60,61].SLC2A1 deficiency syndrome,characterized by a range of phenotypes that include epilepsy,intellectual disability,and ataxia,is a rare neurological disorder that impairs glucose transport across the BBB [62].The transport of glucose in different neural cells is a highly specified process regulated by different SLCs [63].The utilization rate of glucose in astrocytes is higher than in neurons,and stimulation of glucose utilization in astrocytes is mediated by the glutamate transporter SLC1A [64].Glycogen is the major store of glucose in brain and is mainly found in astrocytes.Glycogen turnover and conversion to lactate in the brain is extremely rapid,and is used for glutamate synthesis or producing glucose[65].Astrocyte-neuron lactate transport mediated by SLC16A is required for long-term memory formation [65,66].Further investigation on SLCs will help to understand the homeostatic regulation of energy and glucose metabolism in the brain.
2.2.2.SLCsfunctionintheglutamate/GABA-glutaminecycle
The SLCs regulate the flux of nutrients and metabolites to keep the brain in homeostasis,supply energetic substrates,fulfill neurotransmission,and remove toxins.Among these functions,it is noteworthy that the brain receives about 20%of the total glucose in the body and 80% of the energy is used for maintaining the homeostasis of the glutamate/GABAglutamine cycle [67].In neurons,the neurotransmitters glutamate and GABA are transported to presynaptic terminals by vesicular transporters of SLC17A6/A7 and SLC32A1,respectively.After release and binding with receptors in the postsynaptic neurons,extracellular glutamate and GABA are transported into astrocytes via SLC1A2/A3 and SLC6A11,respectively.Glutamate/GABA is then converted to glutamine and subsequently transferred from mature astrocytes via SLC38A3/A5 and from reactive astrocytes via SLC1A5 [68,69].The glutamine is then imported into neurons via SLC38A1/A2/A7 and SLC1A5 and re-converted to glutamate and GABA (Fig.1) [69–71].The dysfunction of these SLCs may give rise to excitotoxicity caused by accumulation of synaptic glutamate,which can thus be involved in the pathogenesis of various brain disorders,such as AD,PD,epilepsy,autism,and schizophrenia [41].
2.2.3.SLCsfunctioninneurotransmitterreleaseandreuptake
Synaptic transmissions require the controlled release of neurotransmitters packaged in synaptic vesicles.Released transmitters diffuse across the synaptic cleft,bind to specific postsynaptic receptors,and are rapidly cleared from the synaptic cleft by uptake into the presynaptic neuron or glial cells.During this process,neurotransmitter transporters in neuronal and glial plasma membranes and vesicular neurotransmitter transporters that package neurotransmitters into secretory vesicles in presynaptic neurons play important roles.As small molecule neurotransmitters package into synaptic vesicles,SLC18A2/A3 packages acetylcholine,histamine,dopamine,norepinephrine,epinephrine,and serotonin;SLC17A6/A7 packages glutamate;and SLC32A1 packages GABA [72,73].As for small molecule neurotransmitter reuptake,SLC1A1/A2 transports glutamate;SLC5A7 transports choline (released from the breakdown of acetylcholine in the synaptic cleft);and SLC6A transports dopamine,norepinephrine,GABA,and serotonin [52,74,75].Disturbances in the transport of neurotransmitters have been implicated in various brain disorders,such as AD and PD [51].
2.2.4.SLCsfunctionintheBBB
Another noteworthy function of SLCs in the brain is transport across the BBB to strictly control exchanges of substrates between the blood and the brain,including energetic substrates,amino acid,ions and other miscellaneous substrates [27,76].The BBB is the most significant barrier in the brain that prevents approximately 98% of small molecule reagents from passing when treating CNS-related disease [77].Therefore,SLCs in the BBB have a great potential to be utilized for the purpose of improving the delivery of drugs,prodrugs,and nanoparticles into the brain [78–80].
3.SLCs in drug development for brain disorders
Our understanding of the physiological and pathological functions and molecular mechanisms of SLCs has exploded in the last decade [19,81,82].Nevertheless,SLCs still have not been well evaluated in brain disorders and have been under-utilized as drug targets for the treatment of brain disorders.The utilizations of SLCs have been summarized in the treatment of major depression,attention deficit and hyperactivity disorder,epilepsy,movement disorders,psychosis,PD,and other brain disorders [17].Targeting SLCs to ameliorate more brain disorders is becoming a focus of investigations.Particularly,manipulation of SLCs for drug/prodrug transmembrane transport,especially blood-tobrain transport,has also drawn the attention of researchers[83].In the next section,we summarize the typical brain disorders that can be caused by SLCs dysfunction or mutation and manipulation of SLC for improving drug transport.
3.1.SLCs as potential drug targets in treatment of brain disorders
3.1.1.SLCsinepilepsy
Epilepsy is one of the most common neurological diseases affecting approximately 70 million individuals [84].It is characterized by recurrent,unprovoked seizures with multifactorial pathophysiologic mechanisms.Out of the 71 SLC genes expressed in the brain whose mutations or dysfunctions have been reported to be correlated with human brain disorders,about 22% are correlated with epilepsy or epileptic encephalopathy (Table 3).SLC12A2 and SLC12A5 are two typical transporters that have been extensively studied in attempts to understand the mechanism and explore new therapeutic targets of epilepsy [85–87].SLC12A2 mediates chloride ion (Cl−) uptake and favors depolarizing responses to GABA,while SLC12A5 is the main Cl−extruding transporter.Alterations in the balance of SLC12A2 and SLC12A5 activity are correlated with epileptogenesis.The SLC12A2 inhibitor bumetanide has been shown to exert antiepileptic effects [88].Perturbations in the glutamate/GABA-glutamine cycle,such as the disruption of extracellular glutamate clearance from the synaptic cleft,could also contribute to the development of epilepsy [89].Indeed,SLC1A2,which is responsible for regulating extracellular glutamate homeostasis by uptake of glutamate in astrocytes,is reported to cause epilepsy when mutated [90,91].Increased SLC1A2 expression reduces epileptogenic processes in a status epilepticus mouse model and thus enhancing SLC1A2 expression is a potential therapeutic approach for epilepsy [91].The mutation of SLC6A1 and SLC6A11,which are responsible for the reuptake of GABA from the synaptic cleft into neurons and astrocytes,has also been reported in patients with epilepsy [92,93].Tiagabine,which increases the brain levels of GABA by targeting SLC6A1,is used for treating epilepsy [134].Another noteworthy SLC that has a relationship with epilepsy is SLC13A5,whose mutation induces neonatal epilepsy [94–97].SLC13A5,a sodium-coupled tricarboxylate substrate transporter,is expressed at high levels in astrocytes,but is expressed at lower levels in neurons [98].SLC13A5 deficiency results in a loss of citrate transport,causing the short supply of energy,bicarbonate,and biosynthetic precursors in brain [99].Metabolomics profiling of SLC13A5-deficient patients revealed that dysfunction of SLC13A5 function disrupted metabolites of the citric acid cycle and neurotransmitters [100].
3.1.2.SLCsinAlzheimer’sdisease(AD)andParkinson’s disease(PD)
AD is the most common neurodegenerative disease and is a significant concern for aging populations [101].Although the specific pathogenesis mechanisms of AD have not been defined,pathological characteristics that contribute to progressive neurodegeneration are under extensive exploration.Brain glucose metabolism was reported to decline in AD patients [102].SLC2A1 expression is decreased in cerebral microvessels and cortex of AD patients and is associated with Aβdeposition [103–105].Loss of neuronspecific SLC2A3 was found in AD brain along with tau hyperphosphorylation [102].The expression level of SLC2A3 was comfirmed to be regulated by an emerging Alzheimer’s therapeutic target,BDNF (brain-derived neurotrophic factor)[106].SLC2A2 was significantly elevated in AD patients due to activation of astrocytes [102].Dysregulation in glutamate homeostasis has also been demonstrated in AD animal models and AD patients,which suggests a role for glutamate transporters in the onset and procession of AD [107].SLC1A2 is decreased in AD brains,especially in hippocampal tissue and prefrontal cortex from late-stage AD patients [108–110].Abdul et al.showed that SLC1A2 had reduced expression during the procession from mild cognitive impairment to AD [111].It is believed that the study of the transporting mechanism associate with AD will help to clarify the pathogenesis of this disease.
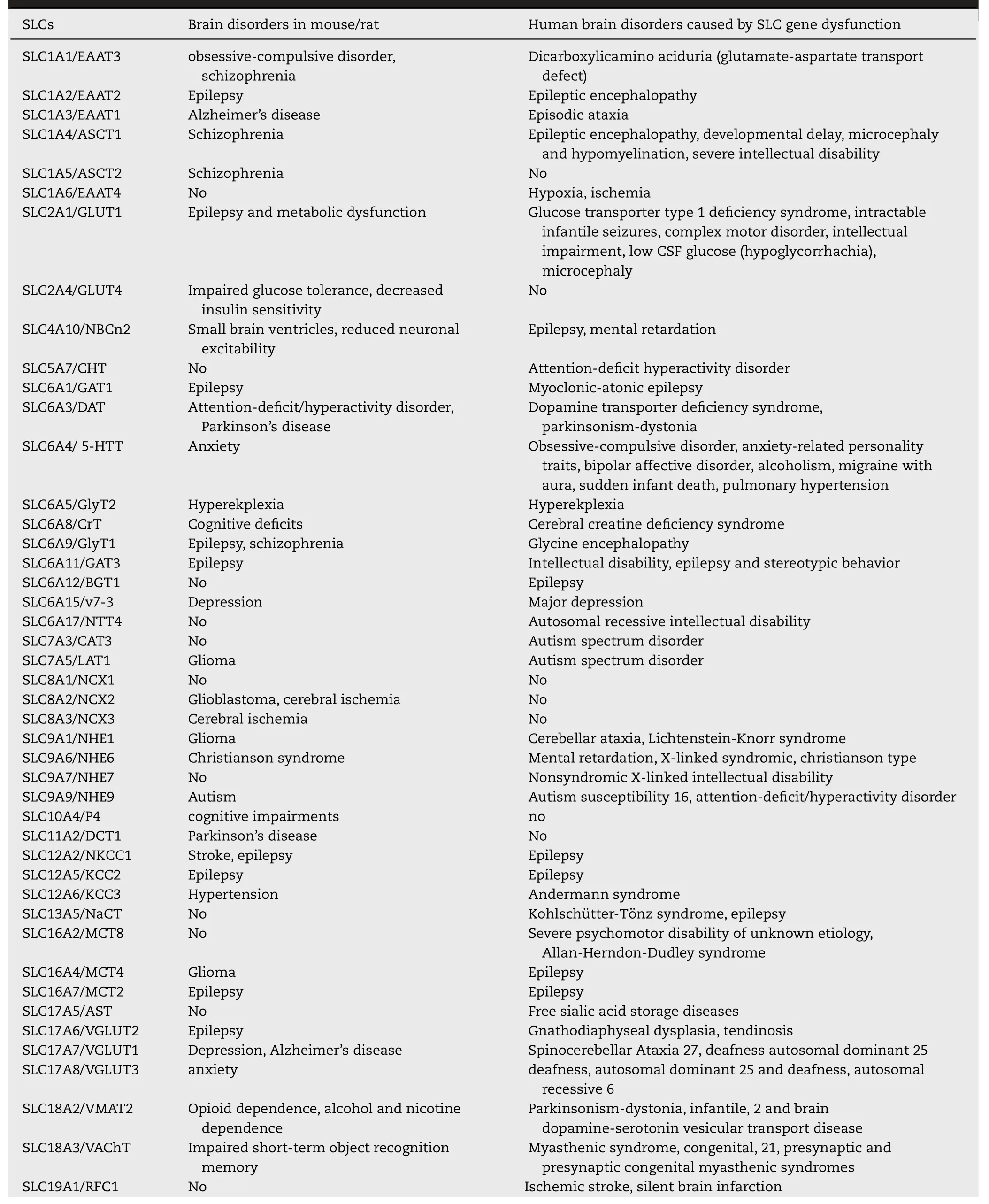
Table 3–Brain disorders associated with SLCs.Examples of brain disorders in SLC knockout/overexpression mice/rats,or mice/rats models of human disease in which SLCs are modified are shown.Human brain disorders caused by SLC gene dysfunction are also shown.Data on human disorders were retrieved from omim.org.
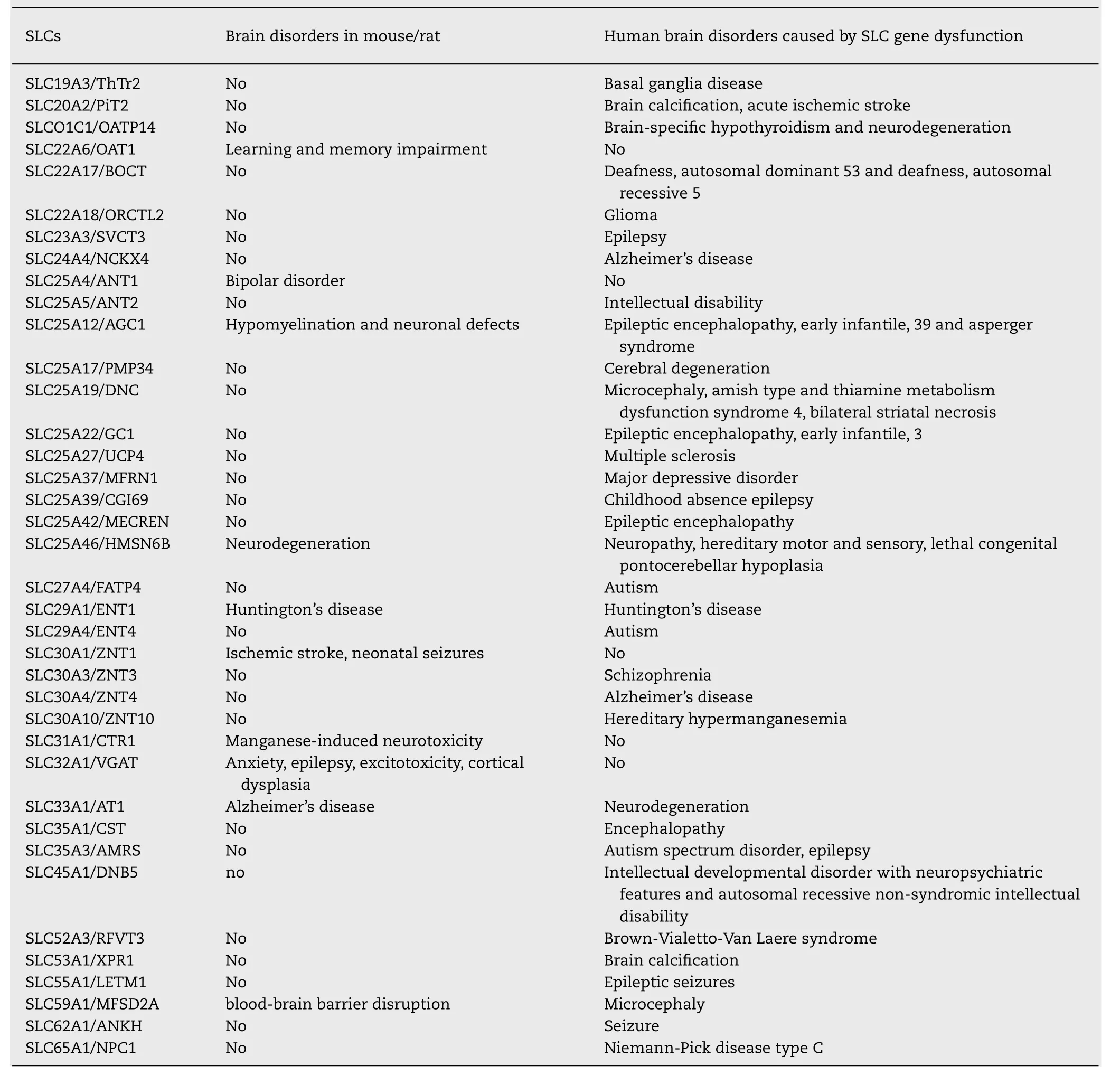
Table 3 (continued)
PD usually starts with the early prominent death of dopaminergic neurons in the substantia nigra and is associated with typical parkinsonian motor symptoms[112,113].A compensatory mechanism related to SLC6A3 mediating the unidirectional rapid reuptake of dopamine dependent on the sodium gradient was shown to be effective[114,115].Dopamine transporter imaging has become a widely used diagnostic tool for PD and SLC7A5 facilitates L-DOPA transport into the brain for the treatment of PD [116–118].
3.1.3.SLCsinautism
Autism spectrum disorders (ASD) are a group of genetic disorders characterized by impairment in social interactions,language,and speech,as well as restricted repetitive behaviors.Gene mutations are important factors in the development of ASD.T ˇarlungeanu et al.identified several patients with autistic traits carrying mutations in SLC7A5[119],a gene encoding a neutral amino acid transporter localized in the BBB.Branched chain amino acids (BCAA),substrates of SLC7A5 at the BBB,regulate GABAergic transmission,which can lead to the development of novel therapeutic strategies for autism [119].Genetic variants of SLC19A1 and SLC25A12,folate and amino acid transporters,respectively,are associated with childhood ASD [120].The fatty acid transporter-encoding gene SLC27A4 p.Gly209Ser mutant results in more fatty acid uptake into an endothelial cell line,and this functional change may be associated with ASD pathophysiology [121].
3.1.4.SLCsinotherbraindisorders
Although the underlying mechanisms of brain disorders have not been well characterized,many case reports have shown that SLCs are significantly involved in the occurrence and development of brain disorders (for a summary,see Table 3),such as attention-deficit hyperactivity disorder,mental retardation,Huntington’s disease (HD),and major depressive disorders.Many drugs/inhibitors targeting SLCs have been tested and utilized in brain disorders.UCPH-101 has been shown to exert sustained inhibition of SLC1A3 [122].Tiagabine exerts anticonvulsant activity via inhibition of uptake by SLC6A1 [123].High throughput screening has led to the discovery of a structurally novel class of inhibitors of SLC6A9 [124].Deutetrabenazine,a novel SLC18A2 inhibitor,is effective for the treatment of involuntary movements in patients with tardive dyskinesia [125].Tetrabenazine,which depletes presynaptic dopamine by blocking SLC18A2,is a US Food and Drug Administration-approved drug for chorea in HD [126].These findings may lead to the development of therapeutic treatments for certain neurological disorders.
3.2.SLCs for improving brain drug delivery
The transport of drugs across the cell membrane using transport systems has been a great success in drug development.The BBB is a bottleneck in brain drug development and delivery as it excludes nearly all largemolecule neurotherapeutics and about 98% of all small molecule drugs [127].In this context,more and more attention has been paid to manipulating transporters in the BBB to promote drug delivery into the brain [18,30,128].SLCs recognize certain substrate structures,and most effective drugs for brain disorders possess N (amine)-containing groups,which suggest SLCs in the BBB may interact with N-containing groups [128].Therefore,incorporating Ncontaining groups into known substrates of SLCs in the BBB will be beneficial for the development of drugs for central nervous system disorders.
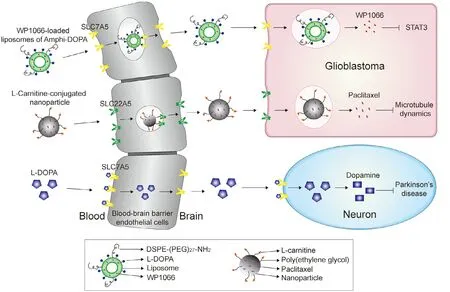
Fig.2–Examples of SLC mediated drug transport across the blood-brain barrier and in glioblastomas and neurons.Three strategies of SLC mediated drug transportation in the brain.1.SLC7A5 mediated liposomal drug carrier system.(1) An L -DOPA functionalized amphiphile is loaded with STAT3 inhibitor WP1066,a drug for the treatment of glioblastoma,and is modified with DSPE-(PEG)27–NH2,a reagent to enhance the circulation stability of the liposomes;(2) SLC7A5 mediates the uptake of Amphi-DOPA into the BBB endothelial cells;(3) Released Amphi-DOPA is recognized by SLC7A5 localized to the membrane of glioblastoma cells and is taken up by tumor cells;(4) WP1066 is released from the Amphi-DOPA and induces tumor cell death by inhibiting the STAT3 pathway.2.SLC22A5 mediates the nanoparticle drug carrier system.(1)L -carnitine-conjugated poly(lactic-co-glycolic acid) (PLGA,biodegradable synthetic polymer) nanoparticles (LC-PLGA NPs) are developed which are loaded with paclitaxel,an anti-glioma drug;(2) SLC22A5 mediates the uptake of LC-PLGA NPs into the BBB endothelial cells;(3) Released LC-PLGA NPs are recognized by SLC22A5 localized in the membrane of glioblastoma cells and are taken up by tumor cells;(4) paclitaxel is released from the LC-PLGA NPs,leading to inhibition of tumor cell proliferation and induction of apoptosis by the anti-microtubule effect.3.SLC7A5 mediates prodrug carrier system.L -DOPA is a prodrug of the neurotransmitters dopamine.It is transported across the BBB and taken up by neurons via SLC7A5.L -DOPA is converted to dopamine for the treatment of Parkinson’s disease.
Research targeting SLCs will help to promote the development of brain disorder drugs;screening candidate drugs for high affinity targeting of the BBB will be crucial for the development of effective treatment of brain disorders[129].Indeed,many drugs that have affinity for receptors and SLCs in the brain have been identified [130,131].Gabapentin,a structural analog of GABA,increases expression of GABAAreceptors [132]and is used in the treatment of epilepsy[133].Although it had been shown that Gabapentin has high permeability across the BBB,the mechanism of this action was not well demonstrated until Dickens et al.showed that SLC7A5 transports Gabapentin as a substrate[134].Another SLC7A5 drug substrate is L -DOPA,which is the most effective drug available for the treatment of PD [135,136].Glioblastomas are the most aggressive primary brain tumor in adults,with only 3–5% survivability beyond 5 years [137].Few successes of systemic chemotherapy against glioblastoma have been reported,and this is partly due to the poor drug delivery into glioblastoma tissue and toxicity of drugs to brain cells [138].SLC7A5 is expressed in both the BBB and glioblastoma and transports L -DOPA as a substrate,which has contributed to the construction of a SLC7A5 mediated liposomal drug carrier system that was prepared from an L -DOPA functionalized amphiphile [131].This strategy provides the potential for systemic chemotherapy of glioblastoma.Nanoparticles conjugated to carrier transporter substrates represent another potential drug carrier system for the treatment of glioblastomas.SLC22A5 is expressed in both BBB and glioblastoma and transports L -carnitine.L -carnitineconjugated nanoparticles were applied to target glioma cells for drug delivery via SLC22A5 (Fig.2) [33].
3.3.SLC structure determination for drug design
Our understanding of the molecular assembly and transport mechanisms of SLCs was limited until a number of crystal structures of SLCs were solved [139].X-ray crystallography,NMR spectroscopy and cryo-electron microscopy have helped to solve SLC structures despite difficulties,such as the poor stability of membrane proteins and purification and crystallization difficulties [139].The revealed architectural features of SLCs provide unprecedented insights into their molecular mechanisms and provide templates for improving preclinical drugs.Drew et al.compared alternating-access mechanisms,including the rocker-switch model,the rockingbundle model,and the elevator model,in relation to the crystal structures of several transporters that have been reported [13].Bai et al.sorted the SLC structures into four groups with different protein folds,including the MFS (major facilitator superfamily) fold,the LeuT (leucine transporter)fold,other antiparallel folds and others [139].SLC7A5,which is crucial for brain drug delivery,as discussed in other parts of this article,forms a disulfide-linked heterodimer with SLC3A2.This SLC7A5-SLC3A2 complex has been revealed by singleparticle cryo-EM and explains the organization of the unique glycoprotein-solute carrier complex [140,141].By comparative modeling,virtual screening,and experimental validation,four SLC7A5 ligands have been identified [142].Computational modeling based on structural biology will accelerate the substrate prediction and drug screening of small molecule libraries for brain drug discovery [19,143].
4.Conclusions and future prospects
There are hundreds of SLCs in the brain that play important roles in the distribution of multiple substrates.The study of SLCs promotes the understanding of the substrates’ roles in brain,as well as the role of transporters in brain homeostasis.SLCs can serve as drug targets or facilitate drug delivery across the BBB and into brain cells.Despite the continuous efforts that have been made to demonstrate the transporting mechanisms,we are far from understanding the SLC transport system in the brain.The physiological function,regulation in disease,and drug deposition of most SLCs expressed in the brain remain to be studied.An understanding of SLCs in aspects of physiology and pharmacology will help to promote drug discovery.Pardridge (2015) introduced a high throughput screening of a small molecule neuropharmaceutical library for drugs interacting with SLCs expressed in the BBB [129],in which a mammalian host cell line overexpressing SLC transporters was used to screen small molecule libraries.Tashima reviewed transporter-conscious drug design to provide advantages in absorption,distribution,excretion,and toxicity of drugs,and especially emphasized the recognition of the N-containing group of substrates by the transporters [128].Novel brain pharmaceutical agent discovery and delivery will be triggered based on the demonstration of transporting mechanisms,drug screening approaches,and transporter-conscious drug design to enhance the quality of life for patients suffering from brain disorders.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by Nation Science and Technology Major Projects for Major New Drugs Innovation and Development (2018ZX09711003-004-002 to L.C.),Ministry of Science and Technology of China National Key R&D Programs (2018YFA0506903 to L.C.),and National Natural Science Foundation of China grants (91857108 to L.C.).
 Asian Journal of Pharmacentical Sciences2020年2期
Asian Journal of Pharmacentical Sciences2020年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- The role of transporters in cancer redox homeostasis and cross-talk with nanomedicines
- Intestinal OCTN2-and MCT1-targeted drug delivery to improve oral bioavailability
- Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis
- Stimulatory effect on the transport mediated by organic anion transporting polypeptide 2B1
- Amino acid transporters:Emerging roles in drug delivery for tumor-targeting therapy
- Glutamine transporters as pharmacological targets:From function to drug design
