人脐带血间充质干细胞通过抑制MEK/ERK通路改善LPS诱导的AECⅡ凋亡机制的研究
刘坚 龙婷婷 李晨彦

[摘要] 目的 探讨人脐带血间充质干细胞(hUC-MSC)对脂多糖(LPS)诱导的肺泡Ⅱ型上皮细胞(AECⅡ)凋亡的影响,及MEK/ERK信号通路在其中的作用。 方法 采用LPS与A549细胞共培养建立AECⅡ凋亡模型;将凋亡模型按随机数字表法分成6组,分别与MSC条件培养基(MSC-CM)、MEK/ERK通路抑制剂(PD98059)及PBS体外共培养,分别为A549+LPS+MSC、A549+LPS+PBS、A549+LPS+PD98059,阴性对照组为A549+PBS、A549+MSC、A549+PD98059,48 h后采用流式细胞术检测A549细胞凋亡情况;对MEK/ERK通路及凋亡相关信号(BAX/BCL-2、FAS、caspase 3及caspase 8)进行qPCR检测,对A549+LPS+MSC、A549+LPS+PBS、A549+PBS、A549+MSC四组组进行Western blot检测。 结果 建立A549+LPS凋亡模型。A549+LPS+MSC、A549+LPS+PD98059组与A549+LPS+PBS比较,凋亡率显著下降。A549+LPS+MSC及A549+LPS+PD98059的MEK、ERK、BAX/BCL-2、caspase 3及caspase 8 mRNA扩增倍数均显著低于A549+LPS+PBS(P < 0.05),而蛋白水平上FAS、BAX、cleaved Caspase 3、p-MEK、p-ERK、A549+LPS+MSC較A549+LPS+PBS明显降低(P < 0.05),而TNF-R1差异无统计学意义(P > 0.05)。 结论 hUC-MSCs可能通过旁分泌作用抑制MEK/ERK信号通路磷酸化,影响内源性线粒体及外源性FAS/FASL途径进而减轻LPS诱导的AECⅡ凋亡。
[关键词] 间充质干细胞;细胞外信号调节激酶;凋亡;肺泡Ⅱ型上皮细胞;脂多糖
[中图分类号] R563 [文献标识码] A [文章编号] 1673-7210(2020)02(c)-0013-06
[Abstract] Objective To discuss the effect of human umbilical cord blood mesenchymal stem cells (hUC-MSC) on alveolar type Ⅱ epithelial cells (AEC Ⅱ) apoptosis induce by lipopolysaccharide (LPS), and the role of MEK/ERK signaling pathway. Methods The AECⅡ apoptosis model was established by LPS co-cultured with A549 cells. The apoptosis model was randomly divided into 6 groups according to random number method, they were co-cultured with MSC conditioned medium (MSC-CM), MEK/ERK pathway inhibitor (PD98059) and PBS, which were named A549+LPS+MSC, A549+LPS+PBS, A549+LPS+PD98059, and the negative control groups were A549+PBS, A549+MSC, A549+PD98059. Apoptosis of A549 cells was detected by Annexin V/PI staining flow cytometry 48 h later. MEK/ERK pathway and apoptosis-related signals (BAX/BCL-2, FAS, caspase 3 and caspase 8) were examined by qPCR. The groups of A549+LPS+MSC, A549+LPS+PBS, A549+PBS, and A549+MSC were selected for Western blot detecting. Results The apoptosis model was established by A549+LPS. Compared with A549+LPS+PBS, the apoptosis rate of A549+LPS+MSC and A549+LPS+PD98059 were decreased significantly (P < 0.05). The mRNA expression levels of MEK, ERK, BAX/BCL-2, caspase 3 and caspase 8 in A549+LPS+MSC and A549+LPS+PD98059 were significantly lower than those in A549+LPS+PBS (P < 0.05). The expression of FAS, BAX, cleaved Caspase 3, p-MEK, and p-ERK protein in the A549+LPS+MSC and A549+LPS+PD98059 were significantly lower than A549+LPS+PBS(P < 0.05). There was no significant different in TNF-R1 among each group (P > 0.05). Conclusion hUC-MSCs may inhibit the phosphorylation of MEK/ERK signaling pathway affecting mitochondria pathway and FAS/FASL pathway by paracrine action, thereby attenuating apoptosis of AECⅡ induced by LPS.
[Key words] Mesenchymal stem cells; Extracellular signal-regulated kinases; Apoptosis; Alveolar type Ⅱ epithelial cells; Lipopolysaccharide
急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)常继发于脓毒症,死亡率高(30%~40%),发病机制复杂,尚缺乏有效的治疗措施[1-2]。脓毒症释放大量炎症因子,肺泡上皮细胞损伤甚至凋亡是ARDS的病理、生理基础[3-4]。ARDS中肺泡Ⅱ型上皮细胞(alveolar epithelial type Ⅱ cell,AECⅡ)的凋亡是AEC死亡的主要机制之一[5]。细胞外信号调节激酶(extracellular regulated protein kinases,ERK)在细胞的分化、增殖及凋亡中起关键作用[6-7],本课题组前期已在脓毒症所致ARDS动物模型中发现MEK/ERK途径的磷酸化影响着AECⅡ凋亡[8]。
因人脐带血来源间充质干细胞(human umbilical cord mesenchymal stem cells,hUC-MSC)有着较高的分化潜能及多种生物学作用,可改善脓毒症引起的ARDS[9-12]。本课题组前期研究发现MSC能够改善脓毒症所致ARDS小鼠中AECⅡ凋亡。本研究選用人类肺泡细胞癌来源并且具有类似AECⅡ代谢特征和形态学特征的A549细胞[13]进行研究,有望从体外试验明确MSC对AECⅡ凋亡在信号通路上的机制。
1 材料与方法
1.1 材料
细胞株:A549细胞株由中国科学院细胞库提供。仪器:流式细胞仪(FACSCalibur,BD);荧光定量PCR仪(7300,ABI)。试剂:LPS(L8880,Solarbio)细胞培养基(RPMI 1640 Hyclone);总RNA提取试剂Trizol Reagent(15596-026,Invitrogen Life Technologies);第一链cDNA合成试剂盒RevertAid First Strand cDNA Synthesis Kit(#K1622,Thermo);山羊抗兔cleaved Caspase3单克隆抗体(bs-1518,Abcam),凯基Annexin V-FITC细胞凋亡检测试剂盒(KGA08,凯基生物);PCR引物(Invitrogen Biotechnology Co.,LTD);cleaved-caspase8(10380-1-AP,PTG);bcl-2(2870S,CST);bax(1163,EPI);TNF-R1(ab2143,Abcam);p-MEK(ab96379,Abcam);ERK(4371,bioworld);p-ERK(4371,cst);MEK(bs-1041R,BIOSS);β-actin(sc-1616R,Santa);Pro-caspase3(bs-1518,Bioworld);FAS(sc-1023,Santa)。
1.2 方法
1.2.1 细胞培养
hUC-MSCs为湘潭市中心医院生殖中心从人脐带血中分离取得,细胞标记及分化潜能在本课题组前期研究中已证实[14]。
1.2.2 建立A549凋亡模型
将对数生长期细胞消化后,按4000细胞/孔接种于96孔板,培养24 h贴壁。继续培养24 h后按随机数字法分为3组,对照组为正常培养的A549细胞;实验组细胞中分别加入不同浓度的 LPS(10、20 μg/mL)并刺激48 h,选择凋亡水平最高的LPS浓度,建立A549凋亡模型。
1.2.3 Annexin-V-FITC/PI染色流式细胞术检测A549细胞凋亡率
分别将A549细胞在对照组培养基和hUC-MSC条件培养基中培养48 h。将细胞消化,离心,去上清。加入1×结合缓冲液400 μL重悬细胞,加入5 μL Annexin-V-FITC混匀,室温避光15 min。加入10 μL PI混匀,冰浴避光5 min。1 h内Annexin V-FITC的绿色荧光通过FITC通道(FL1)检测,PI红色荧光通过PI通道(FL2)检测。
1.2.4 检测MSC及MEK/ERK通路抑制剂(PD98059)对LPS诱导A549凋亡的影响
将对数生长期A549细胞用胰蛋白酶消化,按4000细胞/孔接种于96孔板,按随机数字表分成6组,每组16孔,实验组为A549+LPS+MSC、A549+LPS+PD98059,对照组为A549+LPS+PBS,阴性对照组为A549+MSC、A549+PD98059、A549+PBS。48 h后Annexin-V-FITC/PI染色流式细胞术检测三组细胞凋亡的改变情况。
1.2.5 Rt-PCR及Western blot检测MEK/ERK通路相关基因及蛋白的表达
对6组细胞进行qPCR检测,取A549+LPS+MSC、A549+LPS+PBS、A549+MSC、A549+PBS行Western blot检测。
1.2.5.1 Rt-PCR定量检测 MEK及ERK、BAX、BCL-2、TNF-R1、FAS、caspase 3、caspase 8的mRNA水平 分别收集各组A549细胞,从细胞中分离总RNA,采用Trizol Reagent(Invitrogen Life Technologies),从各细胞样本中取总RNA 5 μg,反转录成cDNA。Rt-PCR筛选候选基因及采用β-actin作为内参。使用Primer Express 2.0软件设计各引物基因序列,β-actin上游引物为5′-GTGACGTTGACATCCGTAAAGA-3′,下游引物为5′-GTAACAGTCCGCCTAGAAGCAC-3′,BAX上游引物为5′-GCCTTTTGCTACAGGGTTCAT-3′,下游引物为5′-TATTGCTGTCAGTTCATCTCCA-3′,BCL2上游引物为5′-TGACTCTCTCGTCGCTACCGT-3′,下游引物为5′-CCTGAAGAGTTCCTCCACACC-3′,CASP3上游引物为5′-GTCTGACTGGAAAGCCGAA-AC-3′,下游引物为5′-GACTGGATGAACCACGACCC-3′,FAS上游引物为5′-GAAGAGACCCCTGTGG-TATTTGA-3′,下游引物为5′-ACACTTTTCCGCTCACAATCAGA-3′。采用Fast Start Universal SYBR Green Master(Rox)(Roche)在荧光定量PCR仪(7300)完成操作。
1.2.5.2 Western blot检测 采用Western blot检测其中A549+LPS+MSC、A549+LPS+PBS、A549+PBS、A549+MSC四组细胞内FAS、BAX、BCL-2、TNF-R1、caspase 3、cleaved caspase 3、p-MEK、p-ERK表达水平。细胞总蛋白提取,使用RIPA裂解缓冲液提取蛋白质,并对蛋白质含量进行定量。将样品(30 μg等蛋白)进行SDS-PAGE,然后转移到PVDF膜上。针对FAS、BAX、BCL-2、TNF-R1、caspase 3、cleaved caspase 3、p-MEK、p-ERK的一抗探测探针,随后与辣根过氧化物酶(HRP)偶联的抗兔二抗(1∶2000)。采用兔单克隆抗β-actin作为上样对照。Quantity One软件分析各蛋白条带灰度值。以目标蛋白与内参β-actin灰度值比值代表各蛋白的相对表达水平。
1.3 统计学方法
采用SPSS 18.0统计学软件进行数据分析,计量资料用均数±标准差(x±s)表示,多组均数比较采用单因素方差分析,两两比较采用LSD检验;计数资料用率表示,组间比较采用χ2检验,以P < 0.05为差异有统计学意义。
2 结果
2.1 AECⅡ凋亡体外模型探索
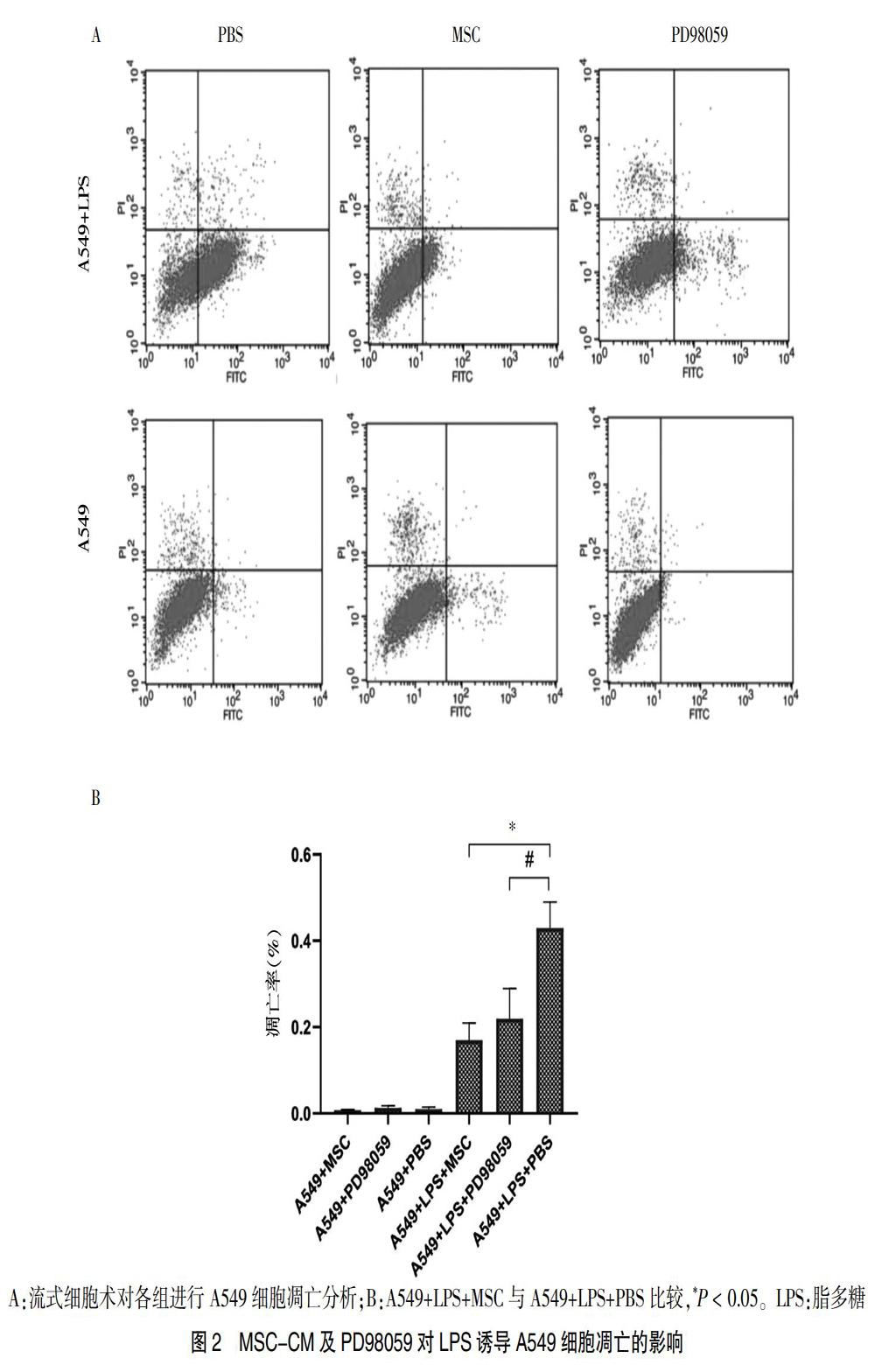
Annexin-V-FITC/PI染色流式细胞术检测各组细胞凋亡结果,如图1所示,因随着LPS浓度继续增大,A549细胞以坏死为主[15],故采用LPS(20 μg/mL)建立诱导A549凋亡模型。
2.2 hUC-MSC和PD98059干预后对LPS诱导的A549凋亡影响
与A549+LPS+PBS比较,A549+LPS+MSC及A549+LPS+PD98059的A549凋亡率显著下降(P < 0.05)。见图2。
2.3 MSC对MEK/ERK通路相关基因及蛋白的表达水平的影响
2.3.1 Rt-PCR定量检测MSC抑制MEK/ERK通路相关基因mRNA表达情况
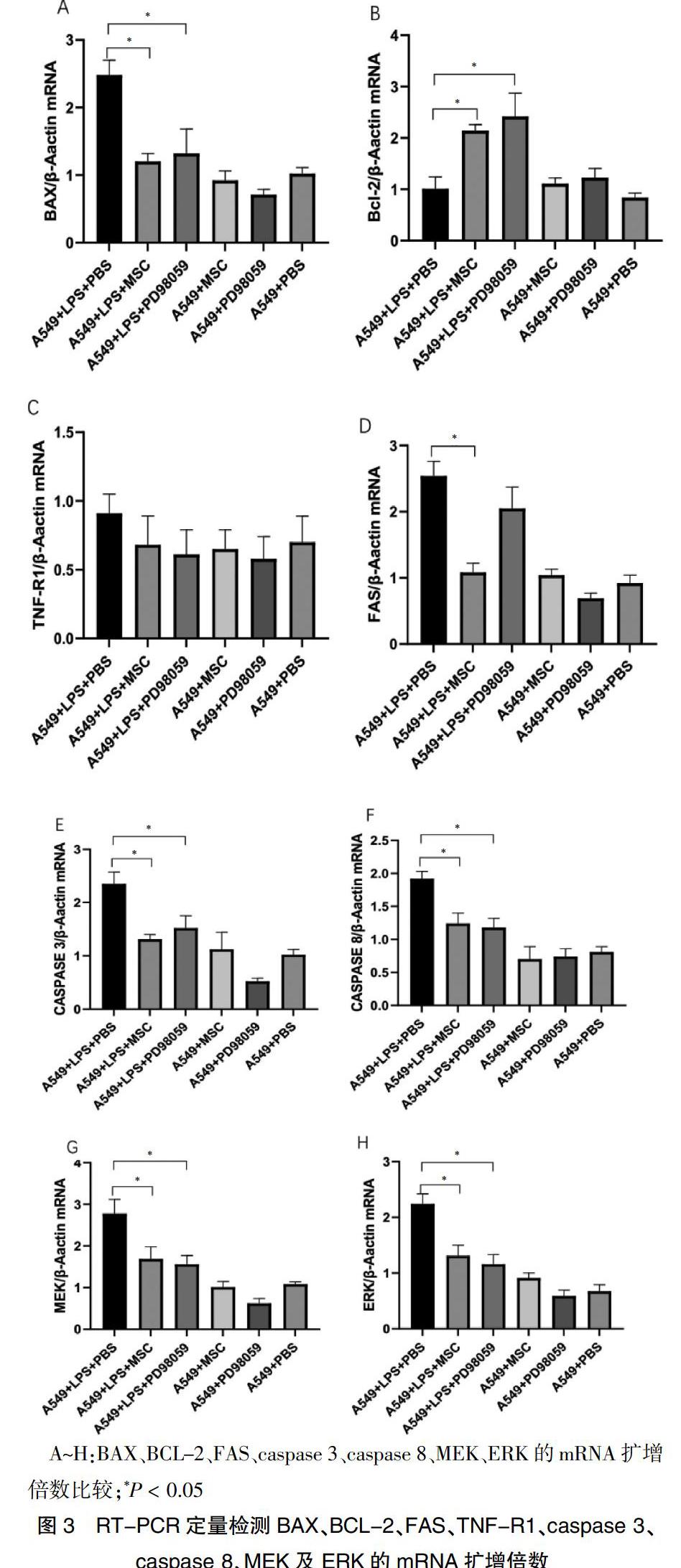
A549+LPS+MSC、A549+LPS+PD98059中A549细胞内BAX、CASPASE 3、CASPASE 8、MEK、ERK的mRNA扩增倍数水平低于A549+LPS+PBS,而BCL-2高于A549+LPS+PBS(P < 0.05)。A549+LPS+PD98059与A549+LPS+PBS的FAS mRNA扩增倍数比较差异无统计学意义(P > 0.05)。而A549+LPS+MSC、A549+LPS+PD98059TNF-R1 mRNA扩增倍数与A549+LPS+PBS组比较,差异无统计学意义(P > 0.05)。见图3。
2.4 MSC对MEK/ERK通路及凋亡相关信号表达水平的影响
选择 A549+LPS+MSC、A549+LPS+PBS、A549+PBS、A549+MSC 进行Western blot检测,BAX及cleaved-caspase 3的蛋白水平,A549+LPS+MSC较A549+LPS+PBS下调(P < 0.05)。与A549+LPS+PBS比较,A549+LPS+MSC的p-MEK及p-ERK水平、p-MEK/t-MEK、p-ERK/t-ERK比值均降低(P < 0.05),而各组TNF-R1差异无统计学意义(P > 0.05)。见图4。
3 讨论
本研究验证了hUC-MSCs可以改善LPS诱导的AECⅡ的凋亡水平,其通过旁分泌改变细胞微环境来抑制FAS/FASL系统及内源性线粒体凋亡途径的激活,而MEK/ERK信号在其中扮演重要角色。
MEK/ERK是Ras丝裂原信号转导下游的核心元件,在细胞凋亡过程中发挥着重要作用,当细胞外刺激物(如LPS、细胞因子)与相应受体结合后,SOS与受体或受体底物上的酪氨酸(Tyr)磷酸化位点结合,在Ras附近形成高浓度的SOS,其促使GTP取代Ras上的GDP而活化Ras,使MEK催化区中Thr和Ser磷酸化激活MEK,发挥调控细胞凋亡的作用[16-17]。根据之前本课题组研究发现的脓毒症所致ARDS中AECⅡ凋亡的机制与MEK/ERK磷酸化相关[8],结合此次体外研究结果,即在LPS诱导A549凋亡模型中,p-MEK/p-ERK蛋白表达增高,本研究认为可能是LPS激活Ras/MEK/ERK信号通路,促进MEK和ERK磷酸化,进而导致细胞色素C的大量释放,形成凋亡体后激活caspase 3,爆发级联反应,最终导致AECⅡ凋亡[18-19]。
MSC通过旁分泌各种生长因子,弥补损伤情况下生物多样性重要成分,发挥调节如NF-κB、PI3K/AKT等重要信号通路的活性,从而改善凋亡的作用[9,20]。本研究结果发现,通过MSC-CM及PD98059与LPS诱导A549共培养,A549凋亡情况均改善,同时MSC与PD98059一致,其Bax/Bcl-2、Fas及MEK、MRK在基因水平與对照组存在明显差异。且经MSC及PD98059干预后,Bax/bal-2的比例及caspase 3也较对照组明显下调。结合MSC干预组的BAX和FAS在蛋白水平的表达上显著低于对照组,且p-MEK和p-ERK的蛋白表达同样弱于对照组,充分说明MSC极有可能是通过抑制MEK/ERK信号通路的磷酸化,从而抑制AECⅡ的凋亡,但其上游相关信号(Raf/Ras)如何调控MEK/ERK的活化尚需进一步的验证。
[参考文献]
[1] Matthay MA,Zemans RL,Zimmerman GA,et al. Acute respiratory distress syndrome [J]. Nat Rev Dis Prim,2019,5(1):18.
[2] Cecconi M,Evans L,Levy M,et al. Sepsis and septic shock [J]. Lancet,2018,392(10141):75-87.
[3] 趙锋利,冼绍祥,罗苑苑,等.参附注射液对脓毒症小鼠免疫调节及炎症因子的影响[J].中国医药导报,2019,16(22):21-25.
[4] Abdulnour R-EE,Gunderson T,Barkas I,et al. Early Intravascular Events Are Associated with Development of Acute Respiratory Distress Syndrome. A Substudy of the LIPS-A Clinical Trial [J]. Am J Respir Crit Care Med,2018,197(12):1575-1585.
[5] Brauer R,Ge L,Schlesinger SY,et al. Syndecan-1 Attenuates Lung Injury during Influenza Infection by Potentiating c-Met Signaling to Suppress Epithelial Apoptosis [J]. Am J Respir Crit Care Med,2016,194(3):333-344.
[6] Zhan J,Liu Y,Zhang Z,et al. Effect of penehyclidine hydrochloride on expressions of MAPK in mice with CLP-induced acute lung injury [J]. Mol Biol Rep,2011,38(3):1909-1914.
[7] Cagnol S,Chambard JC. ERK and cell death:mechanisms of ERK-induced cell death—apoptosis,autophagy and senescence [J]. FEBS J,2010,277(1):2-21.
[8] 刘坚,吕海金,安玉玲,等.间充质干细胞抑制MEK/ERK信号通路改善盲肠结扎穿孔所致急性肺损伤中肺泡Ⅱ型上皮细胞的凋亡[J].中山大学学报:医学科学版,2016, 37(3):367-375.
[9] Harrell CR,Sadikot R,Pascual J,et al. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases:Current Understanding and Future Perspectives [J]. Stem Cells Int,2019,2019:1-14.
[10] Su VYF,Lin CS,Hung SC,et al. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury [J]. Int J Mol Sci,2019, 20(9).
[11] Chen CH,Chen YL,Sung PH,et al. Effective protection against acute respiratory distress syndrome/sepsis injury by combined adipose-derived mesenchymal stem cells and preactivated disaggregated platelets [J]. Oncotarget,2017,8(47):82415-82429.
[12] Salami F,Tavassoli A,Mehrzad J,et al. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils [J]. Immunobiology,2018, 223(12):786-791.
[13] Ryndak MB,Singh KK,Peng Z,et al. Transcriptional profiling of Mycobacterium tuberculosis replicating in the human type Ⅱ alveolar epithelial cell line,A549 [J]. Genom Data,2015,5:112-114.
[14] 陈刚,廖前德,胡优威,等.成骨诱导人脐带间充质干细胞与纳米羟基磷灰石/聚酰胺66支架材料的生物相容性[J].中国组织工程研究,2012,16(16):2931-2934.
[15] Huang C,Zheng H,He W,et al. Ghrelin ameliorates the human alveolar epithelial A549 cell apoptosis induced by lipopolysaccharide [J]. Biochem Biophys Res Commun,2016,474(1):83-90.
[16] Sun Y,Liu WZ,Liu T,et al. Signaling pathway of MAPK/ERK in cell proliferation,differentiation,migration,senescence and apoptosis [J]. J Recept Signal Transduct Res,2015,35(6):600-604.
[17] Yi J,Gao ZF. MicroRNA-9-5p promotes angiogenesis but inhibits apoptosis and inflammation of high glucose-induced injury in human umbilical vascular endothelial cells by targeting CXCR4 [J]. Int J Biol Macromol,2019, 130:1-9.
[18] Kitai H,Ebi H,Tomida S,et al. Epithelial-to-Mesenchymal Transition Defines Feedback Activation of Receptor Tyrosine Kinase Signaling Induced by MEK Inhibition in KRAS-Mutant Lung Cancer [J]. Cancer Discov,2016,6(7):754-769.
[19] Schuh K,Pahl A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury [J]. Biochem Pharmacol,2009,77(12):1827-1834.
[20] 袁哲,吳思梦,王亚君,等.UC-MSC经PI3K/AKT信号通路影响人肺腺癌A549细胞的凋亡和增殖[J].中国肿瘤生物治疗杂志,2019,26(3):260-265.
(收稿日期:2019-10-09 本文编辑:任 念)

