超声波改善乳酸钙-氯化铵-水复合相变材料过冷特性
章学来,刘 璐,张永一川,徐笑锋,汪 翔
·农业生物环境与能源工程·
超声波改善乳酸钙-氯化铵-水复合相变材料过冷特性
章学来,刘 璐,张永一川,徐笑锋,汪 翔
(上海海事大学蓄冷研究所,上海 201306)
针对无机相变材料过冷度高的问题,对乳酸钙-氯化铵-水相变体系做超声震荡处理以及在凝固过程加入超声外场,研究超声波对乳酸钙-氯化铵-水相变体系凝固特性的影响。试验发现,增加超声波震荡后,过冷度降低0.62 ℃,相变过程持续了7 755 s,溶液的相变潜热为276.2 J/g,增加了4.14%;溶液凝固时增加超声波相变体系没有出现过冷现象,相变过程持续了8 256 s溶液的相变潜热为283.5 J/g,增加了6.9%。试验表明,在乳酸钙-氯化铵-水相变体系凝固过程中加入超声场,有利于乳酸钙-氯化铵-水相变体系的性能提高。
超声波;相变材料;热力学性质;乳酸钙;氯化铵;过冷度;相变潜热
0 引 言
相变材料(phase change materials,PCM)是利用材料在随温度变化的过程中发生相变的同时进行潜热储能的物质[1-2]。相变材料分为有机相变材料,无机相变材料和复合相变材料[3-4]。相变储能作为一种高效的能源利用技术被应用于建筑节能[5-6]、空调[7-8]、太阳能[9-12]、冷链物流[13-14]等领域,实现电力移峰填谷,缓解能源应用在时间和空间上不匹配的问题。冷链物流耦合相变储能技术实现对产品的保冷,不仅节约能源,还能降低产品在冷链流通中的耗损[15]。有机相变材料不易出现过冷现象和相分离,材料腐蚀性小,但相变潜热普遍较低。无机相变材料具有相变潜热较大,导热系数高,价格便宜等优点,但普遍存在过冷现象[16-17],添加成核剂虽然能降低过冷度,但需要增稠剂解决分层现象[18-19]。要使无机相变材料更好的应用,就要解决其过冷问题。
研究表明超声波能有效改善气体水合物的结晶,并大大缩短成核诱导期[20],协同成核溶剂时能有效促进有机物系结晶[21],利用超声波照射可以大幅度降低水的过冷度[22]。Hickling基于理论分析认为空化效应产生的正压诱导水成核[23],Hunt等认为是负压作用[24],Hu等在试验中发现虽然超声波能够降低材料的过冷问题控制其成核结晶,但超声的作用是一个随机且伴随着概率性的过程[25]。刘东方对温度为-0.5℃的纳米流体进行玻璃搅拌和超声振荡对比试验,结果表明超声波能稳定的降低材料的过冷度,针对相同浓度的纳米流体超声效果优于搅拌[26]。Liu等研究发现在超声作用下纳米材料的成核时间最优效果能提早90.7%,过冷度能降低69.1%[27]。Kiani等研究了不同超声功率和超声时间对0~−5 ℃的纯水及蔗糖溶液过冷度的影响,发现超声波可以决定不同过冷情况下溶液的成核温度,效果随着超声强度和时间的增加而增加,相比无超声外场的自然蓄冷过程,纯水溶液和蔗糖溶液的过冷度分别降低了3~6.4 ℃和4~6.2 ℃[28-29]。Liu等基于湿润模型建立了一个纳米物理分析模型,得出在超声作用下纳米流体过冷度能较大程度得以降低,同时成核率得以提高[30]。Cogné等研究超声空化泡与溶液过冷度的关系,发现在频率为29 kHz的超声下仅需要2 ns就可以产生1~5 mm的空化泡,随着声强的改变气化泡直径发生变化,诱导过冷溶液成核降低过冷度[31]。
乳酸钙-氯化铵-水相变体系相变潜热高达265.2 J/g,但存在无机相变材料普遍存在的过冷度问题,基于以上分析,本文选用乳酸钙-氯化铵-水相变体系,研究超声波对其凝固过程的影响,为解决相变材料存在的过冷问题提供新思路。
1 材料与方法
1.1 材料和仪器
试验材料:自制的蒸馏水,乳酸钙、氯化铵均为AR分析纯。
试验仪器:电子分析天平(精度0.1mg);安捷伦温度时间采集仪,AGILENT TCHNOLOGY,型号34970A;T型热电偶(精度±0.01 ℃);差示扫描量热仪DSC200F3,德国Netzsch(精度±0.01 ℃);精密天平,METTLER TOLEDO,型号为MS105DU(精度0.01 mg);低温恒温槽,上海恒平有限公司,型号为DC-6515,(精度±0.1 ℃);超声波震荡仪,上海宁商超声仪器公司,型号为SY-300;变功率超声波仪,华永昌科技有限公司,型号为HL-900;数显磁力恒温搅拌器,金坛市城西峥嵘实验仪器厂,型号为HJ-6A。
1.2 样品制备
乳酸钙-氯化铵-水相变体系的制备:用分析天平称取氯化铵和乳酸钙,加入蒸馏水中,配置成0.06 g/mL乳酸钙+0.01 g/mL氯化铵的水溶液30份,将混合物放置于100 mL烧杯中,采用磁力搅拌器搅拌均匀,静置待用。在烧杯上分别标上编号1~30,1~10号为第①组,11~20号为第②组,21~30号为第③组。第①组为对照组;第②组使用超声波震荡仪SY-300做震荡处理,如图1所示,超声次数设置为360次,每工作5 s停止5 s,仪器工作温度上限设置为45 ℃,仪器功率为300 W;第③组不做处理。

1.超声波发生器 2.超声波振子 3.反应室 4.相变材料 5.低温工质 6.可调节支架
1.3 相变材料的性能测定
1.3.1 步冷测试
搭建试验平台1,测试复合相变材料①组和②组的步冷曲线。低温恒温槽运行工质为无水乙醇,温度设置为−20 ℃,为了避免周围环境温度对测量器皿内相变材料温度的扰动及防止粉尘颗粒落入材料影响试验,采用聚氨酯制作的泡沫塞将烧杯顶部进行密封处理,T型热电偶从密封塞中心位置穿过。用Agilent数据采集仪采集温度数据,每隔2 s采集记录一次,待相变材料测试温度达到设定温度完全凝固后停止试验。搭建如图2所示的试验平台2,测试复合相变材料③组的步冷曲线。调整超声波功率为50 W,其余条件与试验平台1条件相同。
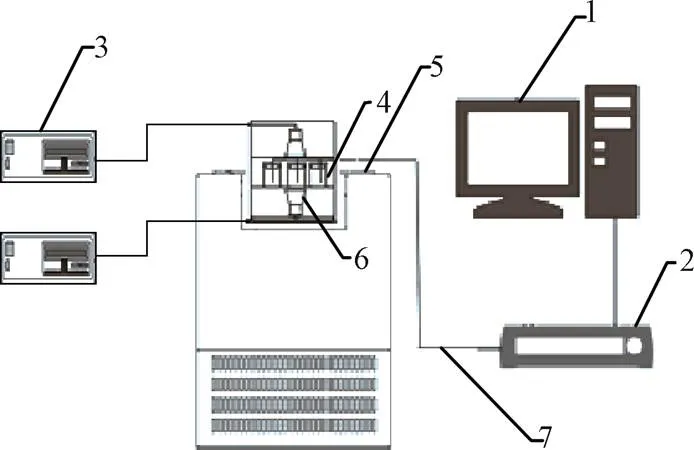
1.电脑 2.Agilent数据采集仪 3.超声波发生器 4.相变材料 5.低温恒温槽 6.超声波振子 7.T型热电偶
1.3.2 DSC测试
乳酸钙-氯化铵-水相变体系的相变温度与相变潜热值的测量使用耐驰公司生产的DSC200 F3来完成。仪器测量前往仪器炉体通入氮气并设定温度为500℃进行干烧,用以消除内部结垢对试验结果的影响。并用6种标准样品进行测试完成仪器温度和灵敏度的校准。使用高精度电子天平(精度0.01 mg)称取5~10 mg的被测材料,将其密封在铝制的试验坩埚中。进行DSC试验时,循环温度起点和终点分别设定为−20 ℃和20 ℃,温度达到设定的温度时分别维持温度恒定3 min,升温及降温速率为5 ℃/min,氮气做保护气,液氮做吹扫气,测得融化曲线,通过软件分析得相变潜热和相变温度。
1.3.3 循环稳定性试验
采用高低温交变试验箱测试样品的稳定性。用烧杯量取20 mL的试验样品,密封放置在高低温交变试验箱内。仪器程序设置为低温冷却至−30 ℃,维持15 min,再加热至30 ℃,维持15 min。循环200次后对样品进行DSC测试。
2 结果与分析
超声波作用会对乳酸钙-氯化铵-水相变体系的过冷度产生影响。为了确定超声波对相变体系的过冷度的影响,做步冷测试。测试结果如图3所示。
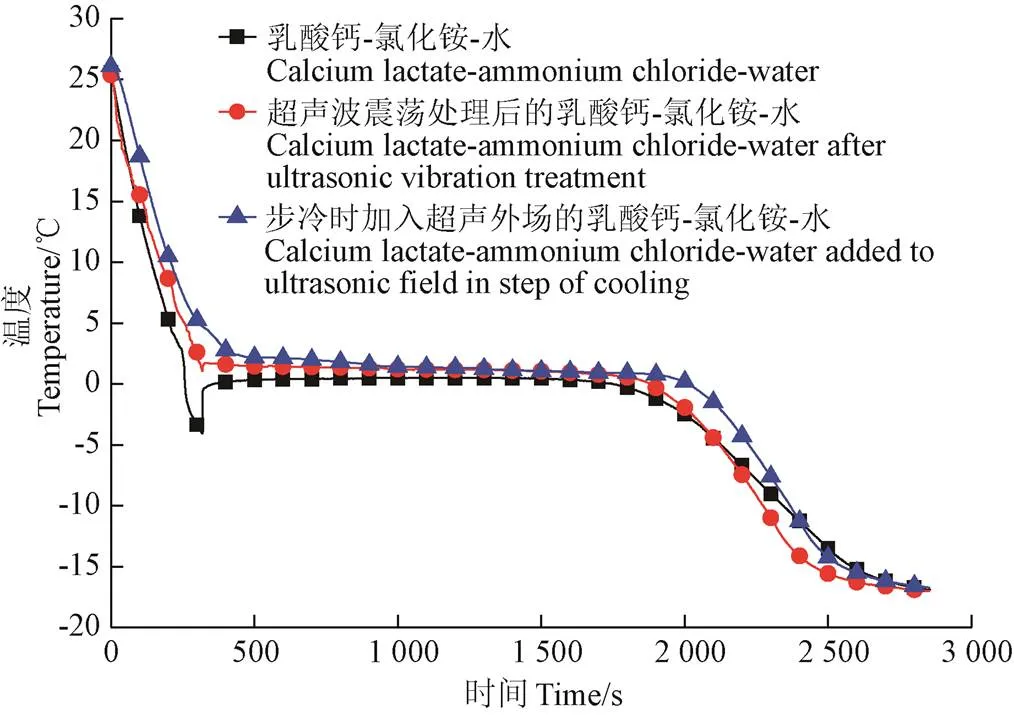
图3 步冷曲线图
从图3的步冷曲线图中,可以明显看出不论是超声波震荡,还是在步冷过程加入超声波外场作用,都可以降低或消除过冷度。相变体系在经过超声处理后,相变温度由−2.4 ℃升高至−2.3 ℃,过冷度由1.35 ℃降低至0.73 ℃;增加超声波相变温度由−2.4 ℃增加至−2.3 ℃,且相变体系没有出现过冷现象。从相变平台看,没有超声外场且没有经过超声处理的相变体系在1 858 s开始发生相变,持续了7 647 s;而增加超声震荡后的相变体系1 778 s开始相变,持续了7 755 s;步冷过程中增加超声外场,相变体系在1 727 s开始相变,持续了8 256 s。这是因为在超声外场的作用下,相变材料中产生了空化气泡,随着空化气泡的成长与破裂引起的微射流和局部高压,给相变材料提供了局部扰动,进而诱导相变材料成核降低过冷度。相比与无超声外场,在超声作用下相变体系的过冷是瞬间完成的,这是因为超声作用下整个液体区域,各处同时产生空化气泡,即而瞬间过冷,相变时间向后延迟。
为了验证超声外场对相变体系相变潜热的影响对经过步冷后的相变体系进行DSC测试,结果如图4。

图4 DSC融化曲线
从图4中可以看出,未作任何处理的乳酸钙-氯化铵-水溶液的相变潜热为265.2 J/g;超声震荡处理过的乳酸钙-氯化铵-水溶液的相变潜热为276.2 J/g,增加了4.14%;在步冷时加入超声外场的乳酸钙-氯化铵-水溶液的相变潜热为283.5 J/g,增加了6.9%。可见,乳酸钙-氯化铵-水相变体系在超声外场的作用下相变潜热明显有增加。这是因为在超声波作用下,相变体系产生了大量气泡,形成空化作用,气泡随着周围的介质的运动不断运动、长大或突然破灭,周围的液体突然冲入气泡从而产生高温高压,并放出热量。
对相变体系经过200循环试验后,相变体系的DSC融化曲线图如图5所示。

图5 循环200次后的DSC融化曲线
从图5中可以看出,未作任何处理的乳酸钙-氯化铵-水溶液的相变潜热为263 J/g;超声震荡处理过的乳酸钙-氯化铵-水溶液的相变潜热为274.7 J/g,在步冷时加入超声外场的乳酸钙-氯化铵-水溶液的相变潜热为281.5 J/g,与循环试验前的相变潜热相比,分别降低了0.83%、0.54%、0.71%。可见,相变体系在经过循环后相变潜热和相变温度均没有发生明显变化,比较稳定。
3 结 论
1)超声波通过对乳酸钙-氯化铵-水相变体系产生空化效应和振荡分散效应降低过冷度。对相变体系进行超声波震荡后,凝固过程相变温度由−2.4 ℃升高至−2.3 ℃,过冷度由1.35 ℃降低至0.73 ℃,相变时间增加;相变体系在凝固过程中增加超声外场,相变温度由−2.4 ℃增加至−2.3 ℃,且相变体系没有出现过冷现象,相变时间增加。乳酸钙-氯化铵-水相变体系凝固时加入超声外场的影响更胜于对相变体系进行超声震荡处理。
2)超声能够提高乳酸钙-氯化铵-水相变体系的相变潜热。试验证明,超声震荡处理过的乳酸钙-氯化铵-水溶液的相变潜热为276.2 J/g,增加了4.14%;在步冷时加入超声外场的乳酸钙-氯化铵-水溶液的相变潜热为283.5 J/g,增加了6.9%。乳酸钙-氯化铵-水相变体系在超声外场的作用下相变潜热明显有增加,在步冷时加入超声外场效果更甚。
3)经过200次冻熔循环测试后,其热物性基本不发生变化。试验结果表明,乳酸钙-氯化铵-水相变体系具有良好的应用前景。
[1] 张东,康韡,李凯莉. 复合相变材料研究进展[J]. 功能材料,2007,38(12):1936-1940.
Zhang Dong, Kang Wei, Li Kaili. Progress in studies of phase change composites[J]. Journal of Functional Materials, 2007, 38(12): 1936-1940. (in Chinese with English abstract)
[2] 曹晓玲,袁艳平,汪玺,等. 肉豆蔻酸/膨胀石墨复合相变材料的制备及性能研究[J]. 太阳能学报,2014,35(8):1493-1498.
Cao Xiaoling, Yuan Yanping, Wang Xi, et al. Preparation and thermal property of myristic acid/expanded graphite composite as phase change materi[J].Acta Energiae Solaris Sinica, 2014, 35(8): 1493-1498. (in Chinese with English abstract)
[3] 张贺磊,方贤德,赵颖杰. 相变储热材料及技术的研究进展[J]. 材料导报,2014,28(13):26-32.
Zhang Helei, Fang Xiande, Zhao Yingjie. Research progress of phase change thermal storage materials and technology[J]. Materials Review, 2014, 28(13): 26-32. (in Chinese with English abstract)
[4] 吴丽彬,陈威,钱静. 有机-无机混合相变材料的热物性研究[J]. 包装工程,2017,38(9):113-117.
Wu Libin, Chen Wei, Qian Jing. Thermophysical property of organic-inorganic phase change mixed material[J]. Packaging Engineering, 2017, 38(9): 113-117. (in Chinese with English abstract)
[5] Hasan M I, Bashir H O, Shadhan A O. Experimental investigation of phase changematerials for insulation of residential buildings[J]. Sustainable Cities & Society, 2018, 36(10): 42-58.
[6] Laaouatni A, Martaj N, Bennacer R, et al. Phase change materials for improving the building thermal inertia[J]. Energy Procedia, 2017, 139(11): 744-749.
[7] Jia J, Lee W L. Experimental investigations on using phase change material for performance improvement of storage- enhanced heat recovery room air-conditioner[J]. Energy, 2015, 93: 1394-1403.
[8] 杨宁,王瑾,柳建华,等. 一种添加纳米颗粒的共晶盐空调蓄冷材料实验研究[J]. 建筑节能,2017,45(1):10-13.
Yang Ning, Wang Jin, Liu Jianhua, et al. Experimental study on a eutectic salt air conditioning cool storage material adding nano-materials[J]. Building Energy Efficiency, 2017, 45(1): 10-13. (in Chinese with English abstract)
[9] Malva C S, Dixon-hardy D W, Crook R. Energy balance model of combined photovoltaic solarthermal system incorporating phase change material[J]. Solar Energy, 2011, 85(7): 1440-1446.
[10] Su D, Jia Y, Lin Y, et al. Maximizing the energy output of a photovoltaic thermal solar collector incorporating phase change materials[J]. Energy & Buildings, 2017, 153(8): 382-391.
[11] 孟娟,吴文潇,成蒙,等. 强化太阳能相变蓄热技术的研究进展[J]. 新能源进展,2019,7(2):155-160.
Meng Juan, Wu Wenxiao, Cheng Meng, et al. Advances in strengthening solar phase change heat storage technology[J]. Advances in New and Renewable Energy, 2019, 7(2): 155-160. (in Chinese with English abstract)
[12] 张喆,方健,熊记,等. 木材太阳能干燥用石蜡相变微胶囊的制备及性能研究[J]. 化工新型材料,2019,47(7):78-81,87.
Zhang Zhe, Fang Jian, Xiong Ji, et al. Preparation and property of MEPCMs for timber drying using solar energy[J]. New Chemical Materials, 2019, 47(7): 78-81, 87. (in Chinese with English abstract)
[13] Carson J K, East A R. The cold chain in New Zealand-A review[J]. International Journal of Refrigeration, 2017, 87(9): 185-192.
[14] Hoang H M, Leducq D, Perez M R, et al. Heat transfer study of submicro-encapsulated PCM plate for food packaging application[J]. International Journal of Refrigeration, 2015, 52(4): 151-160.
[15] 潘欣. 食品相变蓄冷剂的研制[D]. 杭州:浙江大学,2005.
Pan Xin. Development of Food Phase Change Coolant[D]. Hangzhou: Zhejiang University, 2005. (in Chinese with English abstract)
[16] 曾翠华,张仁元. 无机水合盐相变储热材料的过冷性研究[J]. 能源研究与信息,2005,21(1):44-49.
Zeng Cuihua, Zhang Renyuan. Supercooling of salt hydrates as phase change materials[J]. Energy Research and Information, 2005, 21(1): 44-49. (in Chinese with English abstract)
[17] 盛强,邢玉明,王泽. 成核剂对八水氢氧化钡相变材料过冷度的影响[J]. 化工新型材料,2015(2):100-102.
Sheng Qiang, Xing Yuming, Wang Ze. Influence of nucleating agents on the supercooling of Ba (OH)2·8H2O as phase change material[J]. New Chemical Materials, 2015(2): 100-102. (in Chinese with English abstract)
[18] 郑涛杰,陈志莉,刘强,等. 水合盐相变储能材料的增稠剂优选研究[J]. 太阳能学报,2018,39(7):1781-1787.
Zheng Taojie, Chen Zhili, Liu Qiang, et al. Study on optimum agent of thickener for salt hydrate phase change energy storage material[J]. Acta Energiae Solaris Sinica, 2018, 39(7): 1781-1787. (in Chinese with English abstract)
[19] 李琳,李东旭,刘洋,等. 月桂酸-肉豆蔻酸/膨胀石墨封装复合相变储能材料的制备及表征[J]. 太阳能学报,2016,37(10):2627-2632.
Li Lin, Li Dongxu, Liu Yang, et al. Preparationand characterization of lauricacid-myristic acid/expanded graphite composite encapsulated phase change energy storage material[J]. Acta Energiae Solaris Sinica, 2016, 37(10): 2627-2632. (in Chinese with English abstract)
[20] 刘永红,郭开华. 超声波频率对HCFC-141b水合物结晶过程的影响研究[J]. 哈尔滨工业大学学报,2005,37(5):633-635.
Liu Yonghong, Guo Kaihua. Influences of ultrasonic waves frequencies on HCFC-141b hydrate crystallization process[J]. Journal of Harbin Institute of Technology, 2005, 37(5): 633-635. (in Chinese with English abstract)
[21] 赵茜,高大维. 溶剂超声波协同法用于有机物系结晶快速成核[J]. 化工学报,1997,48(5):631-634.
Zhao Qian, Gao Dawei. Study on application of ultrasonic wave and organic solvent in organic system nucleation[J]. Journal of Chemical Industry and Engineering, 1997, 48(5): 631-634. (in Chinese with English abstract)
[22] 王葳,张绍志,陈光明,等. 超声波对水的过冷度影响的试验研究[J]. 制冷学报,2003,24(1):6-8.
Wang Rui, Zhang Shaozhi, Chen Guangming, et al. Study on ultrasound's effect on supercooling of water[J]. Journal of Engineering Thermophysics, 2003,24(1):6-8. (in Chinese with English abstract)
[23] Hickling R. Nucleation of freezing by cavity collapse and its relation to cavitation damage[J]. Nature, 1965, 206(4987): 915-917.
[24] Hunt J D, Jackson K A. Nucleation of solid in an undercooled liquid by cavitation[J]. Journal of Applied Physics, 1966, 37(1): 254-257.
[25] Hu F, Sun D W, Gao W, et al. Effects of pre-existing bubbles on ice nucleation and crystallization during ultrasound- assisted freezing of water and sucrose solution[J]. Innovative Food Science & Emerging Technologies, 2013, 20(4): 161-166.
[26] 刘东方. 潜热型水基氧化石墨烯纳米流体的制备及其过冷特性研究[D]. 重庆:重庆大学,2012.
Liu Dongfang. Study of Preparation and Supercooling Characteristics of Graphene Oxide Nanofluids as Phrase Change Material[D]. Chongqing: Chongqing University, 2012. (in Chinese with English abstract)
[27] Liu Yudong, Liu Yumin, Hu Pengfei, et al. The effects of graphene oxide nanosheets and ultrasonic oscillation on the supercooling and nucleation behavior of nanofluids PCMs[J]. Microfluidics & Nanofluidics, 2015, 18(1): 81-89.
[28] Kiani H, Zhang Z, Delgado A, et al. Ultrasound assisted nucleation of some liquid and solid model foods during freezing[J]. Food Research International, 2011, 44(9): 2915-2921.
[29] Kiani H, Sun D W, Delgado A, et al. Investigation of the effect of power ultrasound on the nucleation of water during freezing of agar gel samples in tubing vials[J]. Ultrasonics Sonochemistry, 2012, 19(3): 576-581.
[30] Liu Y, Su C, Hu P, et al. Containerless nucleation behavior and supercooling degree of acoustically levitated graphene oxide nanofluid PCM[J]. International Journal of Refrigeration, 2015, 60: 70-80.
[31] Cogné C, Labouret S, Peczalski R, et al. Theoretical model of ice nucleation induced by inertial acoustic cavitation. Part 2: Number of ice nuclei generated by a single bubble[J]. Ultrasonics Sonochemistry, 2016, 28(8): 185-191.
Effect of ultrasound on supercooling characteristics of calcium lactate-ammonium chloride-water composite phase change materials
Zhang Xuelai, Liu Lu, Zhang Yongyichuan, Xu Xiaofeng, Wang Xiang
(,,201306,)
Phase change materials can absorb or release a large amount of latent heat in the process of phase change. They have the advantages of large energy storage density, stable energy storage, small size and easy control. They are widely used in the fields of heat storage and temperature control. Supercooling degree generally exists in phase change materials and affects the cold storage performance of low-temperature phase change cold storage materials. Severe supercooling phenomenon will lead to the inability to release the cold amount of phase change cold storage materials and reduce the energy utilization efficiency. Solving the problem of supercooling of cryogenic phase change storage materials is the key to its better application. It has been proved that supercooling of phase change materials can be solved by adding nucleating agent. In addition to salt nucleating agents, nanometer nucleating agents have attracted more and more attention because they can reduce the supercooling degree of materials and improve the thermal conductivity of materials. In addition, the dispersion effect and cavitation effect of ultrasound can optimize the supercooling characteristics. In order to solve the problem of high supercooling degree of inorganic phase change materials, ultrasonic shock treatment was carried out on the calcium lactate-ammonium chloride - water phase change system and ultrasonic field was added in the solidification process to study the influence of ultrasonic wave on the solidification characteristics of calcium lactate-ammonium chloride - water phase change system. It was found that after increasing ultrasonic shock, the supercooling degree decreased by 0.62 ℃, the phase transition process lasted for 7 755 s, and the latent heat of phase transition of the solution was 276.2 J/g, increasing by 4.14%. During the solidification of the solution, the ultrasonic phase change system was added and no supercooling occurred. The latent heat of phase change of 8 256 s solution was 283.5 J/g, an increase of 6.9%. Under the action of ultrasonic external field, cavitation bubbles are generated in the phase-change material. With the growth and rupture of cavitation bubbles, micro-jet and local high pressure are caused, which provide local disturbance to the phase-change material, and then induce nucleation of the phase-change material to reduce the subcooling degree. Compared with ultrasonic field, under the effect of ultrasonic phase transformation system of supercooling is instantaneous, that is because the whole liquid region under ultrasonic and cavitation bubbles everywhere at the same time, namely the moment too cold, phase transition time delayed, bubble ceaselessly as the movement of the surrounding medium movement, grew up or suddenly burst, the surrounding liquid into bubbles produced by high temperature and high pressure, and gives off heat. The results showed that adding ultrasonic field in the solidification process of calcium lactate-ammonium chloride-water phase change system could improve the performance of calcium lactate- ammonium chloride-water phase change system.
ultrasonic; phase change materials; thermodynamic properties; calcium lactate; ammonium chloride; subcooling degree; phase change latent heat
章学来,刘璐,张永一川,徐笑锋,汪翔. 超声波改善乳酸钙-氯化铵-水复合相变材料过冷特性[J]. 农业工程学报,2019,35(18):200-204.doi:10.11975/j.issn.1002-6819.2019.18.024 http://www.tcsae.org
Zhang Xuelai, Liu Lu, Zhang Yongyichuan, Xu Xiaofeng, Wang Xiang. Effect of ultrasound on supercooling characteristics of calcium lactate-ammonium chloride-water composite phase change materials[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(18): 200-204. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2019.18.024 http://www.tcsae.org
2019-03-15
2019-06-06
国家重点研发项目计划(2018YFD0401300);国家自然科学基金(51376115);上海市科委项目(16040501600)
章学来,教授,博导,研究方向为相变储能技术。Email:xlzhang@shmtu.edu.cn
10.11975/j.issn.1002-6819.2019.18.024
TM306
A
1002-6819(2019)-18-0200-05

