Hepatitis B virus X protein enhances hepatocarcinogenesis by depressing the targeting of NUSAP1 mRNA by miR-18b
Zhe Yang, Jiong Li, Guoxing Feng, Yuan Wang, Guang Yang, Yunxia Liu, Shuqin Zhang, Jinyan Feng,Xiaodong Zhang
Department of Cancer Research, College of Life Sciences, Nankai University, Tianjin 300071, China
ABSTRACT Objective:The aim of this study was to investigate the underlying mechanism whereby HBx modulates the targeting of NUSAP1 by miR-18b to enhance hepatocarcinogenesis.Methods:We employed an integrated approach of bioinformatics analysis and molecular experiments in hepatoma cells, HBV transgenic mice, and clinical liver cancer tissues to investigate the role of HBx-regulated miR-18b in the development of liver cancer.Results:In this study, we report that the HBx-mediated tumor suppressor miR-18b modulates hepatocarcinogenesis during the host-HBV interaction. The expression levels of miR-18b were lower in clinical HBV-positive liver cancer tissues and liver tissues of HBV-transgenic mice. Interestingly, HBx inhibited miR-18b expression by inducing the methylation of CpG islands in its promoter. Accordingly, we tested the hypothesis that HBx enhanced hepatocarcinogenesis by increasing the expression of target genes of miR-18b. Moreover, we identified nucleolar spindle-associated protein 1 (NUSAP1) as one of the target genes of miR-18b.NUSAP1 was expressed at high levels in liver cancer tissues. Interestingly, HBx up-regulated NUSAP1 by suppressing miR-18b.Functionally, miR-18b significantly inhibited the proliferation of hepatoma cells by depressing NUSAP1 levels in vivo and in vitro.Conclusions:Thus, we conclude that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b is controlled by HBxmodulated promoter methylation during the host-virus interaction, leading to hepatocarcinogenesis. Our findings provide new insights into the mechanism by which HBx-mediated miRNAs modulate hepatocarcinogenesis.
KEYWORDS Hepatitis B virus; HBx; miR-18b; NUSAP1; HCC
Introduction
Hepatocellular carcinoma (HCC) is a common cancer worldwide and the third leading cause of cancer-related deaths globally1. Chronic hepatitis B virus (HBV) infection is closely related to chronic hepatitis, cirrhosis, and HCC2-4. As a functional trans-activating protein, HBV X protein (HBx)plays important roles in the development of liver cancer5-7.HBx can stimulate transcription, signal transduction, and cell cycle progression by binding to different transcription factors or components of various signaling pathways8-10. In addition,HBx participates in genetic and epigenetic regulation during the development of liver cancer11,12. However, the underlying mechanism by which HBx modulates hepatocarcinogenesis is poorly understood.
MicroRNAs (miRNAs) are endogenous, 21-24 nucleotide RNAs that can play regulatory roles by targeting mRNAs. As a result, the targeted mRNAs are cleaved or posttranscriptionally repressed13-15. More than half of all mRNAs are regulated by miRNAs and each miRNA is estimated to regulate hundreds of mRNAs16-18. Studies of specific miRNAs have shown that they play distinct roles in cellular functions, such as proliferation, development,differentiation, and apoptosis19-23. A previous study reported that miRNAs are critical for essential cellular processes, hostvirus interactions, and virus life cycles24. Many miRNAs have been reported to be involved in the host-virus interactions of more than 50 different infections, including those involving dengue virus, Ebola virus, hepatitis B virus, hepatitis C virus,and rabies virus25. These findings demonstrate the important role of miRNAs in host immunity and the defense against viral infections. Immune dysregulation, instance, during chronic inflammation, can lead to chronic illness and even death26-28. Therefore, a balanced immune response is essential. MiRNAs play an important role in innate immunity by directly targeting viral transcripts29-31. Various miRNAs,such as miR-141, miR-501, and miR-125a-5p have been implicated in the regulation of HBV replication32,33.
It has been reported that miR-18b is able to suppress cell migration and invasiveness in melanoma34. MiR-18b can suppress high-glucose-induced proliferation of human retinal endothelial cells (HRECs) by targeting the IGF-1/IGF1R signaling pathways35. However, the role of miR-18b in HBV-related HCC is still unclear. Some miRNAs are decreased in human tumors due to aberrant hypermethylation of cytosine-phosphate-guanosine (CpG)islands of miRNA genes36,37. Aberrant DNA methylation is also associated with the development of HCC. CpG hypermethylation serves as a mechanism for miRNA silencing, but this field remains largely unexplored. Our group reported that HBx was able to inhibit the tumor suppressor, miR-205 and enhance hepatocarcinogenesis by inducing methylation of the miR-205 promoter38. However,the effect of HBx on miR-18b is undefined. Nucleolar spindle associated protein 1 (NUSAP1) is an important mitotic regulator that has been reported to be essential for many cellular processes39. NUSAP1 plays crucial roles in embryogenesis and cancer40-42and is up-regulated in HCC when compared to nontumor liver tissues43. However, the significance of NUSAP1 in HBV-related liver cancer progression remains poorly understood.
In the present study, we investigated the mechanism by which HBx modulates hepatocarcinogenesis during the hostvirus interaction. Interestingly, we identified that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b was controlled by HBx during the host-virus interaction,leading to hepatocarcinogenesis. Thus, our finding provides new insights into the mechanism by which HBx-mediated miRNAs modulate hepatocarcinogenesis.
Materials and methods
Patient samples
Thirty liver tissues were obtained immediately after surgical resection from randomly selected HCC patients at Tianjin First Center Hospital (Tianjin, China) and Tianjin Medical University Cancer Institute and Hospital (Tianjin, China).Clinicopathological information for the patients was collected from hospital patient records (Supplementary Table S1). Written informed consent for the use of their tissues for research purposes was obtained from all patients in the study. The Institute Research Ethics Committee at the Nankai University approved the study protocol.
Cell lines and cell culture
The HepG2, HepG2-X (cell line with stable HBx transfection), HepAD38 (cell line stably producing HBV),and HepG2.2.15 (a hepatoma HepG2 cell line with integrated full-length HBV DNA), were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA).For HBV replication, HepG2.2.15 cells were seeded at optimal densities and HepAD38 cells were treated without tetracycline for 8 days. LO2 (human immortalized normal liver cell line), H7402 (hepatoma cell line), and H7402-X(cell line with stable HBx- transfection) cells were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin in 5% CO2at 37°C. HepG2-X and H7402-X cells were maintained in 200 μg/mL G418.
HBV transgenic mice and tissue analysis
BALB/c mice and HBV transgenic BALB/c mice (HBV-Tg)containing the HBV genome S, pre-S, and X domains were purchased from Vital River Laboratory Animal Technology(Beijing, China). Animals were maintained under specific pathogen-free conditions. Mice were sacrificed at the end of the experimental period and serum and liver tissues were obtained.
In vivo tumorigenicity assays
Animal transplantation experiments were performed in compliance with the principles of the Declaration of Helsinki.Nude mice were housed and treated according to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Institute Research Ethics Committee at Nankai University. Tumor transplantation experiments were performed as previously described44,45. Briefly, HepG2-X cells were pretreated with control, miR-18b, miR-18b, or pcDNA3.1-NUSAP1 for 24 h.Cells were then resuspended at 2 × 107cells/mL in sterile PBS. Four-week-old male BALB/c athymic nude mice(Experimental Animal Center of Peking, China) were grouped (n = 6 per group) and injected subcutaneously on the shoulder with 0.2 mL of the appropriate cell suspension.Tumor growth was measured 6 days after the injection and thereafter, every 4 days. Tumor volume was evaluated by measuring the length (L) and width (W) with calipers and was then calculated according to the formula, (L× W2)/2. On the thirtieth day, mice were sacrificed and tumors were excised and measured.
Statistical analysis
The correlation between NUSAP1 (or miR-18b) and HBx mRNA/pregenomic (pg) RNA levels in tumor tissues was determined by calculating the Pearson’s correlation coefficient. NUSAP1 expression levels in tumor tissues and adjacent nontumorous tissues were compared using Wilcoxon’s signed-rank test. Statistical significance was assessed by comparing mean values (± standard deviation,SD) using a Student’s t-test for independent groups, with significance assumed at P < 0.05. Each experiment was repeated at least three times.
Results
HBx suppressed miR-18b by inducing hypermethylation of the miR-18b promoter
It has been reported that miR-18b suppresses cell migration and invasiveness in melanoma34. However, the role of miR-18b in HBV-related liver cancer remains unclear. Therefore,we hypothesized that HBx modulates miR-18b during hepatocarcinogenesis. Therefore, we measured miR-18b expression in 30 paired HCC and adjacent nontumorous liver tissues using qRT-PCR. MiR-18b expression was normalized to the expression of an endogenous control (U6 RNA). Our data showed that the expression levels of miR-18b were significantly lower in clinical HBV-related HCC samples, relative to their adjacent noncancerous liver tissues(Figure 1A; P < 0.01, Wilcoxon’s signed rank test). Next, we examined the relationship between HBx and miR-18b in HCC tissues using qRT-PCR. Intriguingly, we found that the expression levels of miR-18b were inversely correlated with those of HBx mRNA/pgRNA (Figure 1B; Pearson’s correlation coefficient, r = -0.5771; P < 0.01). Moreover, our data showed that the expression levels of miR-18b were lower in the livers of HBV-transgenic mice relative to the livers of wild-type mice (Figure 1C), suggesting that HBx may suppress the expression of miR-18b during HBV infection and HCC development. The levels of HBx/pgRNA were also determined in wild-type mice and HBV-transgenic mice using qRT-PCR (Supplementary Figure S1A). Based on these results, we proposed that HBx directly down-regulates miR-18b in liver cells. Next, we transiently transfected HepG2 and H7402 cells with an HBx-expression plasmid(pcDNA3.1-HBx). The overexpression of HBx was validated in the two cell lines (Supplementary Figure S1B). We showed that HBx decreased miR-18b expression in a dosedependent manner (Figure 1D), suggesting that HBx is able to inhibit miR-18b in HCC cells. Given that miR-18b was down-regulated in clinical HCC tissues, we sought to investigate the mechanism by which HBx down-regulates miR-18b in hepatoma cells. It has been reported that HBx downregulates miRNA expression through methylation of the promoter region38. We evaluated methylation levels in the promoter region of miR-18b in HCC cells and clinical HCC tissues (Figure 1E) using bisulfite-sequencing polymerase chain reaction (PCR). Our data showed that the methylation levels of the miR-18b promoter CpG islands were higher in HepG2-X, HepAD38, and HepG2.2.15 cells than in LO2 cells (Figure 1F), suggesting that the miR-18b gene promoter is hypermethylated in HBV-infected hepatoma cells relative to normal liver cells (Supplementary Figure S1C). MiR-18b levels were then measured by qRTPCR in LO2, HepG2-X, HepAD38, and HepG2.2.15 cells.Interestingly, the expression levels of miR-18b were negatively correlated with the methylation status of the miR-18b promoter in all four cell lines (Supplementary Figure S1D). Eight tumor tissues showed high DNA methylation levels (Figure 1G) and DNA methylation levels of all peritumoral tissues were low, suggesting that the miR-18b promoter is hypermethylated in HCC tissues relative to adjacent nontumorous liver tissues (Supplementary Figure S1E). Thus, we conclude that HBx down-regulates miR-18b by inducing the methylation of CpG islands in the miR-18b gene promoter.
MiR-18b inhibited the expression of NUSAP1 by directly targeting the 3'UTR of its mRNA
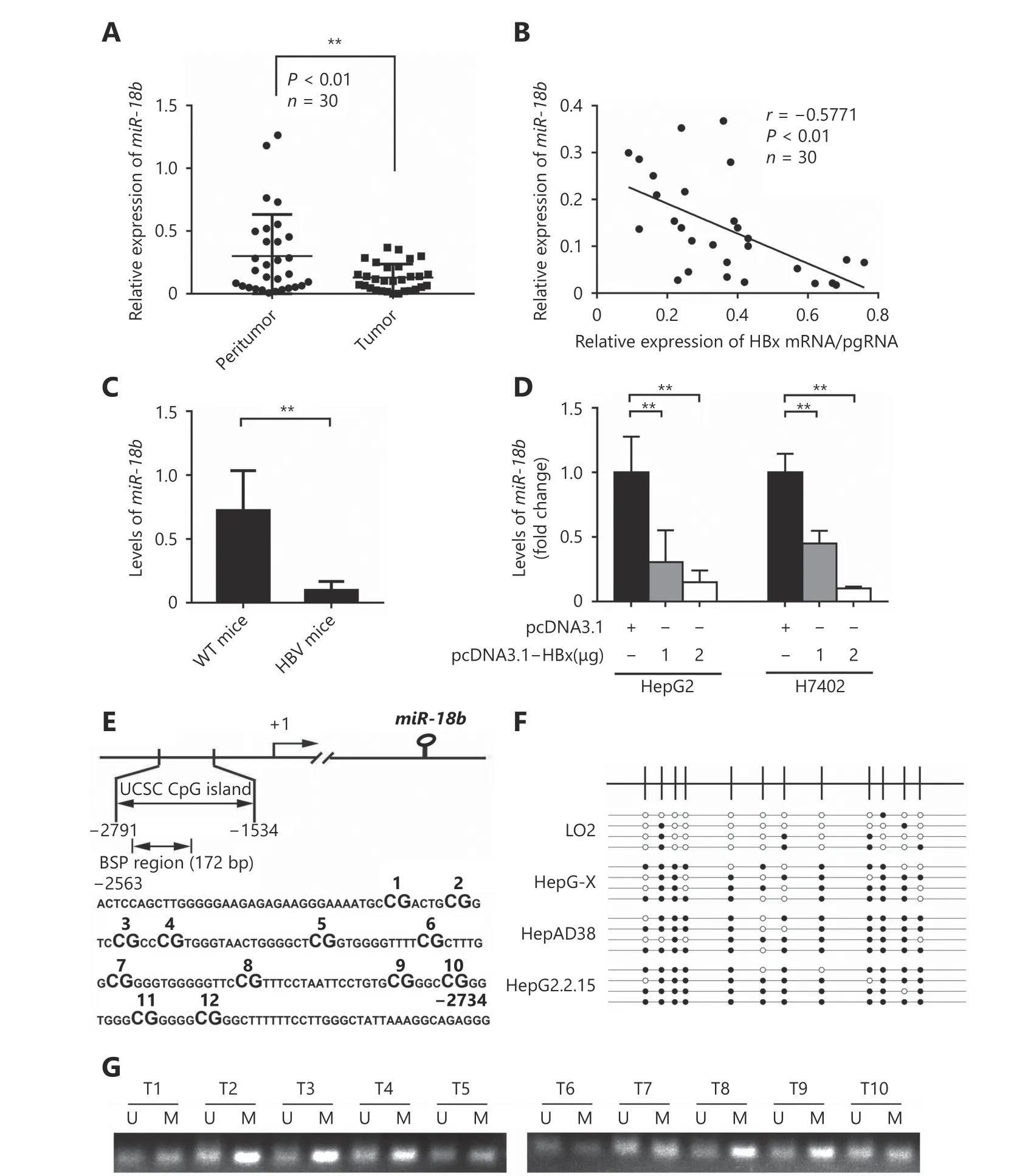
Figure 1 HBx suppresses miR-18b through inducing DNA hypermethylation of miR-18b promoter. (A) Relative mRNA levels of miR-18b were examined by qRT-PCR analysis in 30 pairs of HCC tissues and corresponding nontumorous tissues. **P < 0.01; Wilcoxon's signed-rank test. (B) The correlation between HBx mRNA level and miR-18b mRNA level was analyzed by qRT-PCR analysis in 30 cases of HCC tissues(**P < 0.01, r = -0.5771; Pearson's correlation coefficient). (C) MiR-18b expression was tested by qRT-PCR in HBV-transgenic mice (n = 6). (D)The expression of miR-18b was measured by qRT-PCR analysis after treatment with pcDNA3.1-HBx in HepG2 and H7402 cells. (E) Scheme depicting the genomic localization of miR-18b. The regions analyzed by BSP are indicated. The CpG dinucleotide within the miR-18b promoter regions (-2563 to -1534, 172bp) were numbered as 1-12. (F) The methylation status of the miR-18b promoter in LO2, HepG2-X,HepAD38 and HepG2.2.15 cell lines. The open and filled circles indicate the unmethylated and methylated CpGs, respectively. (G) The methylation status of the miR-18b promoter was examined by MSP analysis in HCC tissues (T).
To further elucidate the molecular mechanism whereby miR-18b affects the pathogenesis of HCC, we used the online miRNA target gene prediction tool, Targetscan(http://www.targetscan.org/) and found that NUSAP1 was a potential target gene for miR-18b. However, the significance of NUSAP1 in HCC is poorly understood. Therefore, we used the cancer microarray database, Oncomine(https://www.oncomine.org/) and Metabolic gEne RApid Visualizer (MERAV, http://merav.wi.mit.edu/) to predict the expression level of NUSAP1 in HCC. These results showed that the expression levels of NUSAP1 were higher in HCC tissues relative to those in normal tissues, suggesting that NUSAP1 is a potential oncogene (Supplementary Figure S2A and S2B). Next, we analyzed the expression of NUSAP1 in clinical HCC tissues using immunohistochemical (IHC)staining of tissue arrays. In HCC tissues, NUSAP1 staining was observed in the cytoplasm and nucleus (Figure 2A).NUSAP1 staining was observed in 68.61% (129/188) of all HCC tissues and in 77.41% (24/31) of grade III HCC tissues,suggesting that NUSAP1 is closely associated with the malignancy of HCC. QPCR analysis revealed that NUSAP1 expression levels were significantly increased in 30 clinical HCC samples, relative to their adjacent nontumor tissues (P< 0.01, n = 30, Wilcoxon’s signed-rank test, Figure 2B).Using the OncoLnc database (http://www.oncolnc.org), we found that a higher level of NUSAP1 expression was associated with a lower survival rate in HCC patients (Figure 2C). Moreover, we found that miR-18b expression levels were negatively correlated with NUSAP1 mRNA levels in HCC tissues (Pearson’s correlation coefficient, r = -0.7278; P <0.01; Figure 2D), suggesting that NUSAP1 may be one of the target genes of miR-18b. To confirm the site-specific repression of NUSAP1 by miR-18b, we constructed a luciferase reporter vector containing the 3'UTR of NUSAP1 mRNA (Figure 3A and 3B). Luciferase reporter gene assays showed that miR-18b directly targeted the NUSAP1 mRNA 3'UTR in HepG2 cells, in a dose-dependent manner, but it did not target a mutated NUSAP1 mRNA 3'UTR (Figure 3C). Conversely, an anti-miR-18b oligonucleotide increased the luciferase activity of pGL3-NUSAP1-3'UTR-wt in a dosedependent manner, but had no effect on the mutant (Figure 3D), suggesting that miR-18b is able to directly bind to the 3'UTR of NUSAP1 mRNA. Furthermore, the over-expression of miR-18b suppressed the expression of NUSAP1 in HepG2 and H7402 cells, at the mRNA and protein level, in a dosedependent manner, and the reverse effect was seen when cells were treated with an anti-miR-18b oligonucleotide (Figure 3E and 3F, Supplementary Figure S2C and S2D). Our data showed that an anti-miR-18b oligonucleotide significantly rescued NUSAP1 levels that were decreased by miR-18b in HCC cells (Supplementary Figure S2E and S2F).Collectively, we conclude that miR-18b is able to inhibit NUSAP1 expression by directly targeting the 3'UTR of its mRNA.
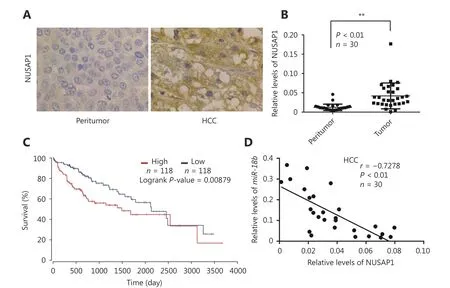
Figure 2 MiR-18b inhibits the expression of NUSAP1 through directly targeting its mRNA 3'UTR. (A) The expression of NUSAP1 was examined by IHC staining in normal tissues and HCC tissues using tissue array, respectively. (B) Relative mRNA levels of NUSAP1 were measured by qRT-PCR analysis in 30 pairs of HCC tissues and corresponding nontumorous tissues. **P<0.01; Wilcoxon's signed-rank test. (C)The survival rate of HCC patients analyzed by OncoLnc database (http://www.oncolnc.org/). (D) The correlation between NUSAP1 mRNA level and miR-18b mRNA level was analyzed by qRT-PCR analysis in 30 cases of HCC tissues (**P<0.01, r=-0.7278; Pearson's correlation coefficient).
HBx up-regulated NUSAP1 in hepatoma cells
Given that HBx was able to down-regulate the targeting of NUSAP1 by miR-18b, we investigated whether HBx could down-regulate NUSAP1 by inhibiting miR-18b. The mRNA levels of NUSAP1 and HBx/pgRNA were examined by qRTPCR in 30 HCC liver tissues. Our data showed that the expression levels of HBx/pgRNA were positively correlated with those of NUSAP1 mRNA in HCC tissues (Pearson’s correlation coefficient, r = 0.7028; P < 0.01; Figure 4A),suggesting that HBx is able to up-regulate NUSAP1.Moreover, our data showed that the expression levels of NUSAP1 were higher in HBV-transgenic mice relative to wild-type mice (Figure 4B), suggesting that HBx may upregulate the expression of NUSAP1 during HBV infection.Furthermore, we confirmed that HBx was able to up-regulate the expression of NUSAP1, in a dose-dependent manner, in HepG2 and H7402 cells transfected with HBx (Figure 4C and 4E). Conversely, HBx knockdown by si-HBx abolished the up-regulation of NUSAP1 in HepAD38 and HepG2.2.15 cells, at both the RNA and protein level, in a dose-dependent manner (Figure 4D and 4F). Thus, we conclude that HBx is able to up-regulate NUSAP1 in hepatoma cells.
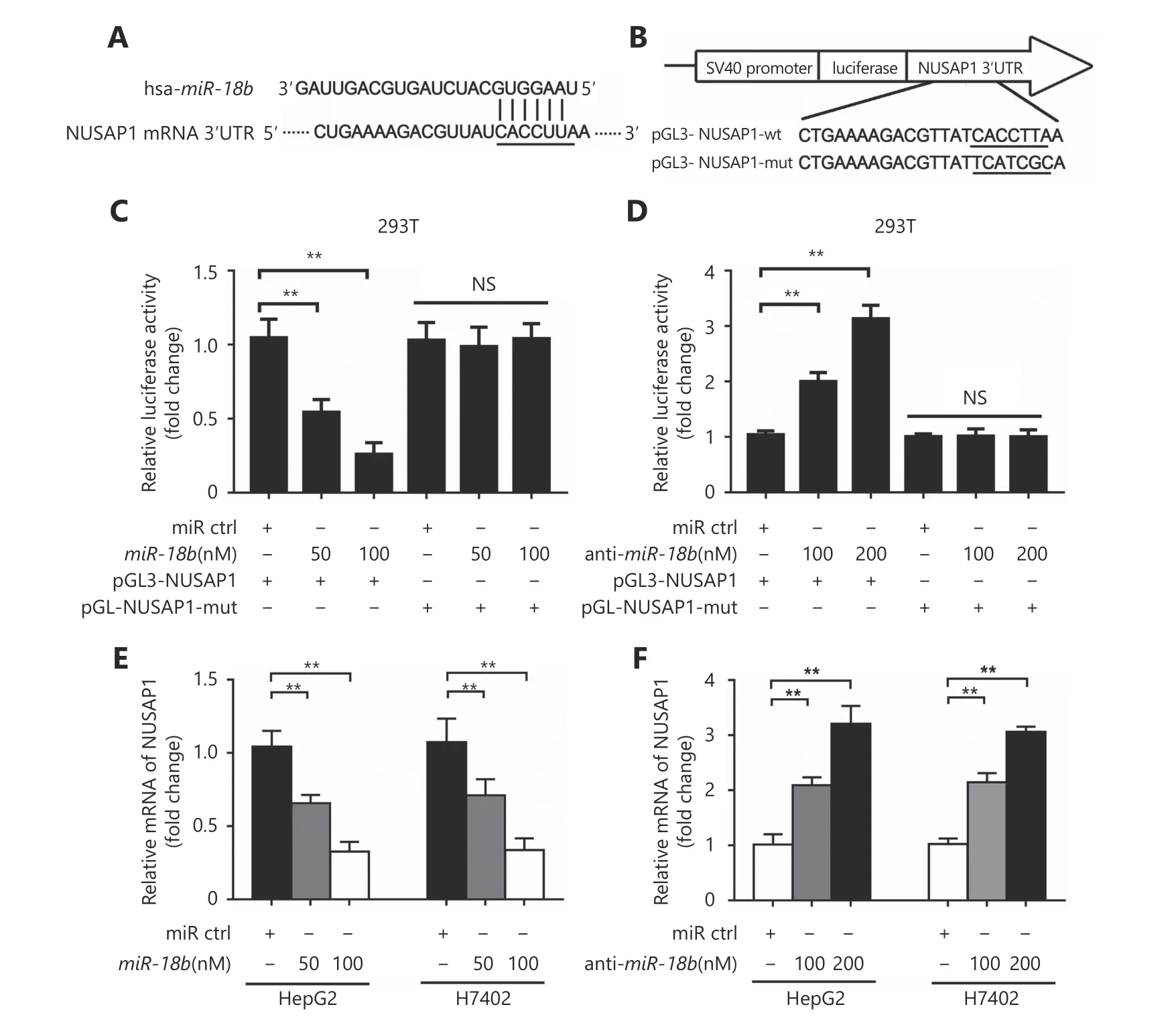
Figure 3 MiR-18b inhibits the expression of NUSAP1 through directly targeting its 3'UTR. (A,B) MiR-18b sequence and the potential MiR-18b -binding sites at the 3'UTR of NUSAP1 mRNA. Seed sequences and mutant are marked. (C,D) The effect of miR-18b (or anti-miR-18b)on the pGL3-NUSAP1-3'UTR-wt and pGL3-NUSAP1-3'UTR-mut reporters was measured by luciferase reporter gene assays in HepG2 cells.(E,F) The effect of miR-18b (or anti- miR-18b) on the expression of NUSAP1 was examined by qRT-PCR analysis in HepG2 and H7402 cells.Error bars indicate SD. Student's t-test, **P<0.01, NS, not significant.
HBx-induced NUSAP1 promoted the proliferation of hepatoma cells in vitro
NUSAP1 expression has been reported to promote cell proliferation in renal cell carcinoma46. We sought to determine whether NUSAP1 could also influence cell proliferation in HCC. MTT and colony-formation assays showed that NUSAP1 promoted cell proliferation in HepG2 cells (Supplementary Figure S3A and S3B). Next, we determined the effect of NUSAP1 targeting by miR-18b on cell proliferation using MTT assays, colony formation assays,and cell cycle analysis in HepG2, HepG2-X, H7402, and H7402-X cells. We found that the proliferation of HepG2-X and H7402-X cells was significantly reduced by miR-18b.However, the over-expression of NUSAP1 could rescue the proliferation of these cells (Figure 5A-5C and Supplementary Figure S4A-4D), suggesting that miR-18b targeting of NUSAP1 is able to suppress the HBx-induced proliferation of hepatoma cells. Taken together, we conclude that HBx-induced NUSAP1 increases the proliferation of hepatoma cells in vitro.

Figure 4 HBx is able to up-regulate NUSAP1 in hepatoma cells. (A) The correlation between NUSAP1 mRNA level and HBx mRNA level was examined by qRT-PCR analysis in 30 cases of HCC tissues (**P < 0.01, r = 0.7028; Pearson's correlation coefficient). (B) MiR-18b expression was measured by qRT-PCR in HBV-transgenic mice (n = 6). (C-F) The expression of NUSAP1 was analyzed by qRT-PCR and Western blot analysis in the cells transfected with pcDNA3.1-HBx plasmid (or si-HBx). Error bars indicate SD. Student's t-test. **P < 0.01.
HBx-induced NUSAP1 facilitated the growth of liver cancer in vivo
To further evaluate the effect of NUSAP1 on tumor growth,we subcutaneously injected pretreated cells into 4-week-old BALB/c athymic nude mice. Interestingly, tumor weight and volume in mice injected with miR-18b-transfected HepG2-X cells were significantly lower than in mice injected with negative control-transfected HepG2-X cells. Over-expression of NUSAP1 rescued the decrease in tumor size in mice injected with miR-18b-transfected HepG2-X cells (Figure 6A-6C), suggesting that the targeting of NUSAP1 by miR-18b is able to suppress the HBx-induced growth of hepatoma cells. In addition, IHC staining was used to determine the expression levels of Ki-67, a marker of proliferation, in tumor tissues from athymic nude mice. Ki-67 expression results were consistent with the results of tumor growth analysis(Figure 6D). Thus, we conclude that HBx-induced NUSAP1 facilitates the growth of liver cancer in vivo.
Discussion
HBV is a widespread virus and chronic HBV infection is a major risk factor for HCC47,48. HBx is a key factor involved in hepatocarcinogenesis. Host miRNAs play an important role in the development of liver cancer associated with HBV infection49. However, the underlying mechanism by which HBx-modulated miRNAs promote liver cancer during the host-virus interaction is still unclear. In this study, we investigated the mechanism by which HBx modulates hepatocarcinogenesis.
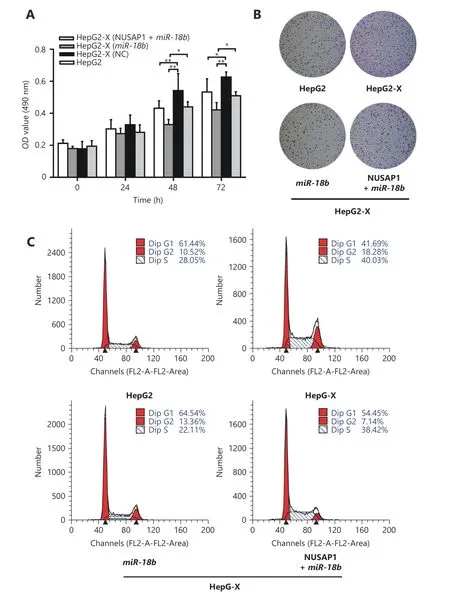
Figure 5 HBx-elevated NUSAP1 promotes the proliferation of hepatoma cells in vitro. (A) The effect of miR-18b and NUSAP1 on HBxinduced cell proliferation was determined by MTT assays in HepG2-X cells. (B) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was measured by colony formation assays in HepG2-X cells. (C) The effect of miR-18b and NUSAP1 on HBx-induced cell proliferation was examined by cell cycle assays in HepG2-X cells. Error bars indicate SD. Student's t-test, *P<0.05, **P<0.01, NS, not significant.
In the early stage of HBV infection, the host can use its own miRNA as a defense against HBV. Some miRNAs,including hsa-miR-210, hsa-miR-199-3p, hsa-miR-125a-5p,and hsa-miR-151-5p, have been shown to affect HBV gene expression in cultured cells by direct binding to viral transcripts50-52. However, the role of miR-18b in hepatocarcinogenesis remains unclear. It has been reported that miR-18b is down-regulated in HCC tissues compared to normal liver tissue53. In addition, miR-18b expression levels decrease during chronic HBV infection54. However, we observed that miR-18b expression levels were low in clinical HBV-related HCC samples, relative to their adjacent noncancerous hepatic tissues and in HBV-transgenic mice,relative to wild-type mice. This implies that HBV may suppress the expression of miR-18b during HBV infection and the subsequent development of HCC.
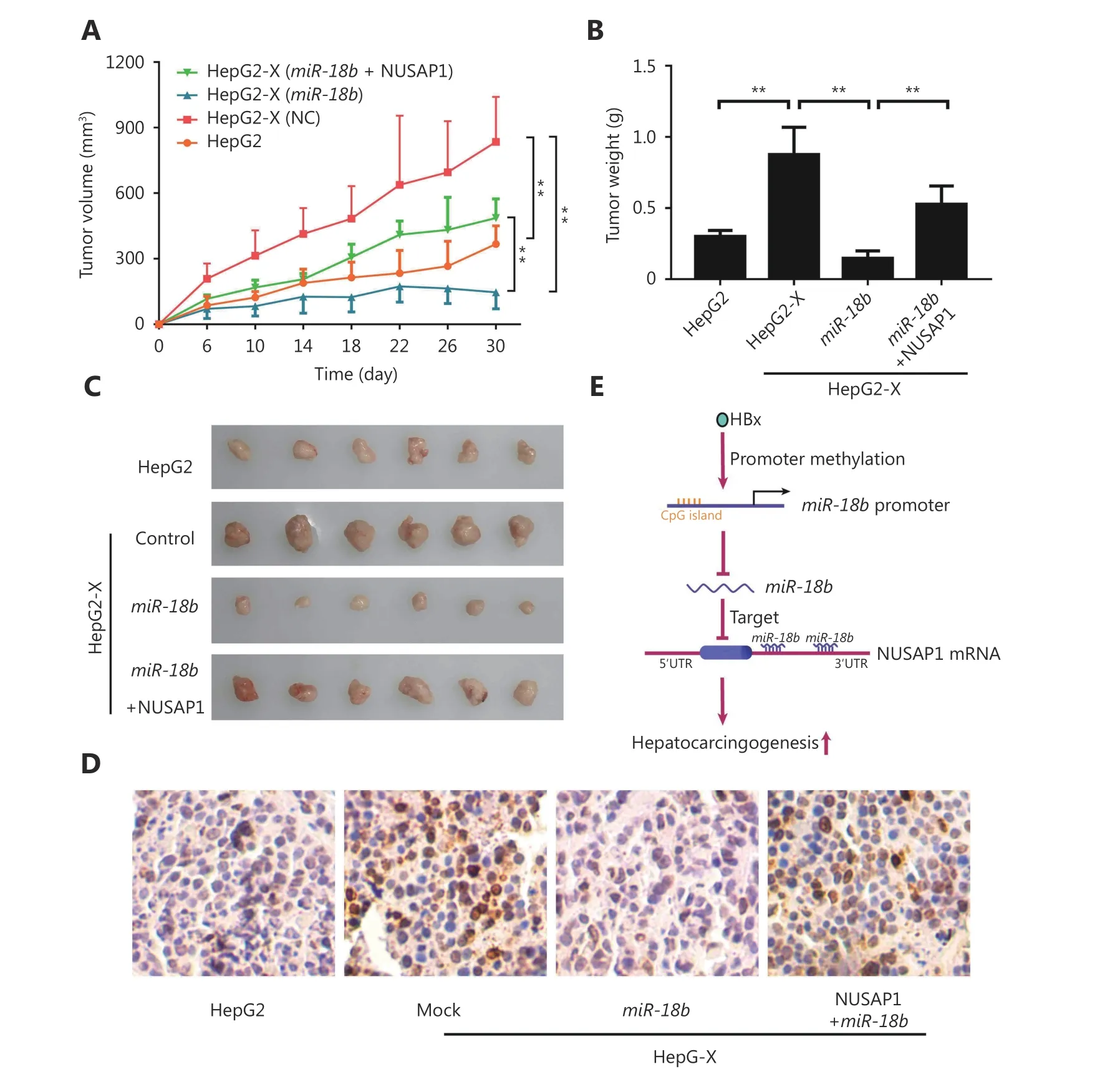
Figure 6 HBx-elevated NUSAP1 facilitates the growth of liver cancer in vivo. (A) Growth curve of tumors transplanted with HepG2 and HepG2-X cells pretreated with miR-18b or co-transfected MiR-18b and NUSAP1 in nude mice. (B) Diagram of average weight of tumors. (C)The photographs of dissected tumors from nude mice were shown. (D) Ki-67, a biomarker of proliferation, was examined by IHC staining in tumor tissues from nude mice, respectively (scale bar: 50μm). (E) A model shows that HBx enhances hepatocarcinogenesis through depressing miR-18b targeting NUSAP1 mRNA.
Our group previously reported that HBx can inhibit the tumor suppressor, miR-205, by inducing methylation of the miR-205 gene promoter38. Accordingly, we hypothesized that HBx may be a key factor in overcoming the host defense against HBV. As expected, HBx was shown to down-regulate the expression of miR-18b by inducing the hypermethylation of the miR-18b gene promoter in hepatoma cells. Therefore,we conclude that HBx can inhibit miR-18b. Next, we evaluated the significance of HBx-inhibited miR-18b in hepatocarcinogenesis. Using a miRNA target gene prediction website, we identified NUSAP1 as a potential target gene of miR-18b. Using the gene prediction database, we found that NUSAP1 was expressed at high levels in liver cancer. It has been reported that NUSAP1 is a microtubule- and chromatin-binding protein. The physiological function of NUSAP1 is to stabilize microtubules, maintain spindle integrity, and further format spindle networks55. NUSAP1 can promote the invasion and metastasis of prostate cancer and increase the aggressiveness of astrocytoma through the Hedgehog signaling pathway56,57. It has been previously reported that NUSAP1 plays crucial roles in various types of cancer40-42. NUSAP1 is up-regulated in HCCs, when compared to nontumor liver tissues43. Our findings in the present study are consistent with these previous reports. It has been reported that miR-18b can modulate many biological functions by targeting different genes.Interestingly, in this study we demonstrated that miR-18b was capable of targeting the 3'UTR of NUSAP1 mRNA,resulting in the down-regulation of NUSAP1 in HCC cells.Therefore, we hypothesized that HBx may up-regulate NUSAP1 expression by down-regulating miR-18b, to promote the development of liver cancer. qRT-PCR analysis showed that the level of NUSAP1 expression was higher in HBV-transgenic mice than in wild-type mice. Functionally,we found that the overexpression of miR-18b significantly inhibited the growth of HepG2-X and H7402-X cells in vitro and in vivo. Thus, miR-18b can down-regulate NUSAP1 in hepatoma cells and HBx can promote the proliferation of hepatoma cells by inhibiting miR-18b. Thereby, HBx can promote the development of liver cancer by also inhibiting other target genes of miR-18b.
Conclusions
We conclude that the targeting of NUSAP1 mRNA by the tumor suppressor miR-18b is controlled by HBx-modulated promoter methylation during the host-virus interaction,leading to hepatocarcinogenesis (Figure 6E).
Acknowledgements
This work was supported in part by grants from National Basic Research Program of China (973 Program, Grant No.2015CB553703), National Natural Science Foundation of China (Grant No. 31670769 and 31470756). All authors would like to acknowledge the First Center Hospital (Tianjin,China) and Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) for providing the patient samples.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年2期
Cancer Biology & Medicine2019年2期
- Cancer Biology & Medicine的其它文章
- Effects of palbociclib on oral squamous cell carcinoma and the role of PIK3CA in conferring resistance
- LAG-3 expression on tumor-infiltrating T cells in soft tissue sarcoma correlates with poor survival
- Genetic polymorphisms and gastric cancer risk: a comprehensive review synopsis from meta-analysis and genome-wide association studies
- Identification of anticancer drugs to radiosensitise BRAFwild-type and mutant colorectal cancer
- Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer
- Factors associated with upstaging in patients preoperatively diagnosed with ductal carcinoma in situ by core needle biopsy
