Factors associated with upstaging in patients preoperatively diagnosed with ductal carcinoma in situ by core needle biopsy
Jing Si, Benlong Yang, Rong Guo, Naisi Huang, Chenlian Quan, Linxiaoxi Ma, Bingqiu Xiu,Yun Cao, Yue Tang, Linxiao Shen, Jiajian Chen, Jiong Wu,3
1Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China;
2Department of Oncology, Fudan University, Shanghai Medical College, Shanghai 200032, China;
3Collaborative Innovation Center for Cancer Medicine, Shanghai 200032, China
ABSTRACT Objective: Patients preoperatively diagnosed with ductal carcinoma in situ (DCIS) by core needle biopsy (CNB) exhibit a significant risk for upstaging on final pathology, which leads to major concerns of whether axillary staging is required at the primary operation. The present study aimed to identify clinicopathological factors associated with upstaging in patients preoperatively diagnosed with DCIS by CNB.Methods: The present study enrolled 604 patients (cN0M0) with a preoperative diagnosis of pure DCIS by CNB, who underwent axillary staging between August 2006 and December 2015, at Fudan University Shanghai Cancer Center (Shanghai, China).Predictive factors of upstaging were analyzed retrospectively.Results: Of the 604 patients, 20.03% (n = 121) and 31.95% (n = 193) were upstaged to DCIS with microinvasion (DCISM) and invasive breast cancer (IBC) on final pathology, respectively. Larger tumor size on ultrasonography (> 2 cm) was independently associated with upstaging [odds ratio (OR) 1.558, P = 0.014]. Additionally, patients in lower breast imaging reporting and data system (BI-RADS) categories were less likely to be upstaged (4B vs. 5: OR 0.435, P = 0.002; 4C vs. 5: OR 0.502, P = 0.001). Overall,axillary metastasis occurred in 6.79% (n = 41) of patients. Among patients with axillary metastasis, 1.38% (4/290), 3.31% (4/121)and 17.10% (33/193) were in the DCIS, DCISM, and IBC groups, respectively.Conclusions: For patients initially diagnosed with DCIS by CNB, larger tumor size on ultrasonography (> 2 cm) and higher BIRADS category were independent predictive factors of upstaging on final pathology. Thus, axillary staging in patients with smaller tumor sizes and lower BI-RADS category may be omitted, with little downstream risk for upstaging.
KEYWORDS Ductal carcinoma in situ; core needle biopsy; axillary staging
Introduction
Ductal carcinoma in situ (DCIS) has increased dramatically owing to advances in the sensitivity of screening mammography, and accounts for > 20% of all newly diagnosed breast cancers1,2. Pure DCIS is a noninvasive disease, with little potential for lymphatic metastasis3,4. The risk for death related to breast cancer has been reported to be as low as 2% within 10 years following diagnosis of DCIS5.Therefore, surgical excision is the primary treatment strategy for patients with DCIS.
Core needle biopsy (CNB) has become a standard tool for the diagnosis of breast lesions and, as such, can obviate more invasive surgical biopsies. However, limitations in the volume of sampling can result in a failure to harvest the most invasive component of a lesion, which in turn can result in an inaccurate preoperative histological diagnosis. For example,when DCIS is diagnosed using CNB, an invasive component may be under-represented due to sampling limitation,resulting in underestimation. According to a previous metaanalysis, approximately 26% of patients diagnosed with DCIS were later upstaged to invasive disease6.
Following diagnosis of DCIS by CNB, a major concern for surgeons is whether axillary staging is required at the primary operation. The National Comprehensive Cancer Network(NCCN) and American Society of Clinical Oncology (ASCO)guidelines both recommend sentinel lymph node biopsy(SLNB) for patients who undergo mastectomy, in whom axillary staging is difficult in stage two of the operation2,7.Additionally, axillary staging should be performed in patients at high risk for upstaging in cases of invasive breast cancer(IBC). Therefore, lesion underestimation when using CNB has significant clinical consequences, especially for axillary staging. Previous studies have identified predictors associated with upstaging, including tumor size, lesion extent,histological grade, and CNB method8-10.
In the current study, we identified clinicopathological factors associated with upstaging in patients preoperatively diagnosed with DCIS by CNB. Our aim was to identify patients with little downstream risk for upstaging, in whom axillary staging can be omitted.
Materials and methods
Patients
The medical records of patients preoperatively diagnosed with pure DCIS by CNB, who underwent axillary staging between August 2006 and December 2015 at Fudan University Shanghai Cancer Center (FUSCC, Shanghai,China), were retrospectively reviewed. The inclusion criteria were as follows: female patients diagnosed with unilateral pure DCIS by CNB; underwent axillary staging; and clinically negative axillary status. Patients who underwent neoadjuvant chemotherapy before surgery or those with a history of breast cancer were excluded. Patients who received CNB in other institutions were also excluded due to the absence of information regarding initial pathological imaging. This study was approved by the Ethics Committee of FUSCC.
Patients underwent selective ultrasonography,mammography, or magnetic resonance imaging (MRI) based on the condition of the lesion. The imaging findings were categorized according to the Breast Imaging Reporting and Data System (BI-RADS) of the American College of Radiology. Ultrasonography and mammography records were reviewed to categorize patients according to the largest diameter of the lesion on ultrasonography and calcification on mammography. All patients underwent CNB before surgery. The biopsy was image-guided and used a 14-gauge core needle, which is the standard procedure in the authors’institute. CNB samples were analyzed by at least two pathologists. Patients in this study underwent surgical excision, including total mastectomy and breast-conserving surgery (BCS). Margin status was evaluated in all patients who underwent BCS. Positive margins were defined as ink on tumor. The final pathological findings were classified as pure DCIS, DCIS with microinvasion (DCISM), or IBC.Microinvasion was defined as an invasive portion no larger than 1 mm. Immunohistochemistry (IHC) was performed on specimens. Estrogen receptor (ER), progesterone receptor(PR), human epidermal growth factor receptor 2 (HER2),and Ki67 were the 4 primary targets tested in all patients. ER and PR were considered to be positive in cases with > 1%staining. HER2 positivity was defined in cases for which IHC was 3+ or 2+ with fluorescence in situ hybridization positivity. The cut-off for Ki67 was 14%.
SLNB was performed at the same time as the surgery.Touch imprint cytology (TIC) was used to evaluate sentinel lymph node (SLN) status intraoperatively. A positive SLN was defined as the presence of either micrometastasis(> 200 cells or > 0.2 mm, but < 2.0 mm) or macrometastasis(> 2.0 mm) identified on hematoxylin-eosin staining, which is the gold standard for histological assessment. Patients with intraoperatively positive SLNs were required to undergo axillary lymph node dissection (ALND). According to the standard ALND procedure, level I and II ALND was performed.
Statistical analysis
Clinicopathological variables were compared between the pure DCIS group and the upstaged group according to the final pathological findings using the chi-squared test for categorical variables. Multivariate logistic regression analyses were performed to investigate risk predictors of upstaging.Two-tailed P values were adopted, and P < 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS version 17.0 (IBM Corporation,Armonk, NY, USA).
Results
Baseline characteristics
The current study included 604 patients. The mean patient age at DCIS diagnosis was 51.00 years (range, 24-83 years).Of the 604 patients, 87.09% (n = 526) presented with lumps.The mean sonographic lesion size was 24.68 mm (range,10-79 mm). The clinicopathological characteristics of the entire cohort are summarized in Table 1. The overall underestimation rate was 51.98% (314/604). Respectively,121 (20.03%) and 193 (31.95%) patients were upstaged to DCISM and IBC on final pathology. Among the total population, 548 (90.73%) underwent mastectomy and 56 (9.27%) underwent BCS. Of all patients, 513 (84.93%)underwent SLNB and 91 (15.07%) underwent ALND.Axillary metastasis was identified in 41 (6.79%) patients based on final paraffin section pathology, of which 85.37%(35/41) had 1-2 positive ALNs and 14.63% (6/41) had≥ 3 positive ALNs.
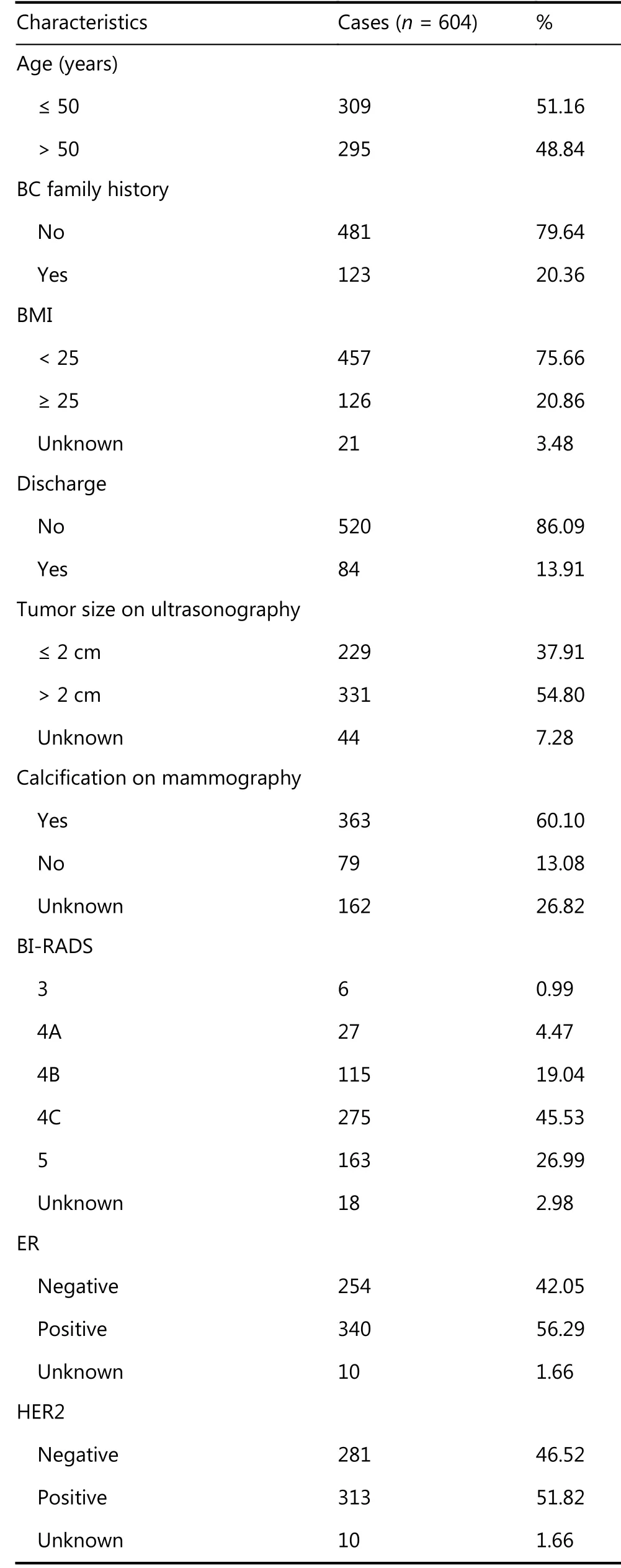
Table 1 Baseline clinicopathological characteristics of the cohort

Continued
Predictive factors of upstaging
Univariate analysis of preoperative characteristics revealed that larger tumor size on ultrasonography (P = 0.005) and higher BI-RADS category (P = 0.009) were associated with upstaging (Table 2). Predictive factors with P < 0.05 in the univariate analysis were included in the multivariate analysis,which revealed that patients with larger tumor size on ultrasonography (> 2 cm) were more likely to be upstaged[odds ratio (OR) 1.558 (95% CI 1.094-2.218); P = 0.014].Additionally, BI-RADS category was an independent factor associated with upstaging. Patients in BI-RADS category 4B(P = 0.002) or 4C (P = 0.001) were 0.435-fold and 0.502-fold less likely, respectively, to be upstaged than patients in BIRADS category 5.
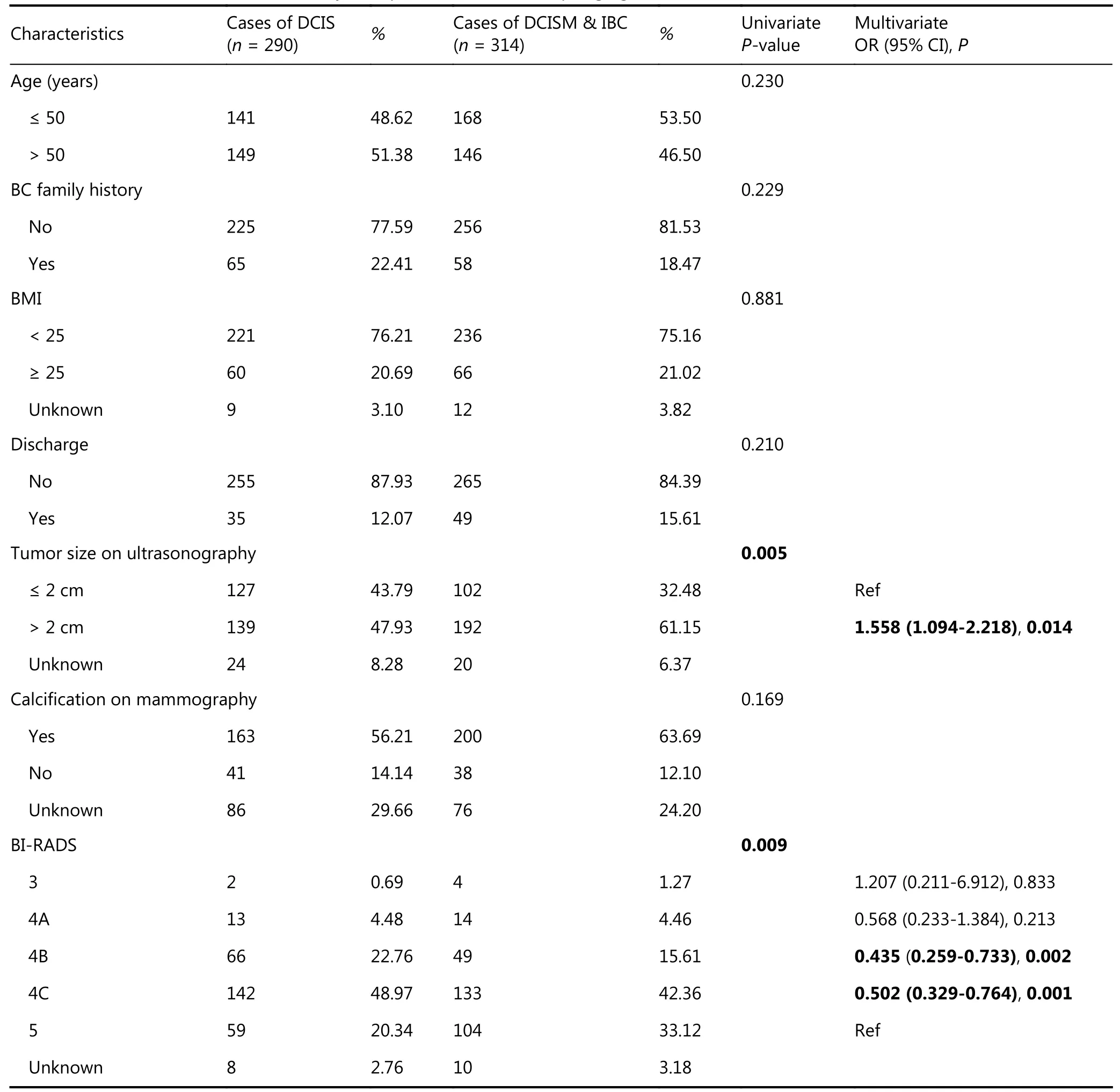
Table 2 Univariate and multivariate analysis of predictive factors of upstaging
Subgroup analysis
Predictive factors for upstaging to DCISM and IBC were analyzed separately. The only factor related to upstaging to DCISM was a family history of breast cancer (P = 0.033).Surprisingly, it was a protective factor for being upstaged to DCISM, which may be due to more active screening in patients with a family history. However, in multivariate analysis of predictive factors for upstaging to IBC (Table 3),larger tumor size on ultrasonography (> 2 cm) was an independent predictive factor of upstaging to IBC [OR 1.762(95% CI: 1.167-2.659); P = 0.007]. Additionally, patients in BI-RADS category 4B (P = 0.026) or 4C (P = 0.001) were 0.520-fold and 0.459-fold, respectively, less likely to be upstaged than patients in BI-RADS category 5.
Axillary metastasis was also correlated with the extent of invasion. Positive ALNs occurred in 1.38% (4/290) of patients with pure DCIS, 3.31% (4/121) with microinvasion,and in 17.10% (33/193) with IBC (P < 0.001). This correlation was not found between patients with DCIS and DCISM (P = 0.198).
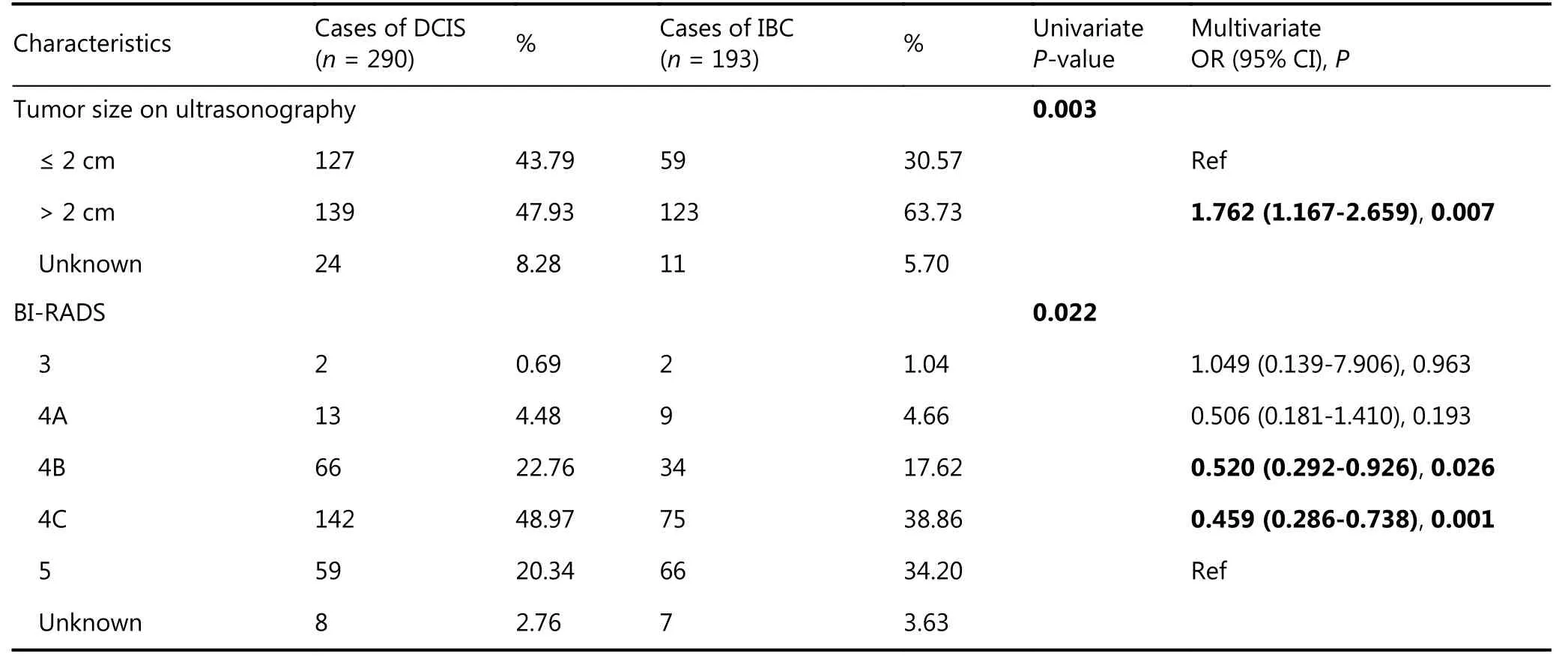
Table 3 Univariate and multivariate analysis of predictive factors of upstaging to IBC
Discussion
The current study identified predictive factors associated with DCIS upstaging. The rate of upstaging to DCISM and IBC was 20.03% and 31.95%, respectively. Independent predictors of upstaging included larger tumor size on ultrasonography (> 2 cm) and higher BI-RADS category.Previous studies reported that approximately 26% of patients diagnosed with DCIS were upstaged to IBC (range, 8.8%-51.5%), and the rate of upstaging to DCISM ranged from 4%-29.6%1,6,11-16. In our study, the rate of underestimation was relatively high, which we attributed to limited tissue volume. In total, 51.98% of patients were upstaged on final pathology, without subgrouping either upstaging to microinvasion or IBC. The variable proportion could be the result of factors related to pathologist interpretation6.
Predictive factors of DCIS upstaging in this study were consistent with those in previous reports. A meta-analysis that included 7,350 cases of DCIS diagnosed by CNB demonstrated that tumor size was one of the strongest independent predictors of underestimation6. Numerous previous studies have shown that large tumor size and palpable mass were associated with risks for upstaging8-10,13.In addition, others reported that suspicious findings on mammography were linked to upstaging on final pathology9,10. Other predictive factors, such as nuclear grade,comedo necrosis, sclerosing adenosis, and CNB method,were also linked to upstaging8,10,13,17. Studies investigating Ki67 as a predictive factor of DCIS upstaging are rare.However, Ki67, as a known proliferation marker, was shown to be associated with recurrence of DCIS, which may be associated with an underlying invasive component18,19.
Theoretically, DCIS is defined on the basis that the cancer has not broken through the basement membrane of the breast duct; thus, it has little potential for metastasis.However, with the possibility of upstaging in preoperative DCIS diagnosis, whether these patients require axillary staging remains controversial. Previous studies have reported overall axillary metastasis rates of approximately 5% in DCIS patients; however, it increased to 10%-20% with preoperative underestimation on final pathology1,8,10,20. In the current study, the overall axillary metastasis rate was 6.79%. Not surprisingly, we found that the axillary metastasis rate was correlated with the extent of invasion. A positive axillary lymph node was identified in 1.38% (4/290) of patients in the DCIS group, but increased to 11.78% (37/314) if underestimation was proven on final pathology. Subgroup analysis revealed that 3.31% (4/121) and 17.10% (33/193) of patients had a positive axillary lymph node in the DCISM and IBC groups, respectively.
It is known that DCIS has a low potential for metastasis;thus, axillary staging can be omitted in patients with pure DCIS, especially those who undergo BCS. For DCIS upstaging in patients with a clinically negative axillary lymph node, the NCCN and ASCO guidelines both recommend SLNB. Thus, when patients who are preoperatively diagnosed with DCIS by CNB undergo axillary staging at the primary operation, surgeons are faced with the dilemma of avoiding a second operation if the final pathology is upstaged, or performing an unnecessary procedure in pure DCIS patients.In this case, axillary staging is only required for patients with predictive factors of upstaging and can be omitted in those who exhibit little risk for upstaging.
In the current study, we found that patients with larger tumor size on ultrasonography (> 2 cm) and higher BIRADS category were more likely to be upstaged on final pathology. In contrast, patients with a smaller mass and in a lower BI-RADS category were less likely to be upstaged, and were more likely to be pure DCIS on final pathology. For these patients, axillary staging may be unnecessary and can be omitted. Moreover, in conjunction with continued progress in adjuvant radiotherapy and systematic therapy, which may be adequate to control axillary status in clinically negative patients, we may be able to further identify patients who can avoid unnecessary axillary staging based only on their clinicopathological predictive factors in the future.
Interestingly, we found that approximately 90% of patients in this cohort underwent mastectomy, which was a relatively high figure compared with other reports. There are two possible explanations. First, Chinese patients have smaller breasts than their western counterparts, and they usually do not have much choice when diagnosed with DCIS, especially those with large tumor size(s), and/or multifocal or multicenter lesions. Second, the high proportion of mastectomies may be the result of improvements in breast reconstruction.
There were some limitations to the present investigation,the first of which was its retrospective design. However, this study had a relatively large dataset with uniform inclusion and exclusion criteria. Second, the sample volume used in preoperative pathological diagnosis in each patient was unknown. Clearly, it is positively correlated with the accuracy of CNB diagnosis. Finally, not all nuclear grades on CNB pathology were reported in our institution. According to previous studies, we believe that histological grade may be an important predictive factor in upstaging8,10. Further assessment is needed to accurately select patients who can safely avoid axillary staging.
Conclusions
For patients preoperatively diagnosed with DCIS by CNB, the present study found that larger tumor size on ultrasonography(> 2 cm) and higher BI-RADS category were independent predictive factors of upstaging. Axillary staging can be omitted in patients with smaller tumor sizes and lower BIRADS category, with little downstream risk for upstaging.
Acknowledgements
This work was supported by grants from Shenkang Center City Hospital Emerging Frontier Technology Joint Research Project (Grant No. SHDC12015119).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年2期
Cancer Biology & Medicine2019年2期
- Cancer Biology & Medicine的其它文章
- Hepatitis B virus X protein enhances hepatocarcinogenesis by depressing the targeting of NUSAP1 mRNA by miR-18b
- LAG-3 expression on tumor-infiltrating T cells in soft tissue sarcoma correlates with poor survival
- Genetic polymorphisms and gastric cancer risk: a comprehensive review synopsis from meta-analysis and genome-wide association studies
- Identification of anticancer drugs to radiosensitise BRAFwild-type and mutant colorectal cancer
- Dorsomorphin induces cancer cell apoptosis and sensitizes cancer cells to HSP90 and proteasome inhibitors by reducing nuclear heat shock factor 1 levels
- Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer
