Loss of PSP94 expression is associated with early PSA recurrence and deteriorates outcome of PTEN deleted prostate cancers
Andreas M. Luebke, Ali Attarchi-Tehrani, Jan Meiners, Claudia Hube-Magg, Dagmar S. Lang, Martina Kluth, Maria Christina Tsourlakis, Sarah Minner, Ronald Simon, Guido Sauter, Franziska Büscheck, Frank Jacobsen, Andrea Hinsch, Stefan Steurer, Thorsten Schlomm, Hartwig Huland, Markus Graefen, AlexanderHaese, Hans Heinzer, Till S. Clauditz, Eike Burandt, Waldemar Wilczak, Doris Höflmayer
1Institute of Pathology, University Medical Center Hamburg-Eppendorf, Hamburg 20246, Germany;
2General, Visceral and Thoracic Surgery Department and Clinic, University Medical Center Hamburg-Eppendorf, Hamburg 20246, Germany;
3Department of Urology, Charité-Universitätsmedizin Berlin, Berlin 10117, Germany;
4Martini-Clinic, Prostate Cancer Center,University Medical Center Hamburg-Eppendorf, Hamburg 20246, Germany
ABSTRACT Objective:Prostate secretory protein of 94 amino acids (PSP94) is a target gene of the EZH2 transcriptional repressor and is often downregulated in prostate cancer; however, its prognostic value is disputed.Methods:Immunohistochemical analysis of a tissue microarray of 12, 432 prostate cancer specimens was performed to evaluate PSP94 expression. Correlation of PSP94 expression with tumor phenotype, patient prognosis, TMPRSS2:ERG fusion status, EZH2 expression and PTEN deletion was studied.Results:PSP94 expression was increased in benign prostatic hyperplasia; however, it was downregulated in 48% and negative in 42% of the 9, 881 interpretable prostate cancer specimens. The loss of PSP94 expression was inversely correlated to EZH2 expression (P < 0.0001) and largely unrelated to the ERG status, but strongly correlated with high Gleason grade, advanced tumor stage, and nodal metastasis (P <0.0001 each). The fraction of PSP94-negative cancer specimens increased from 40% in pT2 to 52%in pT3b-pT4 (P < 0.0001) and from 40% in Gleason 3+3 = 6 to 46% in Gleason 4+3 = 7 and 60% in Gleason ≥4+4 = 8 (P <0.0001). Loss of PSP94 was linked to early prostate-specific antigen recurrence, but with little absolute effect (P < 0.0001).However, it provided additional prognostic impact in cancer specimens with PTEN deletion. Loss of PSP94 deteriorated prognosis of cancer patients with PTEN deletion by more than 10% (P < 0.0001). The combination of PTEN deletion and PSP94 loss provided independent prognostic information that was observed in several subgroups defined by classical and quantitative Gleason grade.Conclusions:The results of our study suggest that combined PSP94/PTEN analysis can be potentially used in the clinical prognosis of prostate cancer.
KEYWORDS MSMB; PSP94; PTEN; prostate cancer; tissue microarray
Introduction
Prostate cancer is the second most prevalent cancer and the fifth leading cause of cancer mortality among men1.Therefore, there is an urgent need for a reliable prognostic method for indolent or aggressive cancer. Currently, Gleason grade and tumor extent on biopsy, pre-operative prostatespecific antigen (PSA), and clinical stage are the established pre-treatment prognostic parameters. Although these parameters are statistically powerful, they are not optimal for individual treatment decisions. The goal is to identify clinically useful biomarkers that will enable a more specific prediction of the aggressive prostate cancer.
Prostate secretory protein of 94 amino acids (PSP94) (also called beta-inhibin or microsemi-noprotein-beta) is a member of the immunoglobulin binding factor family, which is mainly produced by the luminal cells of the prostate glands2. PSP94 are among the most abundant proteins found in the seminal fluid3,4and may have multiple functions that have not yet been fully elucidated. PSP94 has fungicidal effects that may play a role in protecting prostate glands against microbial infection5. It may also be important for fertility as it is reported to bind to the surface of spermatocytes6. However, PSP94 may also function as a tumor suppressor because in vitro studies and in vivo studies in animal models have indicated that PSP94 has a role in cellular growth control7. PSP94 inhibits the secretion of follicle-stimulating hormone8, a known stimulator of prostate cancer growth9,10, and shows growth-suppressing and pro-apoptotic properties in MAT-LyLu (MLL) and PC-3 cells10. Additionally, PSP94 is negatively regulated by the polycomb repressor enhancer of zeste homolog 2 (EZH2),which is upregulated in 50%-60% of prostate cancer cases.Overexpression of EZH2 is strongly associated with tumor aggressiveness and adverse patient prognosis11,12.Accordingly, several studies have reported loss of PSP94 expression in a subset of tumors through immunohistochemical analysis of prostate cancer specimens13-16. However, studies on the correlation between PSP94 levels and prostate cancer phenotype and prognosis are controversial, including studies suggesting better outcome in patients with high17or low PSP94 levels14,18.
We took advantage of our large tissue microarray (TMA)resource that includes more than 12, 000 prostate cancer tissue specimens to examine the role of PSP94 expression.The database attached to our TMA contains pathological and clinical follow-up data. It also includes molecular data on key molecular alterations, such as EZH2 expression,TMPRSS2:ERG fusion, and presence of recurrent deletions,including PTEN, 3p13, 5q21, and 6q15, observed in prostate cancer.
Materials and methods
Patients
We used the data from 12, 432 patients who had undergone radical prostatectomy between 1992 and 2011 at the Department of Urology, and the Martini Clinics at the University Medical Center Hamburg-Eppendorf. Among them 245 patients had been given anti-androgen therapy.Follow-up data were available for 11, 152 patients with a median follow-up of 60 months (range: 1 to 275 months;Table 1). PSA levels were measured post-surgery and recurrence was defined as a postoperative PSA level of 0.2 ng/mL and increasing in subsequent measurements. Tumor stage, Gleason grade, nodal stage, and stage of the resection margin were obtained from the patient’s file. All prostate specimens were analyzed following a standard procedure19.For the TMA, a single 0.6 mm core was taken from a tumor containing tissue block from each patient20. Internal controls included normal prostate tissue and various other tissues. In addition to the classical Gleason grading, quantitative Gleason grading was performed as described previously21.Briefly, for every prostatectomy specimen, the percentage of Gleason 3, 4, and 5 patterns was recorded. Gleason 3+4 and 4+3 cancer specimens were subdivided according to their percentage of Gleason 4. For practical use, we subdivided the 3+4 and 4+3 cancer specimens into 7 subgroups: 3+4 ≤ 5%,3+4 6%-10%, 3+4 11%-20%, 3+4 21%-30%, 3+4 31%-49%, 4+3 50%-60% and 4+3 61%-100% Gleason 4 patterns. In addition, separate groups were defined by the presence of a tertiary Gleason 5 pattern, including 3+4 Tertiary 5 and 4+3 Tertiary 5. The annotated database of this TMA included results on EZH2 expression12, ERG expression, and ERG break-apart fluorescent in situ hybridization (FISH) analysis22,23. It also included PTEN deletion status analyzed using a dual-color FISH probe set that consisted of two Spectrum Green-labeled bacterial artificial chromosome clones (RP11-380G5 and RP11-813O3; Source Bioscience, Nottingham, UK) and a Spectrum Orange-labeled commercial centromere 10 probe (06J36-090;Abbott, Wiesbaden, Germany) as described previously24.Archived diagnostic leftover tissue was used in accordance with the local law (HmbKHG, §12a). The study was approved by the local ethics committee “Ethics commission Hamburg” (Approval No. WF-049/09). All work was carried out in compliance with the Helsinki Declaration.
Immunohistochemistry (IHC)
Freshly cut TMA sections were analyzed on the same day and in one experiment. Incubation with anti-PSP94 mouse monoclonal antibody clone 4A6A6 (Abnova, Taipei, Taiwan;dilution 1:50) was performed; slides were dewaxed and subjected to heat-induced antigen retrieval for 5 min in an autoclave at 121°C in Tris-EDTA buffer (pH 6). Bound antibody was then visualized using the EnVision Kit (Dako,Glostrup, Denmark). Positive and negative tissue control included normal prostate tissue and tonsil, respectively.PSP94 staining was evaluated according to the following scoring system: The staining intensity (0, 1+, 2+, and 3+) and the fraction of positive tumor cells were recorded for each tissue spot. A final score was built from these two parameters according to the following score as previously described25:Negative scores had staining intensity of 0, weak scores had staining intensity of 1+ in ≤ 70% of tumor cells or staining intensity of 2+ in ≤ 30% of tumor cells; moderate scores had staining intensity of 1+ in ≥ 70% of tumor cells, staining intensity of 2+ in > 30% but in ≤ 70% of tumor cells, or staining intensity of 3+ in ≤ 30% of tumor cells; strong scores had staining intensity of 2+ in > 70% of tumor cells or staining intensity of 3+ in > 30% of tumor cells.
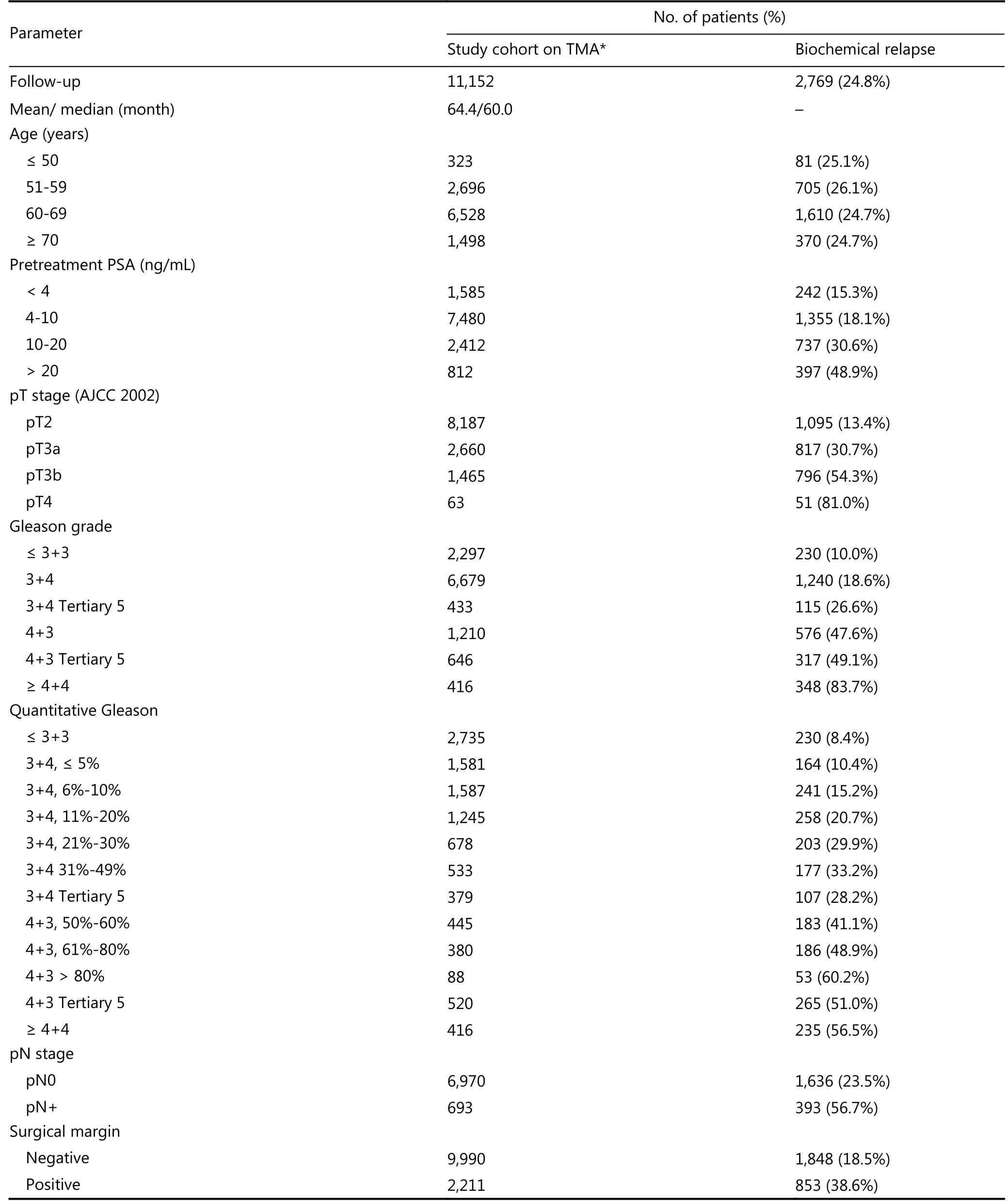
Table 1 Pathological and clinical data of the arrayed prostate cancers
Statistical analysis
Contingency table analysis and the Chi-square test were performed to evaluate the correlation between molecular parameters and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The log-rank test was applied to evaluate significant survival differences between groups. Cox proportional hazards regression analysis was performed to test the statistical independence and significance between pathological, molecular, and clinical variables. JMP 11 (SAS Institute Inc., NC, USA) was used for data analysis.
Results
A total of 9, 882 (88.6%) tumor samples were interpretable in our TMA analysis. Reasons for non-informative cases (1, 270 samples; 11.4%) included lack of tissue samples or absence of unequivocal cancer cells at the TMA spot. PSP94 immunostaining was typically strong in the cytoplasm of normal prostate gland luminal cells. In prostate cancer specimens, strong PSP94 staining was observed only in 926 of the 9, 882 (9.4%) interpretable tissues. Moderate PSP94 staining was observed in 15.2% of the tumor samples and weak PSP94 staining was observed in 33.4% of the tumor samples. PSP94 staining was not observed in 42.0% of the samples. Representative images of PSP94 IHC results are given in Figure 1.
Correlation with tumor phenotype
Unfavorable prostate cancer phenotype was associated with decreased PSP94 expression (Table 2). The fraction of PSP94-negative tumor specimens gradually increased from 39.9% in pT2 to 43.2% in pT3a and 51.5% in pT3b-pT4 (P <0.0001). Similar correlation was observed with the classical and the quantitative Gleason grade. The fraction of PSP94-negative tumor specimens gradually increased from 40.4% in Gleason 3+3 = 6 to 46.3% in Gleason 4+3 = 7 and 59.5% in Gleason ≥ 4+4 (P < 0.0001). Similar results were obtained when PSP94 expression was combined with PTEN deletion analysis. Four combinations of tumor subset were defined:PSP94-positive (≥ weak staining) tumor specimens with normal PTEN copy numbers, PSP94-negative tumor specimens with normal PTEN copy numbers, PSP94-positive tumor specimens with PTEN deletion, and PSP94-negative tumor specimens with PTEN deletion. We observed that PSP94-negative tumors with PTEN deletion was associated with adverse tumor phenotypes. All data are summarized in Table 2.
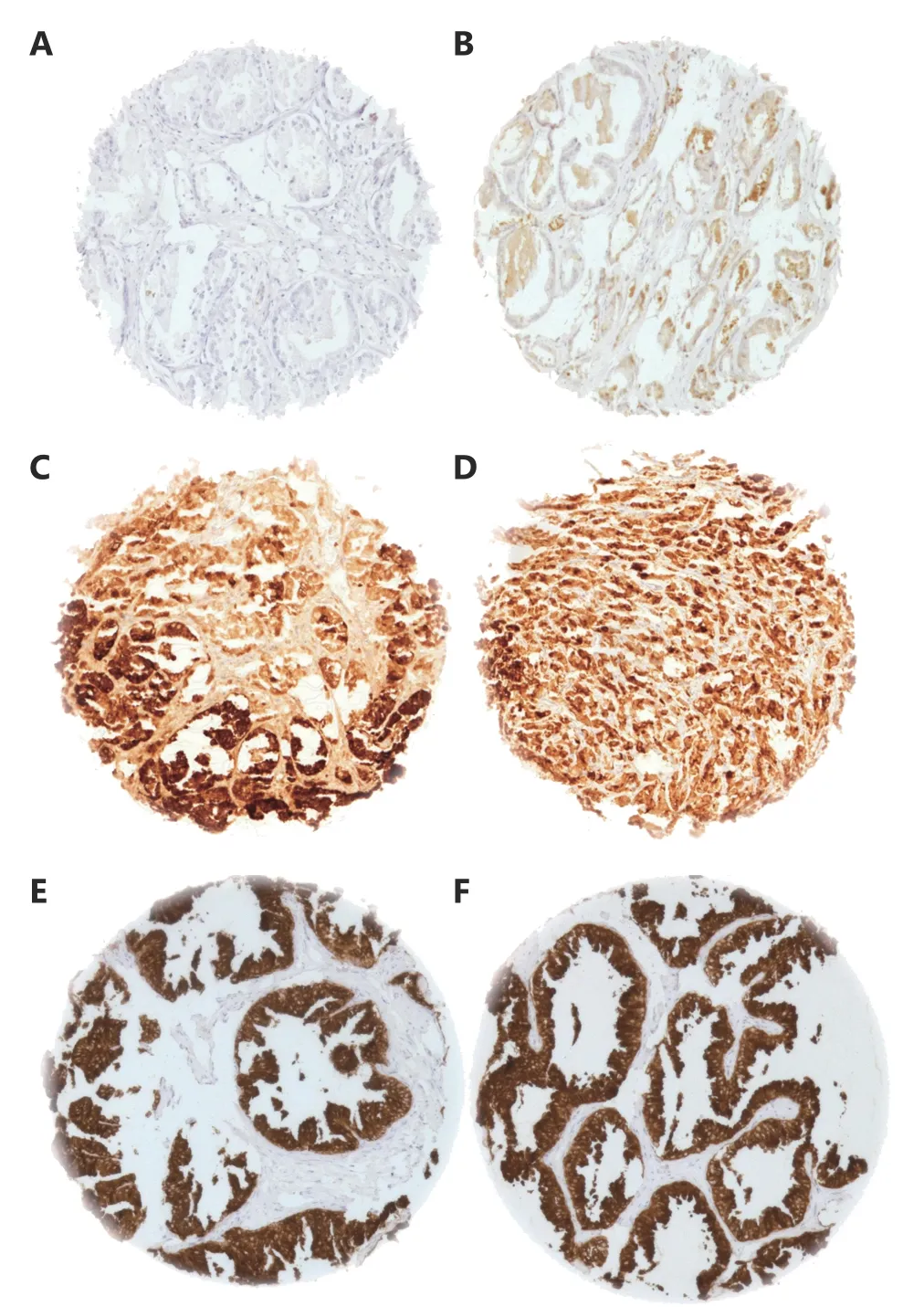
Figure 1 Representative images of (A) negative, (B) weak, (C)moderate (D) strong PSP94 staining of prostate cancer and (E, F)normal prostate. Note the strong positive staining of normal prostate epithelium. Spot size is 600 µm at 100 x magnification.
Correlation with molecular changes
Data on TMPRSS2-ERG fusion status obtained by FISH were available for 5, 721 patients and those obtained by IHC were available for 8, 878 patients. PSP94 staining was comparable in tumor specimens with and without TMPRSS2-ERG rearrangement or ERG expression. Loss of PSP94 expression was observed in 42% of the tumor specimens with ERG fusion and in 44% of the tumor specimens without ERG fusion. Similarly, loss of PSP94 expression was observed in 39% of ERG-positive and 45% of ERG-negative tumor specimens (Figure 2) by immunohistochemical analysis. Due to the large number of tissue specimens analyzed, the difference between ERG-positive and ERG-negative tissue specimens were statistically significant (P < 0.0001).Comparison of IHC data on EZH2 expression from an earlier study using this TMA12demonstrated a strong negative correlation between EZH2 and PSP94 in 8, 194 cancer tissue specimens with interpretable results for both proteins. Loss of PSP94 expression was observed in 68%, 52%, and 41% of cancer tissue specimens with strong, weak, and moderate EZH2 expression, respectively. Only 40% of tumors lacked detectable EZH2 staining (P < 0.0001, Figure 3). PSP94 expression did not correlate with PTEN deletions (P = 0.3378 in all cancer specimens, P = 0.3779 in ERG-negative and P =0.6036 in ERG-positive cancer specimens, data not shown).
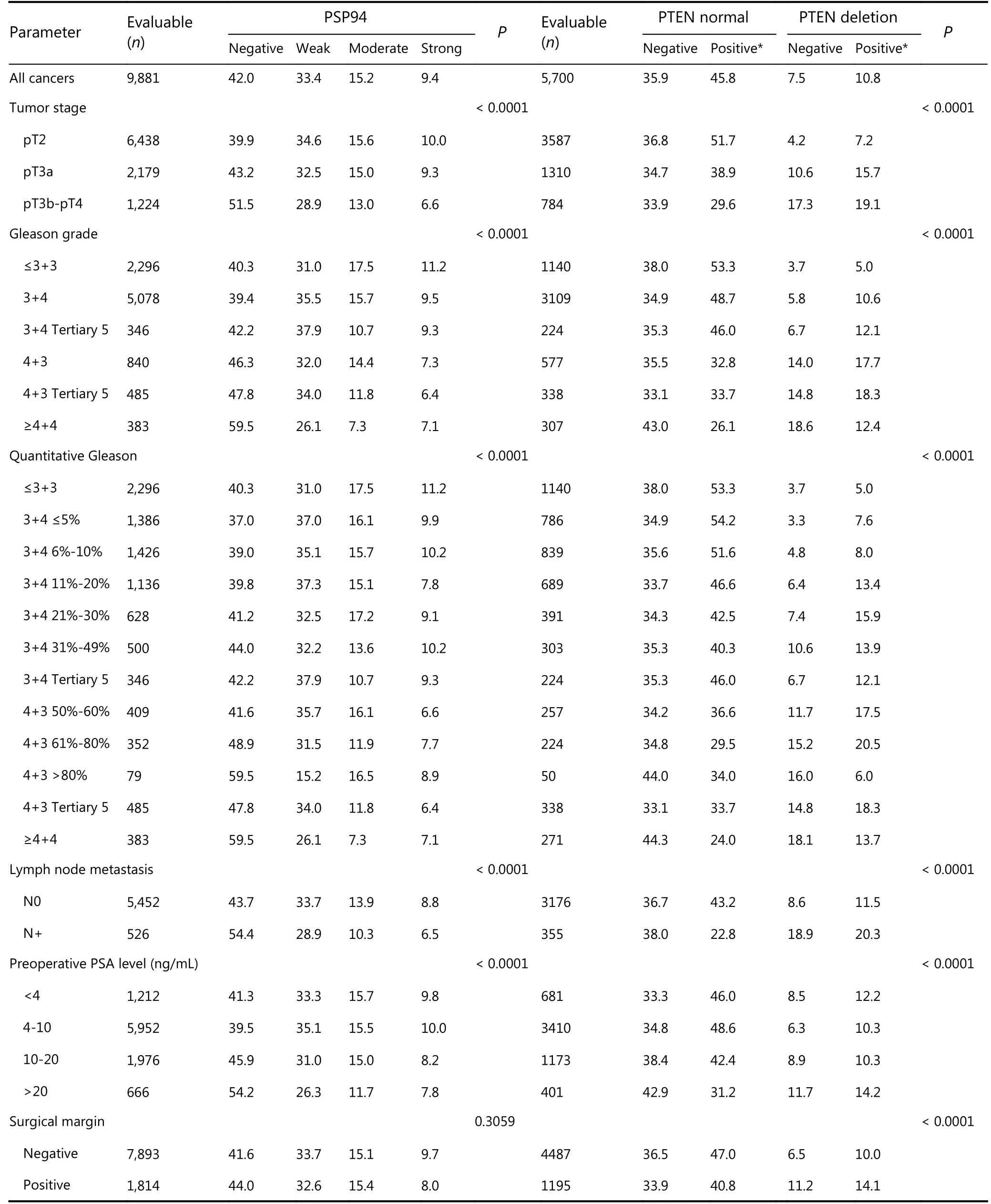
Table 2 Association between cancer phenotype and PSP94 expression alone and in combination with PTEN status
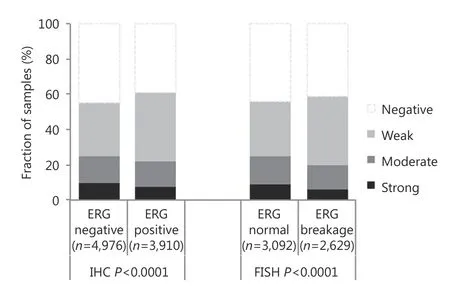
Figure 2 Relationship of PSP94 expression with ETS-related gene(ERG) fusion probed by immunohistochemistry and FISH.
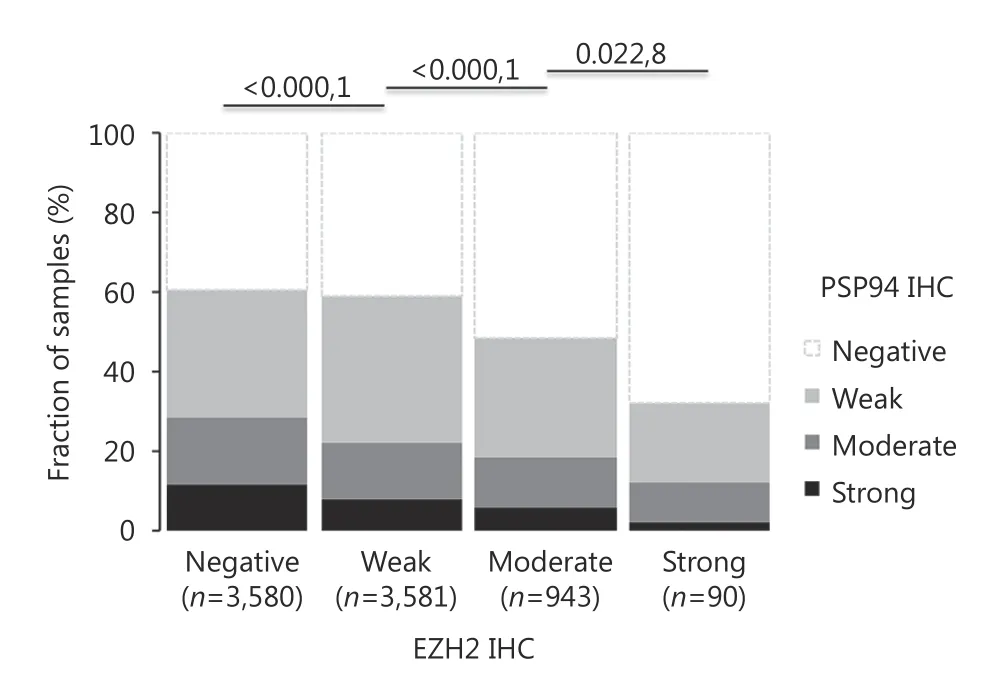
Figure 3 Inverse association between EZH2 and PSP94 expression (P < 0.0001).
Correlation with PSA recurrence.
Follow-up data were available for 9, 168 patients with interpretable PSP94 immunostaining on the TMA. Loss of PSP94 expression was associated with a slightly reduced time for biochemical recurrence when all specimens were simultaneously analyzed (P < 0.0001, Figure 4A). Because of the similar clinical behavior of cancer specimens with weak,moderate, and strong PSP94 expression, these three groups were subsequently combined into “positive” group for further analyses. Further analyses considering ERG, PTEN,and EZH2 status revealed a comparable (mild) prognostic impact of PSP94 expression in ERG-positive and ERGnegative tissue specimens (Figure 4B and 4C) and did not show marked additional value of PSP94 measurement in specimens with varying EZH2 expression levels (Figure 4D).However, there was a striking prognostic impact of loss of PSP94 expression in specimens with PTEN deletion.Prognosis deteriorated by > 10% points between specimens with PTEN deletion expressing PSP94 and specimens with PTEN dele-tion lacking PSP94 (P < 0.0001, Figure 4E). The combined analysis of PTEN deletion and PSP94 expression loss even exhibited significant prognostic differences in several tumor subsets characterized by identical classical(Gleason 3 + 4, P < 0.0001, Figure 5A) or quanti-tative Gleason grade (11%-20% Gleason 4, P = 0.0015, Figure 5B-H).
Multivariate analysis
To further estimate the prognostic value of PSP94 expression loss in combination with PTEN, four multivariate models were calculated representing different clinical scenarios(Table 3). Scenario 1 was utilizing all postoperatively available parameters including pathological tumor stage,pathological lymph node status (pN), surgical margin status,preoperative PSA value and pathological Gleason grade obtained after the morphological evaluation of the entire resected prostate. Scenario 2 was utilizing all postoperatively available parameters with the exception of nodal status. The rational for this approach was that the indication and extent of lymph node dissection is not standardized in the surgical intervention of prostate cancer and that excluding pN in multivariate analysis can markedly increase case numbers.Two additional scenarios were included to model the preoperative status. Scenario 3 included PSP94 expression in combination with PTEN deletion, preoperative PSA, clinical tumor stage and Gleason grade obtained on the prostatectomy specimen. In scenario 4, the preoperative Gleason grade obtained on the original biopsy was combined with preoperative PSA, cT stage and PSP94 expression in cancer subtypes with PTEN deletion. These analyses revealed that the combined analysis of PSP94 and PTEN provided independent prognostic information in all scenarios (P ≤0.0003 each, Table 3).
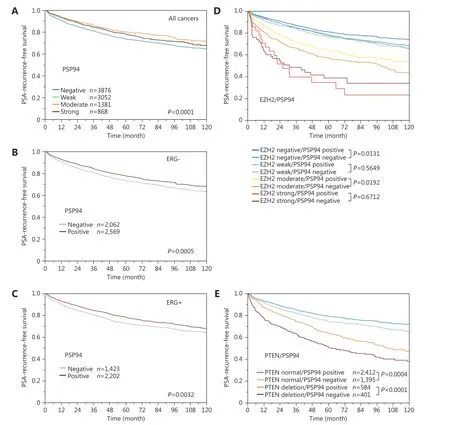
Figure 4 Prognostic impact of PSP94 in (A) all cancers and subsets of cancers defined by (B) absence of TMPRSS2: ERG fusion. (C) presence of TMPRSS2: ERG fusion. (D) PSP94 expression and different EZH2 expression levels. (E) PSP94 expression and PTEN deletion status.
Discussion
Our study demonstrated that the loss of PSP94 expression can predict unfavorable tumor phenotype and early PSA recurrence. This is particularly true in subsets of prostate cancer characterized by deletions in PTEN.
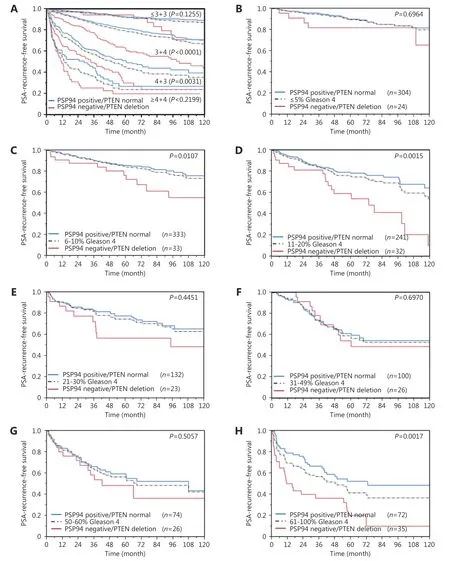
Figure 5 Prognostic impact of combining PSP94 expression and PTEN deletion data in subsets of cancers defined by Gleason score: (A)Impact of normal (i.e., PSP94 positive and PTEN normal, blue line) and inactivated (i.e., PSP94 loss and PTEN deletion, red line) PSP94/PTEN as compared to classical Gleason score categories (indicated by black dotted lines). (B-H) Impact of negative (red line) and positve (blue line) PSP94 expression as compared to the quantitative Gleason score categories (indicated by black dotted lines) defined by subsets of cancers with (B) ≤ 5%, (C) 6%-10%, (D) 11%-20%, (E) 21-30%, (F) 31%-49%, (G) 50%-60%, and (H) 61%-100% Gleason 4 patterns.
In this study, the fraction of PSP94-positive specimens was 57.9%, which is in line with several earlier studies reporting the positive rate of 7.7%, 14.0%, 38.5%, 61%, 63.4% and 67.8% after analyzing cohorts of 88-779 patients13-16,18,26. The wide range of PSP94-positive rates in these studies is most likely attributable to the use of different antibodies,laboratory protocols and scoring criteria. This reflects the inherent lack of standardization of immunohistochemical studies and is also seen for most other proteins that are studied by multiple research groups. The wide range of PSP94-positive rates observed does not seem to be the result of multiple PSP94 epitopes or alternative splicing variants as reported by Xuan et al.27because the immunogenic part of the molecule is linear and located at the N-terminal end28.
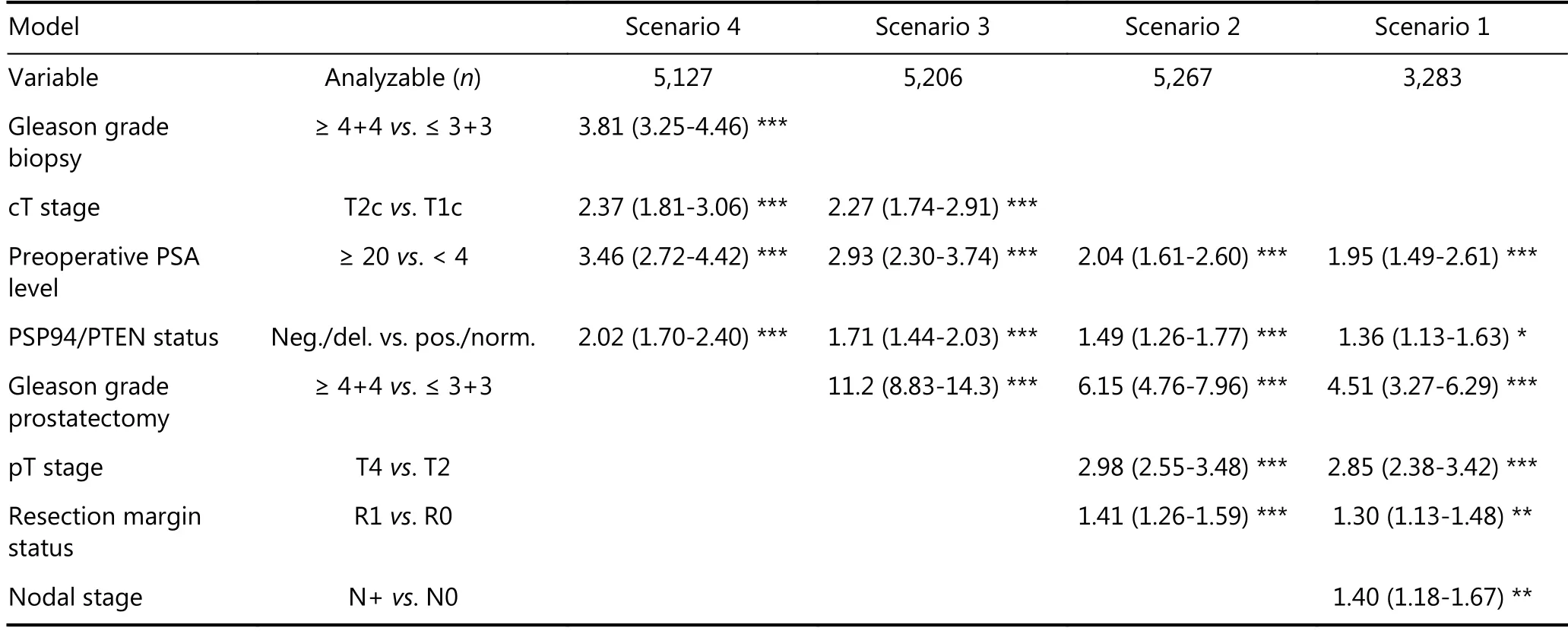
Table 3 Hazard ratios (95% confidence intervals) for biochemical relapse after prostatectomy for established risk factors and PSP94/PTEN status
Ubiquitous and strong PSP94 expression in benign prostate epithelium observed in our study is consistent with the observation of earlier studies reporting uniformly strong and diffuse staining in both normal and hyperplastic glands14,15,26,29. Overall, these data demonstrate that a fraction of prostate cancer specimens lost physiological PSP94 expression during malignant transformation.
Our successful analysis of 9,168 prostate cancer specimens,which included outcome data, revealed that the loss of PSP94 expression in prostate cancer is significantly correlated to unfavorable tumor phenotype and poor clinical outcome.Notably, the risk for PSA recurrence after 5 years differed only between 28% in PSP94-negative and 20% in strongly PSP94-positive cancer specimens when all tumors were analyzed irrespective of the above-mentioned deletions.These small differences may explain why earlier studies on smaller patient cohorts have arrived at variable conclusions with respect to the prognostic impact and correlation with tumor phenotype. Two studies on 96 and 779 patients reported a similar cor-relation between reduced PSP94 expression and poor patient prognosis as observed in our study14,18. Another study, which analyzed 59 cancer tissue specimens reported an inverse correlation for good prognosis17. Additionally, other studies have reported that PSP94 expression did not correlate to tumor phenotype and/or disease outcome13-16,18,26. This also includes a study by Hoogland et al.13reporting that PSP94 expression, as determined in core needle biopsies, was not related to the risk of finding significant prostate cancer in subsequent radical prostatectomy specimen in a set of 147 patients.
The molecular database attached to our TMA enabled us to study the correlation between PSP94 expression and unique molecular features of the tumor specimens. For this study, we selected EZH2 as it is known to interact with PSP94 1723781030and TMPRSS2:ERG fusion, which is the most common molecular change observed in prostate cancer31.Additionally, we selected PTEN deletion because this represents the strongest prognostic feature24,32in prostate cancer that can be reliably assessed. Finding a strong inverse correlation between PSP94 and EZH2 aids in evaluating their functional relationship and provides a strong indirect evidence for the validity of the assays used in this study. The polycomb repressor EZH2 was previously shown to epigenetically downregulate PSP9430and was a strong predictor of poor patient prognosis in our earlier study using the same TMA12. The highly significant statistical association found between ERG and PSP94 expression in this study is more due to the very high number of specimens analyzed than due to the strong biological effects. In absolute numbers, the fraction of PSP94-positive cancer specimens differed only by less than 5% between ERG-positive and ERG-negative cancer specimens. This concurs with the result of studies involving global transcriptome analyses in prostate cancer, which did not reveal a significant difference in PSP94 mRNA levels between ERG-negative and ERG-positive tumors33,34. Additionally, the PSP94 promoter does not carry an ERG binding site according to the eukaryotic promoter database35, and other interactions between these proteins have not been reported. Mild correlation between parameters measured by immunohistochemistry may be due to a fraction of samples that were non-reactive to immunohistochemical staining resulting in “negative”staining results for all parameters measured.
The striking prognostic impact of PSP94 loss in prostate cancer patients with PTEN deletion, a subgroup already characterized by poor prognosis, was the most remarkable finding in this study. The strong joint prognostic effect of these features suggests a functional interaction between disrupted PSP94 and constitutively activated AKT signaling.This is supported by the studies which report that PSP94 expression is related to a regulatory loop involving Lin28b/Let736, an upstream regulator of PTEN/AKT signaling37. We observed that the loss of PSP94 was statistically unrelated to PTEN deletions, which might suggest that these two molecular changes do not leverage each other.The Gleason grade is the strongest prognostic parameter for prostate cancer. We have previously shown that the percentage of adverse Gleason patterns in prostatectomy specimens, i.e. the “quantitative” Gleason grade, has striking prognostic impact in prostate cancer patients and enables a further clinically relevant risk assessment within and beyond Gleason 3+4 and also 4+3 cancer specimens21. The combined analysis of PSP94 and PTEN deletion can further stratify the prognostic groups defined by the classical Gleason score, and the quantitative Gleason score, highlighting the potential of this biomarker combination. Because lack of FISH signals and lack of expression are comparatively easy to measure,they might have the potential for a routine application.
The tumor suppressive effects of PSP94 was used for the development of PSP94-derived anti-cancer agents a decade ago38. The synthetic peptide PCK3145, which corresponds to amino acids 31-45 of PSP94, exhibit anti-metastatic activity39and inhibits proliferation not only in prostate cancer cells40,41but also in ovarian, breast and colon cancer cells42,43.PCK3145was shown to inhibit the secretion of the metastasisrelated protein matrix metallo-proteinase-939, to suppress angiogenesis by interfering with the vascular endothelial growth factor (VEGF) signaling44, and to decrease malignancy-associated hypercalcemia38. Clinical phase I and phase IIa trials have shown that the drug is safe with minimal side effects45, and that PCK3145 downregulate the levels of metastasis-associated plasma matrix metallopro-teinase 9 in patients with hormone refractory prostate cancer45. Based on our study, it would be interesting to investigate whether drugs mimicking PSP94 will be particularly effective in tumors with defects in AKT and/or MAPK signaling.
In summary, our study identified the loss of PSP94 expression as a predictor of unfavorable tumor phenotype and early PSA recurrence that is particularly powerful in cancer tissues with PTEN deletion. The combination of PSP94 loss and PTEN deletion is easy to measure and may be applicable in clinical practice.
Acknowledgements
This work was supported by a grant from the Federal Ministry of Education and Research (Grant No.01KU1505B). We are grateful to Janett Lütgens, Sünje Seekamp and Inge Brandt, for excellent technical assistance.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年2期
Cancer Biology & Medicine2019年2期
- Cancer Biology & Medicine的其它文章
- Effects of palbociclib on oral squamous cell carcinoma and the role of PIK3CA in conferring resistance
- Genetic polymorphisms and gastric cancer risk: a comprehensive review synopsis from meta-analysis and genome-wide association studies
- Identification of anticancer drugs to radiosensitise BRAFwild-type and mutant colorectal cancer
- Hepatitis B virus X protein enhances hepatocarcinogenesis by depressing the targeting of NUSAP1 mRNA by miR-18b
- Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer
- Factors associated with upstaging in patients preoperatively diagnosed with ductal carcinoma in situ by core needle biopsy
