腹腔镜下胃癌根治术后肺炎的危险因素及预后价值
简锦亮 杨春康 林振孟
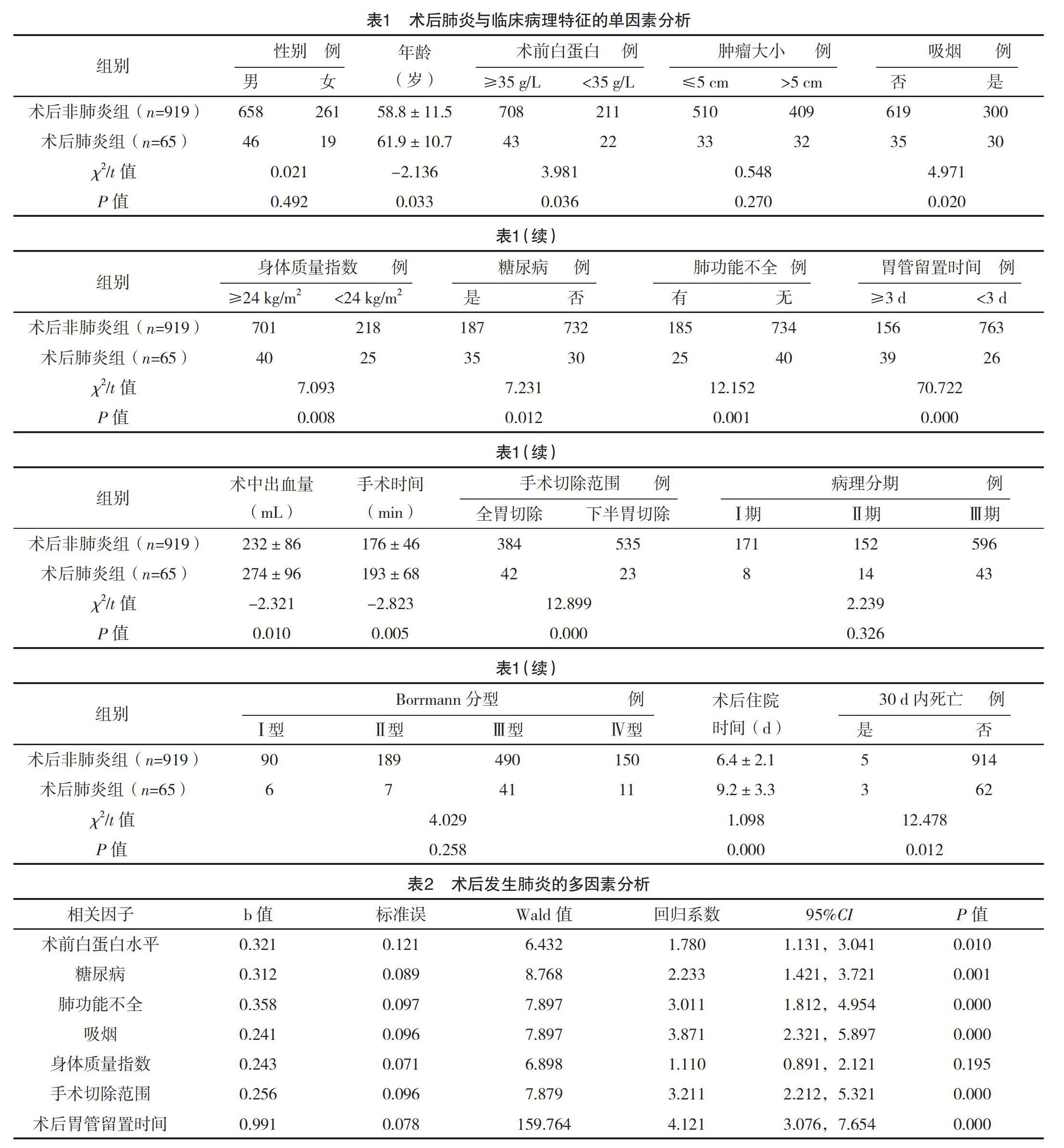
【摘要】 目的:研究腹腔鏡下胃癌根治术后发生肺炎的高危因素及对预后的影响。方法:回顾性分析2003年1月-2013年12月于福建医科大学附属肿瘤医院行腹腔镜下胃癌根治术的984例患者的临床病理资料,观察术后肺炎与多个因素的关系,评价肺炎的高危因素及对预后的影响。结果:共65例(6.6%)患者术后出现肺炎,平均术后(6.0±2.1)d发生肺炎。术后肺炎患者30 d内死亡率高于非肺炎患者(P<0.05)。术后肺炎患者的住院时间长于非肺炎患者(P<0.05)。单因素分析显示,术后肺炎与年龄、糖尿病、身体质量指数(BMI)、吸烟史、肺功能不全、术前白蛋白水平、术中出血量、手术时间、手术切除范围以及术后胃管留置时间有关(P<0.05)。多因素分析显示,吸烟史、术前白蛋白、肺功能不全、手术切除范围、糖尿病以及术后胃管留置时间是影响肺炎的独立危险因素(P<0.05)。术后肺炎组患者的3、5年生存率与非肺炎组比较,差异均无统计学意义(P>0.05)。结论:术后肺炎与多种因素有关,术前详细评估患者的状况,及时消除高危因素有利于降低术后并发症的发生。
【关键词】 腹腔镜手术 胃癌根治术 术后肺炎 预后
[Abstract] Objective: To study the risk factors of pneumonia after laparoscopic radical gastrectomy and its effect on prognosis. Method: The clinicopathological data of 984 patients who underwent laparoscopic radical gastrectomy in the affiliated tumor hospital of fujian medical university from January 2003 to December 2013 were retrospectively analyzed, the relationship between postoperative pneumonia and multiple factors was observed to evaluate the risk factors of pneumonia and its effect on prognosis. Result: A total of 65 patients (6.6%) developed pneumonia after surgery, with an average of (6.0±2.1) d. Mortality of pneumonia patients within 30 days after surgery was higher than that of non-pneumonia patients (P<0.05). Postoperative stay of pneumonia patients was longer than that of non-pneumonia patients (P<0.05). Univariate analysis showed that postoperative pneumonia was associated with age, diabetes, body mass index (BMI), smoking history, pulmonary dysfunction, preoperative albumin level, intraoperative blood loss, operative time, surgical resection area, and postoperative gastric tube indwor (P<0.05). Multivariate analysis showed that smoking history, preoperative albumin, pulmonary dysfunction, surgical resection range, diabetes and postoperative gastric tube indwor were independent risk factors for pneumonia (P<0.05). There were no statistically significant differences in 3-year and 5-year survival rates between the postoperative pneumonia group and the non-pneumonia group (P>0.05). Conclusion: Postoperative pneumonia is related to a variety of factors, preoperative detailed assessment of the patients condition and timely elimination of high-risk factors are beneficial to reduce the incidence of postoperative complications.
[Key words] Laparoscopic surgery Radical gastrectomy Postoperative pneumonia Prognosis
First-authors address: Fujian Cancer Hospital, Fuzhou 350014, China
doi:10.3969/j.issn.1674-4985.2019.32.001
胃癌根治术是目前治愈胃癌的唯一方式,但胃癌手术具有解剖复杂、血运丰富、清扫淋巴结难度大的特点,术后容易出现肺炎、吻合口瘘、肠梗阻、腹腔感染等并发症[1-2],术后仍有较高的死亡率。当前医师追求完整切除肿瘤的同时,尽量减轻患者痛苦,减少术后应激反应,从而减少手术并发症、缩短住院时间、提高患者的满意度[3]。随着微创理念的深入及医疗器械的发展,腹腔镜技术日益成熟。即使腹腔镜具有并发症少、创伤小、恢复快等优点[4],但日本和韩国开展了腹腔镜下胃癌切除术患者仍有较高的肺部并发症[5]。本文探讨腹腔镜下胃癌根治术后肺炎的危险因素及远期价值,重视高危患者围手术期的预防,降低术后肺炎的发生。现报道如下。
1 资料与方法
1.1 一般资料 回顾性分析2003年1月-2013年12月于福建医科大学附属肿瘤医院行腹腔镜下胃癌根治术的984例患者的临床病理资料。目前,国内外对术后肺炎的诊断标准并未统一,许多研究机构定义为外科手术后30 d内新发的肺炎,包括即使出院后但仍发生肺炎的患者,肺炎诊断需同时满足以下条件:(1)两次及两次以上影像学检查(对无心、肺基础疾病等,可行一次检查)。(2)至少符合以下一项:无其他明确原因的发热(T>38 ℃);静脉血WBC>12×109/L或<4×109/L;年龄≥70岁出现神志改变且无其他明确原因。(3)至少符合以下两项:新出现的咳嗽、呼吸困难或呼吸频率加快;新出现的脓痰或痰的性状变化;呼吸道分泌物增多;肺部啰音或支气管呼吸音;需要医护辅助吸痰次数增多;既有的咳嗽、呼吸困难或呼吸急促加重;气体交换情况恶化;氧需求量增加或需机械通气支持[6]。纳入标准:(1)腹腔镜下手术;(2)标准的D2淋巴结清扫术;(3)术后病理明确为腺癌;(4)术前无肺炎;(5)临床病理资料完整。排除标准:(1)术后病理为间质瘤、淋巴瘤、良性肿瘤等;(2)因幽门梗阻、出血急诊行手术治疗;(3)术前新辅助化疗。其中男704例,女280例,平均年龄(59.0±11.5)岁。采用门诊、电话方式进行随访,随访截止时间为2018年12月1日,平均随访时间(61±15)个月。本研究通过本院伦理委员会审批。
1.2 方法 通过回顾性研究的方法,收集984例患者的临床病理资料。通过门诊、电话等方式进行随访,术后第1个月、术后2年内每3个月随访1次、术后3~5年每6个月随访1次。
1.3 观察指标 观察患者出现术后肺炎的情况、30 d内死亡率、住院时间,对术后肺炎与临床病理特征进行单因素和多因素分析。
1.4 统计学处理 采用SPSS 24.0统计软件进行统计,计量资料符合正态分布图采用t检验,符合偏态分布采用Wilcoxon秩和检验;计数资料单因素分析采用卡方检验,单因素分析中有意义的参数采用Logistic回归进行多因素分析。生存分析采用Kaplan-Meier法并绘制生存曲线,log-rank法进行生存差异检测。P<0.05表示差异有统计学意义。
2 结果
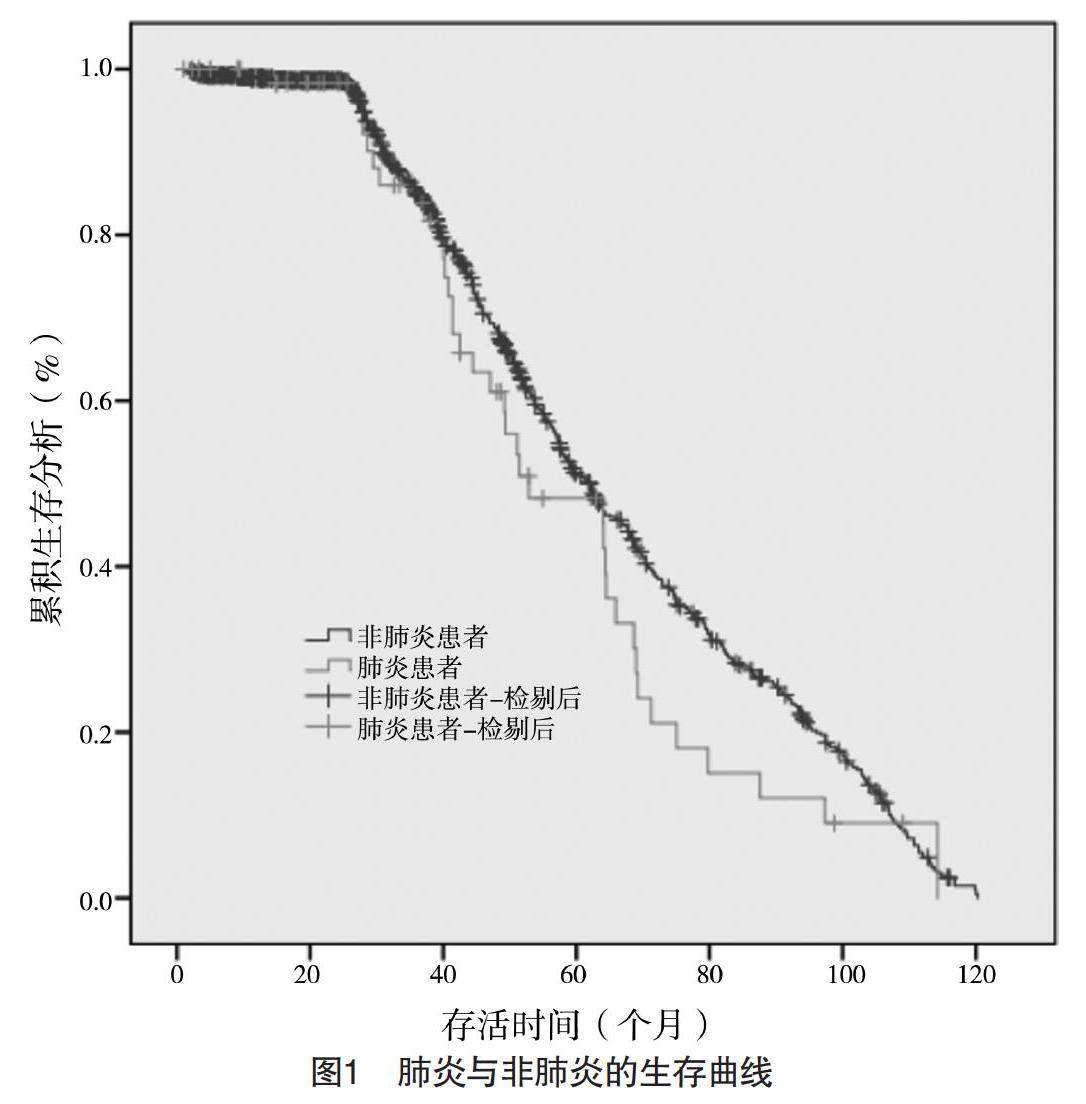
共有6.6%(65/984)患者术后出现肺炎,术后4~13 d发生肺炎,平均(6.0±2.1)d。术后肺炎患者30 d内死亡率高于非肺炎患者,差异有统计学意义(P<0.05)。术后肺炎患者的住院时间为(9.2±3.3)d,长于非肺炎患者的(6.4±2.1)d,差异有统计学意义(P<0.05)。单因素分析显示,术后肺炎与年龄、糖尿病、肺功能不全、身体质量指数(BMI)、吸烟史、术前白蛋白水平、术中出血量、手术时间、手术切除范围以及术后胃管留置时间均有关(P<0.05)。多因素分析显示,吸烟史、术前白蛋白、肺功能不全、术中出血量、手术时间、糖尿病、手术切除范围以及术后胃管留置时间是影响肺炎的独立危险因素(P<0.05)。见表1、2。术后肺炎组和非肺炎组患者的3年生存率分别为81.5%(53/65)和84.7%(778/919),两组比较差异无统计学意义(字2=0.344,P=0.621)。术后肺炎组和非肺炎组患者的5年生存率分别为47.7%(31/65)和50.9%(468/919),两组比较差异无统计学意义(字2=0.188,P=0.701),见图1。
3 讨论
自1994年Kitano首次实施腹腔镜辅助下远端胃切除术治疗早期胃癌后,微创技术已广泛应用于胃癌手术中,特别是当前医疗设备、缝合装置、技术娴熟加速该手术的普及[7]。腹腔镜具有创伤小、术后住院时间短、术后疼痛轻等特点[8]。胃癌术后由于腹部切口疼痛、麻醉以及阿片类止痛药物的使用,使患者由深慢呼吸变为浅表呼吸,使肺泡萎陷及呼吸功能不全,容易并发肺不张、肺炎等并发症[9-10]。本研究腹腔镜下根治性胃癌切除术后肺炎发生率为6.6%,严重影响患者术后康复及出院时间,本研究虽然肺炎对患者3、5年生存率不受影响,但肺炎患者30 d内死亡率高于非肺炎患者。
吸烟是术后肺炎独立危险因素,长期吸烟可使支气管黏膜的纤毛受损、变短,影响纤毛的清除功能。而且,黏膜下腺体增生、肥大,黏液分泌增多,成分也有改变,容易阻塞细支气管[11]。因此,对于长期吸烟患者,如果病情允许,术前应戒烟2周以上,且需要加强围手术期呼吸锻炼,如咳嗽、深呼吸训练、端坐位腹式呼吸、术前呼吸机伸展训练等。本研究中糖尿病患者更易患肺炎,原因考虑糖尿病患者中性粒细胞趋化、吞噬、抑菌能力下降,T淋巴细胞和B淋巴细胞增殖及活力低下,造成患者抵抗力下降;并且糖尿病患者微血管病变,术后易引起代谢紊乱[12]。术前白蛋白水平低下也是术后肺炎不利因素,白蛋白是人体血浆中重要蛋白质,是人体营养水平的标志。术前白蛋白水平低下是由于肿瘤慢性消耗以及患者进食减少,患者长期处于负氮平衡,患者体质减弱,抵抗力下降。另一方面,血清低白蛋白容易引起肺间质水肿,液体积聚于肺组织细胞外间隙,使肺泡受压、萎缩,萎陷而多水的肺组织适合细菌增殖,进而引起肺炎[13-14]。术后长期留置胃管也易并发肺炎,留置胃管影响患者有效的咳嗽、咳痰,浅表无力的咳嗽只会增加患者痛苦而无法使肺充分膨胀[15]。全胃切除患者肺炎发病率高于远端胃切除。由于全胃切除患者切除范围更大,创伤更大,机体恢复慢,手术时间及出血量更多;全胃切除时刺激膈肌,使膈肌运动受限,进而肺通气容量下降,引起肺不张和肺炎[16]。手术时间和術中出血量均影响术后肺炎,全身麻醉诱导后,呼吸系统立即发生改变:呼吸肌肌力减弱,肺容量减少进而形成肺不张。胃癌术后呼吸系统可能需要6周后才恢复术前状态;同时长时间麻醉造成肺部免疫系统受损,进而导致肺部感染[17-18]。单因素分析显示,BMI与术后肺炎有关,但多因素分析显示BMI不是术后肺炎的独立危险因素。主要是由于:(1)BMI高的患者常合并糖尿病、高血压等基础疾病,术后易发生肺炎;(2)BMI患者内脏脂肪分布增多,手术难度增大,手术及麻醉时间延长。年龄与术后肺炎有关,年龄大患者常合并肺功能不全、代谢性疾病,术后愈合能力弱,体质差[19]。术前肺功能不全患者本身供氧能力弱,气管上皮细胞功能受限,杯状细胞增生,分泌物增多,术后容易导致肺炎[20]。
總之,术后肺炎与多种因素有关,医护人员需要重视评估患者的功能,对高危人群采取一系列针对性的处理,加强围手术期肺部锻炼、术中精细操作、减少出血及手术时间,减少术后肺炎的发生。
参考文献
[1] Katai H,Mizusawa J,Katayama H,et al.Short-term surgical outcomes from a phase Ⅲ study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer:Japan linical Oncology Group Study JCOG0912[J].Gastric Cancer,2017,20(4):699-708.
[2] Yasuhiko M,Koji T,Yuji T,et al.Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study[J].Medicine,2016,95(11):e3125.
[3] Lauren M,Postlewait M D,Shishir K.Response:increased complications associated with feeding jejunostomy in gastrectomy for gastric cancer: Chicken or the egg?[J].Journal of Surgical Oncology,2016,112(1):121.
[4] Lei Q C,Wang X Y,Zheng H Z,et al.Laparoscopic versus open colorectal resection within fast track programs: an update meta-analysis based on randomized controlled trials[J].Clin Med Res,2015,7(8):594-601.
[5] Inaki N,Etoh T,Ohyama T,et al.A Multi-institutional prospective phase Ⅱ feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer(JLSSG0901)[J].World J Surg,2015,39(11):2734-2741.
[6]中华预防医学会医院感染控制分会第四届委员会重点部位感染防控学组.术后肺炎预防和控制专家共识[J].中华临床感染病杂志,2018,11(1):11-19.
[7] Takuro S,Yukinori K,Yasuhiro M,et al.Which is a more reliable indicator of survival after gastric cancer surgery: Postoperative complication occurrence or C-reactive protein elevation?[J].Surgical Ncology,2015,112(8):894-899.
[8] Beyer K,Baukloh A K,Kamphues C.Laparoscopic versus open gastrectomy for locally advanced gastric cancer: a systematic review and meta-analysis of randomized controlled studies[J].World J Surg Oncol,2019,17(1):886-890.
[9] Takeshi K,Naoki H,Takeshi S,et al.Prognostic Significance of Complications after Curative Surgery for Gastric Cancer[J].Annals of Surgical Oncology,2014,21(3):891-898.
[10] Hayashi T,Yoshikawa T,Aoyama T,et al.Impact of infectious complications on gastric cancer recurrence[J].Gastric Cancer,2015,18(2):368-374.
[11] Jun K,Shuhei K,Daisuke I,et al.Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer[J].International Journal of Clinical Oncology,2016,21(5):920-926.
[12] Nyberg M,Gliemann L,Hellsten Y.Vascular function in health, hypertension, and diabetes: effect of physical activity on skeletal muscle microcirculation[J].Scand J Med Sci Sports,2015,25(4):60-73.
[13] Daniel D,Bohl M D,Mary R,et al.poalbuminemia Independently Predicts Surgical Site Infection,Pneumonia, Length of Stay, and Readmission After Total Joint Arthroplasty[J].The Journal of Arthroplasty,2016,31:15-21.
[14] Fecso A B,Bhatti J A,Stotland P K,et al.Technical performance as a predictor of complications in laparoscopic gastric cancer surgery:another piece of the puzzle[J].Journal of the American College of Surgeons,2017,225(4):92.
[15] Climent M,Hidalgo N,Vidal ?,et al.Postoperative complications do not impact on recurrence and survival after curative resection of gastric cancer[J].European Journal of Surgical Oncology,2016,42(1):132-139.
[16] Tzani P,Chetta A,Olivieri D.Patient assessment and prevention of pulmonary side-effects in surgery[J].Curr Opin Anaesthesiol,2011,24(1):2-7.
[17] Miskovic A,Lumb A B.Postoperative pulmonary complications[J].British Journal of Naesthesia,2017,118(3):317-334.
[18] Suzuki H,Matsuda Y,Noda M,et al.Management of De Novo Mycobacterial Infection After Lung Transplantation Without Rifampicin: Case Series of a Single Institution[J].Transplantation Proceedings,2018,50(9):2764-2767.
[19] Takeuchi D,Koide N,Suzuki A,et al.Postoperative complications in elderly patients with gastric cancer[J].Journal of Surgical Research,2015,198(2):317-326.
[20] Yang C K,Teng A,Lee D Y,et al.Pulmonary complications after major abdominal surgery: national surgical quality improvement program analysis[J].Surg Res,2015,198:441-449.
(收稿日期:2019-08-20) (本文編辑:张爽)

