Screening and verification of the factors influencing somatic embryo maturation of Larix olgensis
Yue Song•Shujuan Li•Xiaoming Bai•Hanguo Zhang
Abstract With embryogenic callus of Larix olgensisis,we investigated the effects of inositol,glutamine,casein hydrolysate,carbohydrate,abscisic acid and silver nitrate concentration on the maturation of the somatic embryo.Three dominant factors emerged,and we developed a response surface model based on the Box–Behnken design.We defined the optimal conditions for the maturation of somatic embryos.The contents of abscisic acid,silver nitrate,sucrose and casein hydrolysis significantly affected the amount of maturing embryos,but inositol,maltose and glutamine had no effect.By establishing a response surface model with multiple factors,we predicted that the optimal number of L.olgensis somatic embryos was 204±4 g-1 on basal medium,containing 18.28 mg L-1abscisic acid,5.46 mg L-1silver nitrate and 82.67 g L-1sucrose.In the verification experiments,the addition of20 mg L-1 abscisic acid,5 mg L-1silver nitrate and 80 g L-1sucrose to BM yielded an average of 202.06 somatic embryos per gram.These results should guide large-scale breeding of L.olgensis.
Keywords Larix olgensis·Embryogenic callus·Somatic embryo maturation ·Box–Behnken design
Introduction
Larix olgensis is a fast-growing coniferous timber species that is widely planted in northeast China.Its growth is not restricted by edaphic conditions and it can grow well even in arid or swampy soils(Yang et al.1994).Pest and diseases have further caused a decline in the yield of L.olgensis seeds in recent years.At present,cutting propagation is still the main asexual reproduction method for larch for forestry production.However,the rooting rates of cutting propagation are relatively low and are often affected by genotype of parent(clone),environment and other factors(Lv et al.2005;Liu et al.2007).Somatic embryogenesis is not only used in studies of totipotency or morphological processes,but also as an effective method for large-scale propagation of plants,especially for superior genotypes that are difficult to propagate using conventional asexual reproduction(Li et al.2007).It is important to screen and verify the factors that influence somatic embryo maturation of L.olgensis.
During somatic embryo maturation of conifers,many factors directly affect the processes of cell differentiation,somatic embryo morphogenesis and maturation.Somatic embryogenesis and plant regeneration systems have been developed for species including Larix kaempferi,Larix decidua,Larix principis-rupprechtii and the hybrid larch L.olgensis×L.kaempferi(Von et al.1990;Qi 2000;Lv et al.2005;Wang et al.2009).Song et al.(2016)reported preliminary results on somatic embryogenesis of L.olgensis,but the key factors influencing somatic embryo maturation in L.olgensis have not been reported.
We studied the effects on the maturation of somatic embryos of L.olgensis of inositol,glutamine,casein hydrolysate,carbohydrate,abscisic acid and silver nitrate concentration.Our study objectives were to identify compounds that most strongly influenced the somatic embryogenesis of L.olgens using the single factor and orthogonal test,and to de fine optimal conditions for maturation of somatic embryos by building a response surface model based on the Box–Behnken design.
Materials and methods
Plant material
Immature seeds of L.olgensis were collected on 27 June 2014 from Qingshan Seed Orchard in Heilongjiang Province of China about 60 d after pollination.After surface sterilization,immature zygotic embryos were separated as initial explants and were placed in embryogenic callusinducing medium.After 6 weeks,the embryonic callus(Fig.1a)was induced and the embryonic suspensor mass(ESM)(Fig.1b)was observed by microscopy as separate from the embryonic callus.The embryogenic calli induced from immature seeds were propagated in a proliferation medium and subcultured every 2 weeks as described by Song et al.(2016).
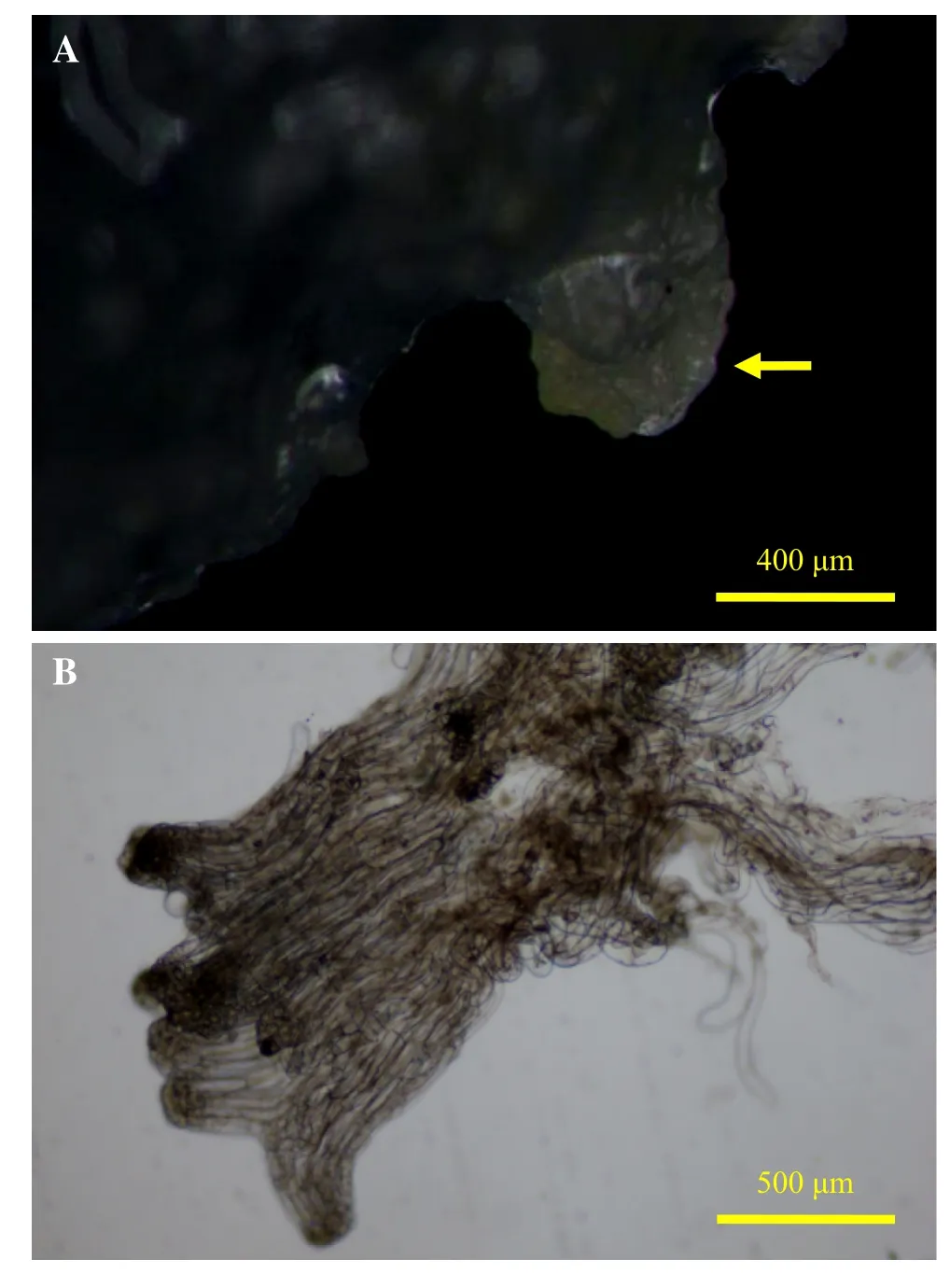
Fig.1 Embryogenic callus and microstructure of the embryonic suspensor mass.The embryonic callus(a),as the arrow pointing that is like a crystal,shows colorless transparent and the surface is full of filamentous bump;the microstructure of the embryonic suspensor mass(b)
Embryogenic callus-inducing medium contains basal medium (BM),1 g L-1glutamine,0.5 g L-1casein hydrolysate,1 g L-1inositol,2 mg L-12,4-D (2,4-Dichlorophenoxyacetic Acid),0.5 mg L-16-BA(6-Benzylaminopurine),0.5 mg L-1KT (Kinetin),25 g L-1sucrose and 6 g L-1agar.The proliferation medium comprises BM,0.15 mg L-12,4-D,0.05 mg L-16-BA,0.05 mg L-1KT,and 6 g L-1agar,at a pH value adjusted to 6.00±0.02.All cultures were maintained at 25±1°C under darkness conditions.
Maturation of somatic embryos
Embryonic calli were placed on a pre-culture medium for about 14 days,then transferred to a maturation medium to promote the maturation of somatic embryos.After 8 weeks,the somatic embryos were counted.Compared to the basal medium,the pre-culture medium contained half or a quarter of BM salts,with all vitamins,60 g L-1sucrose,1 g L-1glutamine,0.5 g L-1casein hydrolysate and 10 g L-1inositol,but without plant growth regulators.The maturation medium contained all BM salts,80 g L-1PEG4000, 0–1 g L-1inositol, 0–1 g L-1glutamine,0–0.5 g L-1casein hydrolysate (Fig.2),30–90 g L-1sucrose or maltose(Fig.3),10–30 mg L-1abscisic acid(Fig.4)and 0–10 mg L-1silver nitrate(Fig.5).Culture conditions in the mature medium were the same as during the proliferation of embryogenic calli.A single factor design was applied,and each treatment was repeated three times.The preliminary results identified the dominant factors that were then tested in further experiments.

Fig.2 Effects of organic ingredients concentration on the amount of somatic embryos.Different letters indicate significant differences at 0.05 level.For each vertical bar represents the standard deviation of the mean’s multiple range test.In addition to the factors tested,the medium also contained 5.0 mg L-1silver nitrate,20.0 mg L-1 abscisic acid and 60.0 g L-1sucrose
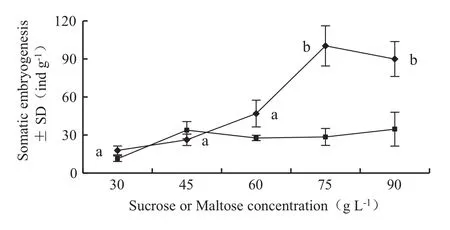
Fig.3 Effects of sucrose and maltose concentration on the amount of somatic embryos.‘Black diamond’representative of sucrose;‘black square’representative of maltose;different letters indicate significant differences at 0.05 level.For each vertical bar represents the standard deviation of the mean’s multiple range test.In addition to the factors tested,the medium also contained 5.0 mg L-1silvernitrate,20.0 mg L-1abscisic acid,0.50 g L-1inositol,and 0.50 g L-1 casein hydrolysate and 0.25 g L-1glutamine
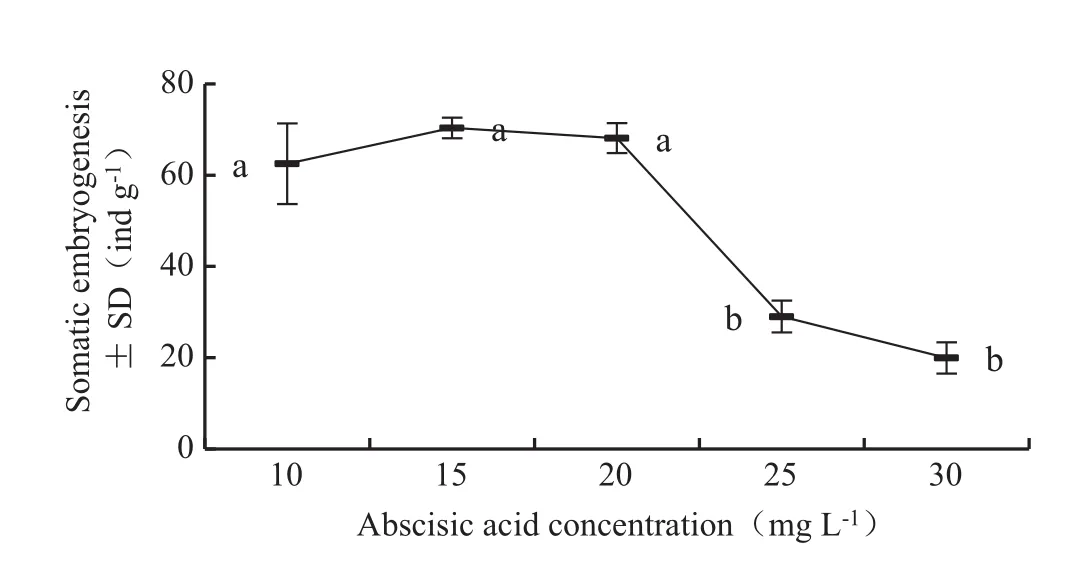
Fig.4 Effects of abscisic acid concentration on the amount of somatic embryos.Different letters indicate significant differences at 0.05 level.For each vertical bar represents the standard deviation of the mean’s multiple range test.In addition to the factors tested,the medium also contained 5.0 mg L-1silver nitrate,0.50 g L-1inositol,0.50 g L-1casein hydrolysate,0.25 g L-1glutamine and 60.0 g L-1 sucrose
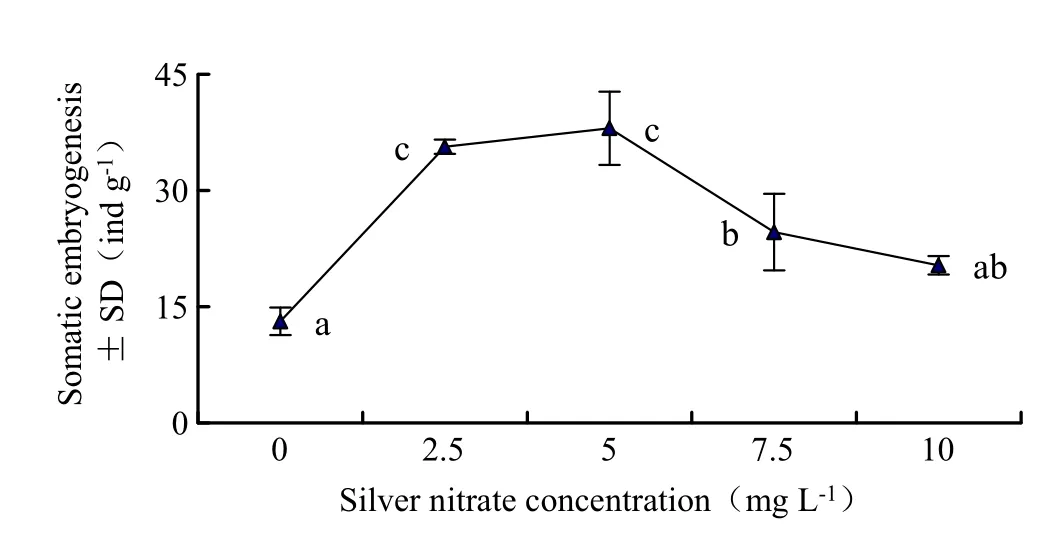
Fig.5 Effects of silver nitrate concentration on the amount of somatic embryos.Different letters indicate significant differences at 0.05 level.For each vertical bar represents the standard deviation of the mean’s multiple range test.In addition to the factors tested,the medium also contained 20.0 mg L-1abscisic acid,0.50 g L-1 inositol,0.50 g L-1casein hydrolysate,0.25 g L-1glutamine and 60.0 g L-1sucrose
Establishment and validation of the response surface model
A response surface model was developed based on our preliminary results.The number of somatic embryos was the response value.Three factors(abscisic acid,silver nitrate and sucrose contents)were identified as the dominant factors(Table 1).The maturation medium of these experiments also contained 80 g L-1PEG4000,0.5 g L-1inositol,0.5 g L-1glutamine and 0.25 g L-1casein hydrolysate.To test and verify the reliability of the optimization results,we selected the adjacent integer values of the optimized values for the prediction and further compared with the predicted results.
Each treatment was repeated for three times,each time using three groups of inoculated embryogenic calli,each group of calli weighed about 0.35 g.Cultures were maintained at 25± 1°C in the dark.
The number of somatic embryos per gram of embryogenic calli(ind.g-1)=number of somatic embryos/fresh mass of embryogenic calli.Data were analyzed using PASW 18(IBM,Armonk,NY,USA)and Minitab 17(Minitab,State College,PA,USA)ANOVA and Duncan’s multiple range test were performed on the results of the experiments.
Results
Effect of organic compounds on somatic embryogenesis
The number of somatic embryos decreased with increasing contents of glutamine and casein hydrolysate in the maturation medium(Fig.2).Casein hydrolysate at 0.25 g L-1significantly reduced the number of somatic embryos(p=0.003).Neither glutamine nor inositol affected the number of mature somatic embryos,but the addition of the two ingredients was bene ficial to the morphology of the late stage of somatic embryos,which can reduce the percentage of abnormal embryos(not shown).
Effects of carbohydrate on somatic embryogenesis
The number of somatic embryos of L.olgensis initially increased and then decreased with increasing sucrose concentration. There were 100.32±27.46 somatic embryos obtained from each gram of embryogenic calli in the maturation medium supplemented with 75 g L-1sucrose.However,the number of somatic embryosdeclined significantly when sucrose was replaced with the same concentration of maltose.Only 28.45±11.62 somatic embryos were obtained when maltose was added to the medium.The results of variance analysis show that the effect of sucrose concentration on somatic embryos maturation was very significant(p=0.003),and the effect of maltose concentration was not significant(p=0.251).

Table 1 Experimental factors and levels
Effect of abscisic acid on somatic embryogenesis
The concentration of abscisic acid in the maturation medium significantly affected(p=0.000)the number of somatic embryos.Abscisic acid at 10–20 mg L-1in the medium was suitable for somatic embryo maturation of L.olgensi (Fig.4).Of all the tested concentrations,15 mg L-1abscisic acid had the strongest effect on promoting the somatic embryogenesis,with an average of 70.36±3.87 somatic embryos per gram.But the number of embryos significantly decreased when the concentration of abscisic acid was more than 25 mg L-1.
Effect of silver nitrate on somatic embryogenesis
The results indicated that adding 0.25–0.5 mg L-1silver nitrate(AgNO3)in culture media was more suitable for somatic embryo maturation of L.olgensis(Fig.5).Especially the amount of somatic embryos peaked when silver nitrate was added at0.5 mg L-1.An average of 38.04±8.20 somatic embryos can be obtained from the embryonic calluses for each gram.Thus,the number of somatic embryos decreased when silver nitrate was increased to 0.75 mg L-1.Variance analysis shows that silver nitrate concentration significantly affected somatic embryogenesis(p=0.000).
Response surface model and significance test
The results obtained from 15 experimental runs were carried out according to the Box–Behnken design summarized in Table 2.The proposed second-order polynomial fit the data in Table 2,and multiple linear regressions were used to determine the optimum maturation conditions to achievethe maximum number of somatic embryos of L.olgensis.The effects of sucrose,abscisic acid and silver nitrate concentration were quantitatively evaluated using response curves.By a multiple regression analysis on the experimental data,the following second-order polynomial was obtained,and the model adequately reflected the relationship between the number of somatic embryos and sucrose,abscisic acid and silver nitrate concentration as Eq.(1):
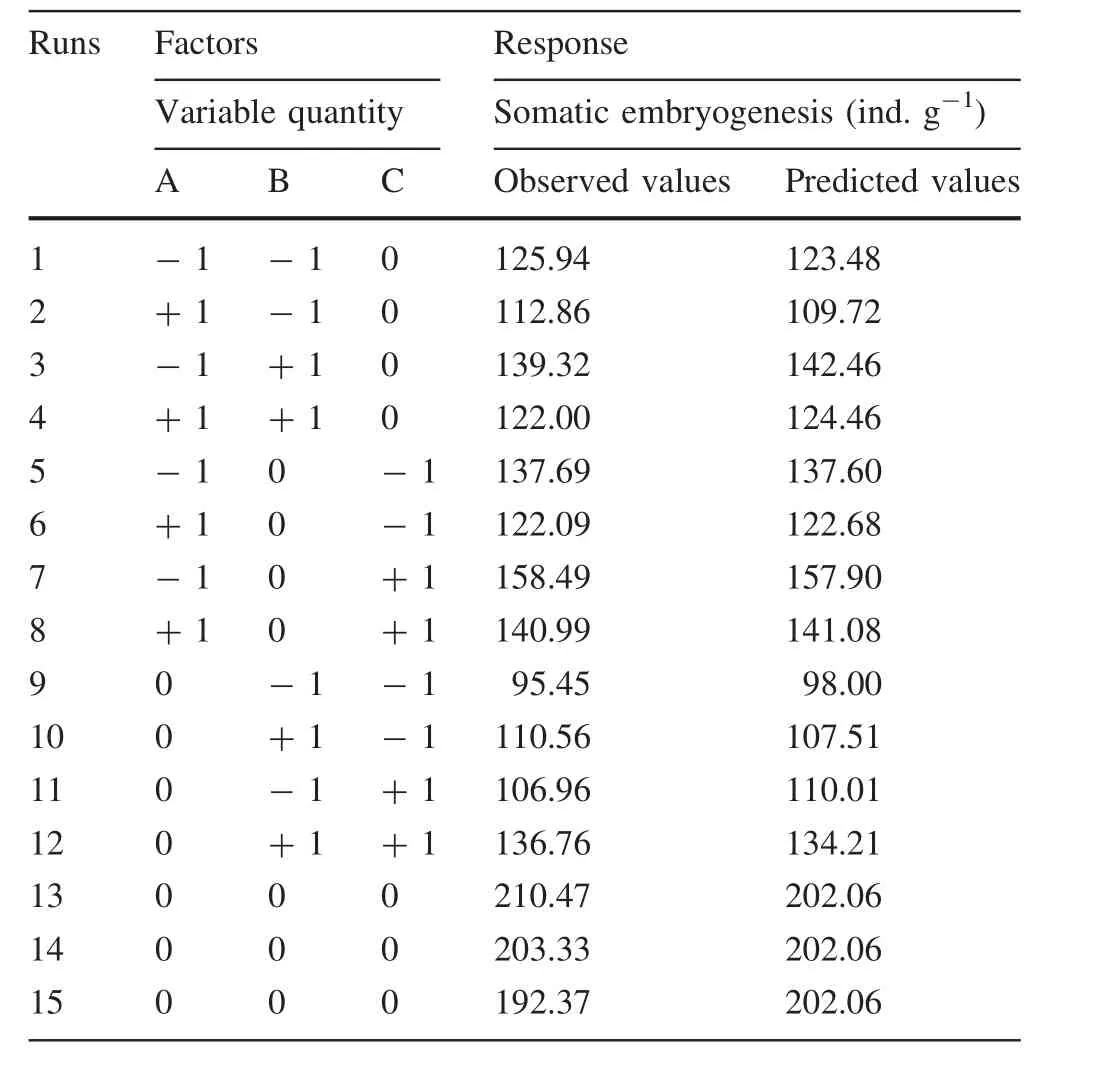
Table 2 Experimental design and results
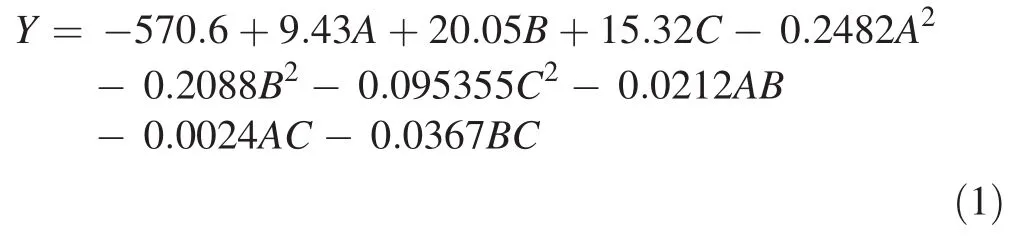
where Y in the formula was predicted response.A,B and C were independent variables,on behalf of abscisic acid,silver nitrate and sucrose concentration,respectively.A2,B2and C2are linear effects.The AB,AC and BC were interaction terms.The predicted values of the number of somatic embryos per gram of calluses using above equation were given in Table 2 along with the average experimental data of three repetitions.
The significance of the fit of the second-order polynomial for the number of somatic embryos was assessed with an analysis of variance(ANOVA)(Table 3).The R2(coefficient of determination)of the model was 0.987,and the adjusted R2is 0.963,which indicates that the model could adequately represent the real relationship between the variables under consideration.An R2of 0.987 means that 98.70%of the variability was explained by the model.The coefficient of variation(CV),indicates the degree of experimental precision,was 4.81%.This low CV suggests a high reliability of the experiment.
Values of P less than 0.01 indicate the model terms are extremely significant.Values greater than 0.05 indicate the model terms are not very significant.A model F of 41.86 and a very low probability less than 0.000 imply significant model fit.The ‘lack of fit’F of 0.26 implies that there was insignificant lack of fit.The ‘lack of fit’P of 0.853 implies that there was an 85.3%chance that the ‘lack of fit’’F could be due to noise.
In the regression model,the terms A (p=0.021),B (p=0.017),C (p=0.010),A2(p=0.001),B2(p=0.000)and C2(p=0.000)were significant with a probability of 95%.However,the model terms AB(p=0.767),AC(p=0.894)and BC(p=0.329)were not significant,indicating that there was no interaction between abscisic acid and silver nitrate concentration or sucrose concentration or between silver nitrate and sucrose contents on the number of somatic embryos produced per gram during the maturation culture stage.
Interaction analysis and optimization of maturation culture conditions
To optimize the variables that influence the production of somatic embryos from embryogenic callus of L.olgensis,response surface plots were generated from the regression model.The three-dimensional plots were generated by keeping one variable constant at the centre point and varying the others within the experimental range.The resulting response surfaces shows the effect of abscisic acid,silver nitrate and sucrose concentration on the number of maturation somatic embryos.Figures 6,7 and 8 represents the response surfaces and the contour plots for the optimization of the number of somatic embryos obtained in the maturation stage.From the three figures,we can see that the response value increases first and then decreases with the concentration change in abscisic acid,silver nitrate and sucrose concentration,and there was a maximum of the response value in a certain range.
The optimal maturation conditions of somatic embryos can be obtained through the optimal model.The number of somatic embryos obtained was 204±4 g-1when the abscisic acid concentration was 18.28 mg L-1,the silver nitrate concentration was 5.46 mg L-1and the sucrose content was 82.63 g L-1.The number of somatic embryos was between 194 and 214 g-1when the confidence interval is 95%.For convenience,abscisic acid concentration was set at 20 mg L-1,silver nitrate at 5 mg L-1and sucrose at 80 g L-1in our experiments.The average number of somatic embryos generated was about 202.06 g-1,consistent with the fitted value of 206(Fig.9),indicating that the model fits the actual situation well.
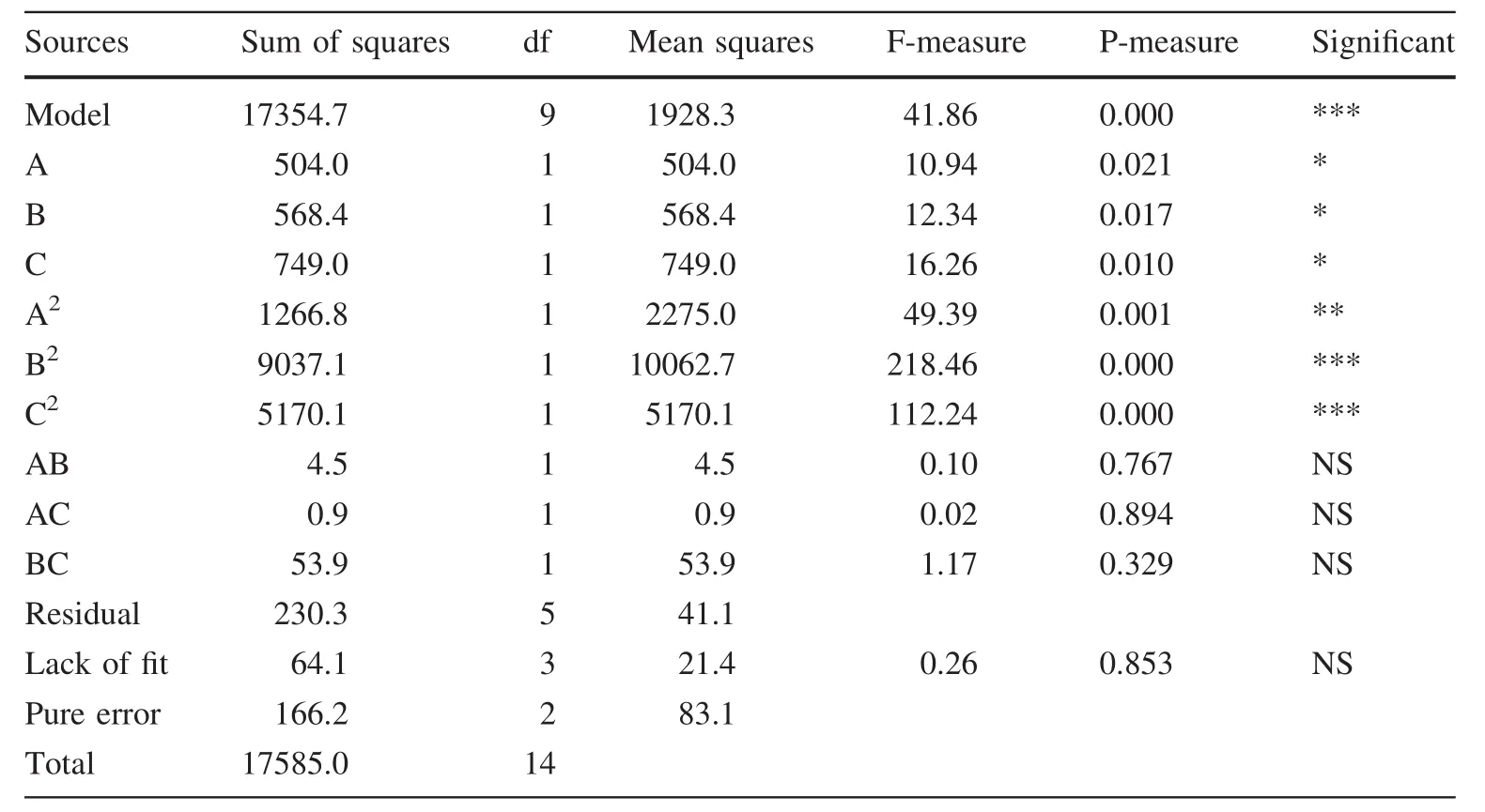
Table 3 Variance analysis of the effects of sucrose,abscisic acid and AgNO3concentrations on somatic embryogenesis of Larix olgensist

Fig.6 Response surface plot and the corresponding contour plot showing the effects of abscisic acid and silver nitrate concentration on somatic embryogenesis of Larix olgensis
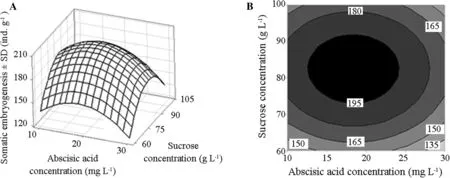
Fig.7 Response surface plot and the corresponding contour plot showing the effects of abscisic acid and sucrose concentration on somatic embryogenesis of Larix olgensis

Fig.8 Response surface plot and the corresponding contour plot showing the effects of silver nitrate and sucrose concentration on somatic embryogenesis of Larix olgensis
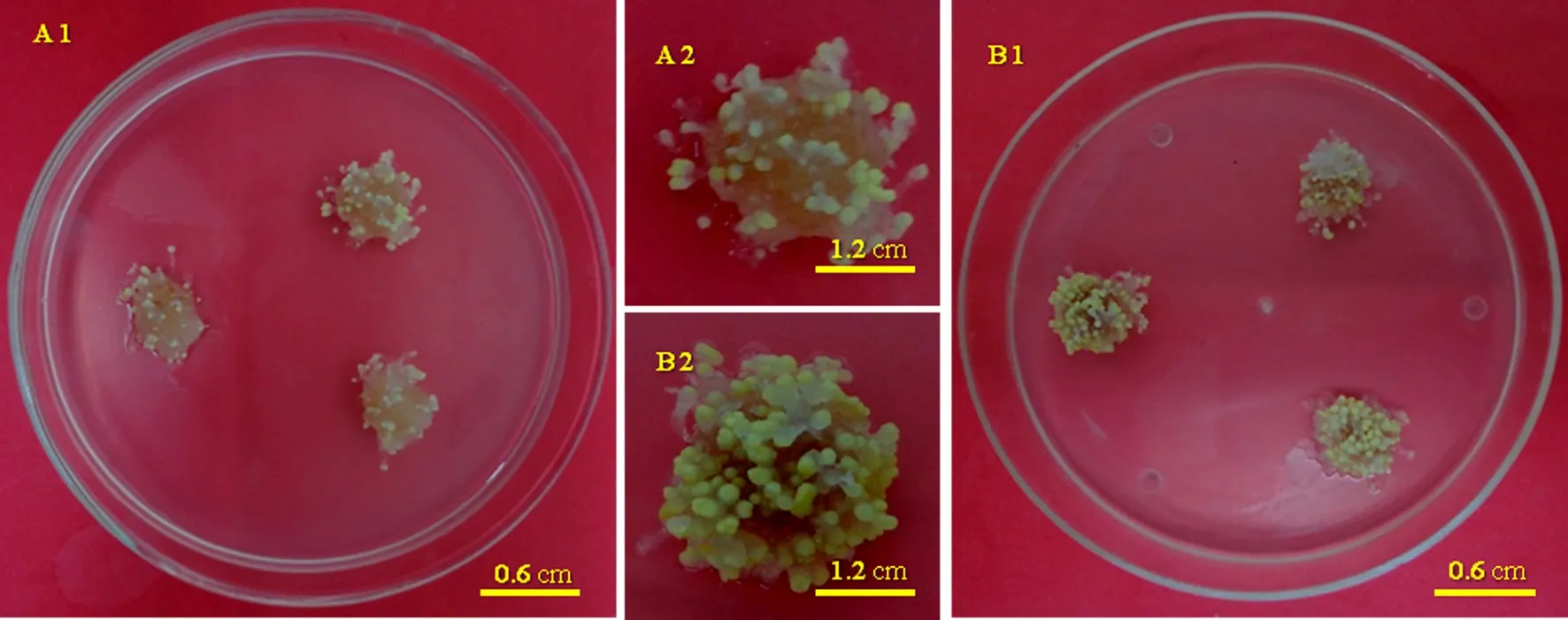
Fig.9 The situation of somatic embryogenesis of Larix olgensis before and after optimization of maturation culture medium.A1 and A2 show the situation of somatic embryogenesis of L.olgensis before optimization,mature medium contains 15 mg L-1abscisic acid,10 mg L-1silvernitrate and 60 g L-1sucrose,70.36±3.87 embryos per gram of embryogenic callus;B1 and B2 show the situation after optimization,202.06±9.12 embryos per gram of callus
Discussion
Somatic embryogenesis is influenced by many factors,such as culture medium,environmental conditions,plant growth regulating substances,explants types and sources.(Mee et al.2016).Most studies related to somatic embryogenesis have focused on the effects of organic components in the culture medium(Perez and Sommer 1987;Tuskan et al.1990;Yu et al.2011).Casein hydrolysate and glutamine improve the ratio of ammonium nitrogen to nitrate nitrogen when added to the differentiation medium;these favor somatic embryogenesis or organogenesis in some conifers(Wang et al.2009).Inositol can regulate the osmotic pressure of the medium,and its derivatives are involved in diverse cellular processes,including intracellular signal transduction,synthesis and transport of auxin,membranerelated anchoring of proteins and plant cell inverse regulation(Bandurski 1986;Luo et al.2011).
In this study,we found that the number of somatic embryos of L.olgensis peaked when 1 g L-1inositol was added to the maturation medium without any casein hydrolysate or glutamine.This result contrasts with the report of Wang et al.(2009)that inositol,casein hydrolysate and glutamine had significant effects on somatic embryogenesis of hybrid larch(L.olgensis×L.kaempferi),and optimal contents of the three components were 250,250 and 750 mg L-1,respectively.Their results are not the same as ours perhaps due to the use of different species(or hybrids)and variations in their responses to the organic components.Our test results suggested that proper concentrations of additives are important for somatic embryogenesis in L.olgensis.
Some studies indicated that the somatic embryogenesis of conifers is influenced by the type and concentration of the carbohydrate(Li et al.1998;Zhang et al.2014),which is critical as the carbon source in the maturation medium.In a previous study,Qi(2000)found that the quantity and quality of embryos of Larix principis-rupprechtii were affected by sucrose concentration.Zhang et al.(2014)reported that the higher concentration of sucrose did not benefit somatic embryogenesis of Picea;the majority of somatic embryos obtained were small,and the germination rate was low when the sucrose concentration was lower.Sugars not only supply energy,but also regulate the osmotic pressure.Although low concentration of sucrose is favorable for synchronization of development,it is not favorable for generating high-quality somatic embryos.Levels of sucrose that are too high cause cytoplasm-cell wall separation,so that most of the embryonic cells die(Zhang et al.2014).
In comparison to the sucrose,maltose is more beneficial to the morphogenesis of radiculodium of the somatic embryo of loblolly pine,the degree of maturation and the rate of rooting of the somatic embryos of Larix principisrupprechtii is improved when sucrose is replaced with 3%maltose(Li et al.1998;Qi et al.2004).In our study,as the main carbon source,the maltose did not increase the number of somatic embryos generated,perhaps because the embryogenic tissue of L.olgensis was not able to absorb and utilize maltose.
Abscisic acid plays an important role in somatic embryogenesis of many kinds of plants,especially conifers(Mundy et al.1990).Optimal concentrations of exogenous abscisic acid improve the quality and frequency of somatic embryos,which may be related to specific gene expression during somatic embryogenesis(Vales et al.2007).In some conifers,such as Picea glauca,Pinus pinaster,Pseudotsuga menziesii and hybrid larch,specific mRNAs(cDNAs)or protein may result during somatic embryogenesis in response to exogenous abscisic acid(Dong and Dunstan 1996,1997;Dong et al.1997;Sonia et al.2005).Furthermore,Vales et al.(2007)reported that maturation of somatic embryos of loblolly pine may be regulated through the expression of genes responsive to abscisic acid.
Previous research on somatic embryos of Larix species showed a remarkable maturation frequency in response to 40 μmol L-1(about 10.57 mg L-1)abscisic acid in L.laricina(Klimaszewska 2011),15.51 mg L-1in L.principis-rupprechtii(Qi et al.2004),40 mg L-1in L.kaempferi×olgensis(Wang et al.2009)and in L.leptolepis(Wang and Yang 2010).In these experiments,the relatively low concentration of abscisic acid was more favorable for somatic embryo maturation in L.olgensis.So we speculated that the interspecies specificity might be one reason that different concentrations of abscisic acid are suitable for somatic embryo maturation for different species of larch.
A number of studies have also shown that ethylene and polyamines play important roles in the development of somatic embryos of plants(Kumar et al.2008;Kepczynska et al.2001;Ptak et al.2010;Mauri and Manzanera 2011;Lu et al.2011).An increase in polyamines is considered to be a prerequisite for somatic embryogenesis and might mediate the effect of hormones on somatic embryogenesis(Galston 1983).As the medium for hormone action,polyamine act as a second messenger during somatic embryogenesis of plants.In addition,hydrogen peroxide formed by the oxidation of polyamines can also affect cell differentiation(Yoda et al.2006).As a precursor,S-adenosyl methionine may be involved in the biosynthesis of endogenous ethylene and polyamines in plants(Bais et al.2001;Bhatanagar et al.2001;Puga-Hermida et al.2006).As an inhibitor of ethylene synthesis,silver ions can eliminate the inhibition of ethylene,promote the synthesis of polyamines and increase the frequency of somatic embryogenesis(Even et al.1982;Pegg and Poso 1983).In our experiment, adding 5.46 mg L-1silver nitrate improved the somatic embryogenesis of L.olgensis.However,silver nitrate at certain levels can be toxic to plant cells can significantly decrease the number of somatic embryos generated.
Conclusion
In this study of the effects of inositol,glutamine,casein hydrolysate,carbohydrate,abscisic acid and silver nitrate concentration on somatic embryo maturation by L.olgensis,three dominant factors of abscisic acid,silver nitrate and sucrose were determined.By establishing a response surface model for somatic embryogenesis of L.olgensis,we predicted the best amount of somatic embryos was 204±4 g-1on basal medium,containing abscisic acid of 18.28 mg L-1,silver nitrate of 5.46 mg L-1and sucrose of 82.63 g L-1.In verifying experiments,the average number of somatic embryos was 202.06 g-1when adding 20 mg L-1abscisic acid,5 mg L-1silver nitrate and 80 g L-1sucrose,consistent with the predicted value.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Temperature characteristics of wood during microwave treatments
- Tin-based metal bath heat treatment:an efficient and recyclable green approach for Pinus massoniana wood modification
- Decay resistance and dimensional stability improvement of wood by low melting point alloy heat treatment
- A novel image segmentation approach for wood plate surface defect classification through convex optimization
- Ectomycorrhizal fungus enhances drought tolerance of Pinus sylvestris var.mongolica seedlings and improves soil condition
- First report of leaf-spot disease caused by Sphaeropsis visci on Asian mistletoe[Viscum coloratum(Kom.)Nakai]in China
