Ectomycorrhizal fungus enhances drought tolerance of Pinus sylvestris var.mongolica seedlings and improves soil condition
Dachuan Yin·Ruiqing Song·Jinyu Qi·Xun Deng
Abstract Mongolian pine is an important afforestation species widely used for ecological management in northeast China.The environment in this region is very unstable and the flora are regularly subjected to drought stress.This paper reports on the influence of inoculation with the Suillus luteus on seedlings under different water conditions.Both inoculated and non-inoculated ectomycorrhizal fungi(ECMF)-S.luteus seedlings were maintained under well watered or water-stress conditions for 3 months.The S.luteus colonization rate under water stress was higher than that in well-watered conditions.Under water stress,inoculated seedlings had greater growth than non-inoculated seedlings.In addition,under water stress,S.luteus-inoculated seedlings had greater superoxide dismutase and peroxidase activity,higher soluble protein content,lower proline content,and lower malondialdehyde content than non-inoculated seedlings.S.luteus colonization increased the rhizosphere soil-enzyme activity and the rhizosphere soil nutrition content under both well-watered and waterstress conditions.Given the positive impact on seedling growth and physiology,S.luteus shows potential for use in the arid and semi-arid regions of northeast China for afforestation.
Keywords Pinus sylvestris var.mongolica ·Suillus luteus·Water stress
Introduction
Desertification is one of the major environmental issues of the twenty- first century and affects environmental quality worldwide(Yang et al.2005;Ravi et al.2010).Some environmental problems,such as climate change and the loss of biodiversity caused by land desertification,have been documented in China(Wang et al.2008).As a protective measure,shelter forest systems have been established in areas subject to potential desertification(Zheng et al.2012).Water stress is one of the most important factors limiting plant growth and yield(Karaba et al.2007).Although different plant species vary in their sensitivity and response to a decrease in water,all plants have an encoded capability for stress perception,signaling,and response(Bohnert et al.1995).
Horqin Sandy Land,is a semi-arid,sandy region in North China located between the Greater Khingan Mountains in the north and the Nuluerhu Mountains in the south,and between the Songliao Plain in the east and the Seven Mountains in the west.At the beginning of the 1950s,Mongolian pine(Pinus sylvestris var.mongolica)was introduced,as the main tree species in the shelter forest system in this region,because of its tolerance to drought resistance.
In drought environments,plants usually modify their growth and physiology(Ruiz-Lozano 2003)to increase their drought tolerance.Diverse environmental stresses affect plant processes,leading to the loss of cellular homeostasis and the formation of reactive oxide species(ROS),which causes oxidative damage to membranes,lipids,proteins,and nucleic acids(Huang et al.2006;Jaleel et al.2009).Effective antioxidant enzymatic systems for protection against oxidation damage include catalase(CAT),peroxidase(POD),and superoxide dismutase(SOD)to minimize the deleterious effects of ROS.Several soil microorganisms associated with plants may also help alleviate water stress symptoms.For example,ectomycorrhizal fungi(ECMF),which are associated with the roots of Pinaceae plants,can be introduced at the time of planting and may stimulate the growth of plants and contribute to enhanced tolerance to abiotic stress(Abbaspour et al.2012;Miller 1982).
ECMF can improve plant growth by increasing the absorption of mineral nutrients and water,and increasing plant resistance to pathogens and environmental stresses(Bending and Read 1995;Smith and Read 2008).A bene ficial effect of ECMF on the biological control of larval root herbivores has also been reported(Edda et al.2010;Baar and Stanton 2000;Mucha et al.2006;Sharma et al.2010;Shaw et al.1995;Vinale et al.2004).Recently,the growth of ECMF-inoculated plants before afforestation under drought conditions has been focused on arid and semi-arid areas of China(Yin et al.2014;Yooyongwech et al.2013).For example,Suillus luteus helps P.sylvestris var.mongolica to become established in Horqin ecosystems,by increasing plant growth and tissue nutrients(Wang et al.2012).However,few studies have analyzed the effects of S.luteus on P.sylvestris var.mongolica drought tolerance under water-deficit conditions in arid and semi-arid areas.
This study aims to assess the influence of S.luteus on the growth and physiological performance of Mongolian pine(P.sylvestris var.mongolica)seedlings.It compares the growth,chlorophyll concentration,antioxidant enzyme activity(catalase CAT,peroxidase POD,superoxide dismutase SOD),osmotic adjustment substances(soluble protein,soluble sugar,malondialdehyde MDA,free proline),rhizosphere soil enzymes activity(catalase,urease,phosphatase)and rhizosphere soil nutrient(N,P,K)content of ECMF-(S.luteus)inoculated and non-mycorrhizal Mongolian pine seedlings under water stress and in well watered conditions.Rhizosphere soil catalase,urease,and phosphatase activity are the direct embodiment of soil biological activity:thus,it is important to measure the soil biological activity at the same time when analyzing seedlings.This work studies Mongolian pine seedlings’growth and physiological parameters and how they influence the plants’ability to endure water stress and reflect the effects of S.luteus.The results of such information are anticipated to help improve the success rate of reforestation programs in arid and semi-arid areas of China.
Materials and methods
Organisms and growth conditions
The S.luteus was isolated from Horqin Sandy Land under the P.sylvestris var.mongolica forest from a basidiome in the ZhanggutaiLiaoning Province,NortheastChina(42°35′–42°47′N,122°23′–122°40′E).It was grown on MMN agar medium at pH 6.6[NaCl 0.025 g/L,(NH4)2-HPO40.25 g/L,KH2PO40.50 g/L,FeCl30.050 g/L,CaCl20.50 g/L,MgSO40.15 g/L,thiamine 0.1 0 g/L,casamino acids 1.0 g/L,malt extract 10 g/L,glucose 10 g/L,and agar 20 g/L],as described by Brundrett et al.(1996).The S.luteus on MMN agar medium was cut with a sterile puncher(φ=10 mm)after culturing for 20 days.Suspension cultures of S.luteus were obtained by transferring the mycelium inoculum to liquid MMN medium(MMN medium without agar).Three-week suspension cultures,maintained in the dark at 27°C under agitation(120 r/min),were used to inoculate the seedlings.The seeds(collected from Zhanggutai Liaoning Province)were surface sterilized with potassium permanganate(0.5%,v/v)for 30 min,then washed three times with sterile distilled water.They were then germinated on sterile moistened gauze at 25°C for 5 days.
After germination,the seedlings were transferred to plastic pots(18× 18 cm;20–30 seeds per pot) filled with a sterile culture medium,vermiculite/soil/sand(1:2:1,v/v/v)mixture which was sterilized in a high-temperature autoclave for 2 h under 121°C.The basic soil parameters are N 550 mg/kg,P 9.2 mg/kg,K 85 mg/kg,pH 7.2.The pots were kept under greenhouse conditions(day/night thermal regime of 23/9± 2°C and 14 h light/10 h dark photoperiod),watered every 2 days for 1 month,and the seedlings were then inoculated with the fungi.
Experimental design and seedling inoculation
For all treatments,including the control, five pots(~20 seedlings per pot)were prepared,giving a total of 100 seedlings per treatment.
There were four treatments:(1)well-watered with S.luteus(W+S);(2)well-watered without S.luteus(W-S);(3)water stress with S.luteus(D+S);(4)water stress without S.luteus(D-S).The following weighing method was adopted:well-watered,75–80% of relative field capacity;water stress,and 35–40% of relative field capacity.The inoculations were performed by transferring 50 mL of the fungal suspension culture to the planting hole,where it was introduced at the root system level.The treatments without S.luteus were inoculated in an equivalent sterilization medium.All treatments were arranged at random under the greenhouse conditions given above.
Sampling and analysis of seedlings
The seedlings were harvested 3 months after inoculation.Harvesting was performed without damaging the root system,which was carefully washed to remove the soil.A total of 30 seedlings per treatment were randomly selected(e.g.,30 seedlings out of 100 per variant).For each seedling,the plant height,collar diameter,and biomass at harvest were calculated.The biomass was calculated based on fresh weight and dry weight.Once the fresh weight had been measured,the seedlings were oven dried at 85°C for 5 h.
Twenty sections of seedling root were selected randomly and cut into 1 cm pieces.The mycorrhizal colonization rate observed with a microscope no stained.The colonization rate was determined using the following ration:colonization rate(%)=mycorrhizal seedlings/total seedlings×100.Results are given as a mean.
The content of photosynthetic pigment was determined following the method described by William and Paul(1985).Fresh leaf tissues(0.5 g)were ground in 10 mL acetone,followed by centrifugation at 10,000 rpm for 5 min.The absorbance was recorded at 663 and 645 nm.These experiments were repeated three times.
The content of soluble protein was determined following the method described by Christos et al.(2008).One hundred mg of Coomassie Brilliant Blue(CBB)G-250,100 mg/L reagent was added to 50 mL of 95%ethanol and mixed until dissolved.One hundred mL of 15 mol/L H3PO4was added to~500 mL of distilled H2O and mixed well.Roots,stems,and leaves(0.5 g each)were ground in 10 mL distilled water,and placed in a centrifuge at 10,000 rpm for 5 min.Finally,1 mL supernatant was added to 5 mL CBB reagent and mixed well.The absorbance was recorded at 595 nm.
The content of soluble sugars was analyzed using the anthrone method described by Yemm and Willis(1954).The fine powder of roots,stems and leaves(about 100 mg)were homogenized in 3 mL of 80%ethanol and incubated in an ultrasonic bath for 30 min at 80°C.After centrifugation(6000×g,25 °C,10 min),the supernatant was collected.The pellet was extracted as above,and the supernatant was collected and combined with the previous one.After adding 2 mL of anthrone reagent to the supernatant,the mixture was heated in boiling water for 7 min.After the mixture was cooled to room temperature,the absorbance of the mixture was recorded spectrophotometrically at 620 nm.
The CAT and POD enzymes were extracted using 0.1 g of roots,stems,and leaves in 10 mL of 50 mmol/L phosphoric acid buffer solution,pH 7.0.The extract was centrifuged at 10,000 rpm for 20 min at 4°C,and the activities of both CAT and POD in the supernatant were analyzed.
The SOD enzyme was extracted using 0.1 g of root,stem,and leaves in 10 mL of 50 mmol/L phosphoric acid buffer solution,pH 7.8.The extract was centrifuged at 10,000 rpm for 20 min at 4°C,and the activity of total SOD in the supernatant was analyzed(Zhang 2003;Zhang et al.2011).All antioxidant enzyme activities were calculated following the method described by Song et al.(2005).
MDA and free proline play a key role as osmolytes in the control of water retention in plants under water stress,and so were chosen as variables to con firm the bene fits of S.luteus inoculation.
Malondialdehyde(MDA)levels were measured as described by Wasowicz et al.(1993),0.2 g of roots,stems,and leaves fresh tissues were placed in a tube containing 1 mL distilled water.After the addition of 1 mL of 29 mmol/L acetic acid solution,samples were placed in a water bath and heated for 1 h at 95–100 °C.After the samples were cooled under running water,25 μL 5 mol/L HCl was added,and the reaction mixture was stopped by agitation for 5 min with 3.5 mL of n-butanol.After centrifugation(10,000 rpm for 5 min),the separated butanol phase was removed and the fluorescence was measured in a spectrofluorometer(Schimadzu-RF-5000,Kyoto,Japan)using 525 nm for excitation and 547 nm for emission.
Proline in the plant tissues was analyzed using the method described by Bates et al.(1973):each 0.5 g of frozen roots,stems,and leaves fresh tissues were ground in liquid N2.The powder was then mixed into 1 mL aqueous sulfosalicylic acid(3%,w/v).This solution was then mixed with an equal volume of glacial acetic acid and ninhydrin reagent and held at 95°C for 1 h.The reaction was terminated by placing the container in an ice bath for 15 min,and the reaction solution was mixed with 2 mL of toluene.The absorbance of the chromophore was measured at 520 nm with a UV–VIS spectrophotometer(HACH DR/4000;model 48000,HACH Co.,Loveland,Colorado,USA)and compared with a standard.
Analysis of rhizosphere soil enzymes
Soil catalase,urease and soil phosphatase were selected as representative rhizosphere soil enzymes.The rhizosphere soil samples were collected from the root zone within 5 mm using a brush,passed through a 1 mm mesh screen,and air dried at 25°C.Samples were stored in darkness at 4°C until assayed.Five grams of the air-dried soil sample were used for each assay.The catalase activity was determined using the potassium permanganate method with 1 g of the soil titrated using KMnO4 solution at a concentration of 1×10-3mol/L(Guang 1986).The method used for the assay of urease activity in soils was described as Zantua and Bremner(1975).
Soil phosphatase activity was determined according to the method described in Tabatabai(1982).The activity of the enzymes is expressed as a soil dry weight and is given in units of l g p-nitrophenol produced g-1soil h-1.These experiments were repeated three times.
Analysis of soil nutrition
Soil samples were air dried at 25°C.A quantity of 0.5 g of the sample was added to a 100-mL Kjeldahl flask,with 5 mL concentrated sulfuric acid.The contents were gently shaken,and then heated in a digestion furnace.After cooling,5 mL of 30%(w/v)H2O2was added to the solution.The mixture was gently shaken,and then heated again for 20 min.The H2O2step was repeated until the liquid became clear,and the flask was then heated for 10 min to eliminate the excess H2O2.Distilled water was then added to make a volume of 100 mL.The solution was then analyzed for nitrogen,available phosphorus,and potassium content.
Total N was determined using the Kjeldahl method,while available phosphorus was determined colorimetrically using a spectrophotometer.A 25 mL volume of the solution was placed in a 50 mL volumetric flask and two drops of 2,6-DNP indicator was added,followed by 6 mol/L NaOH until the solution became yellow,indicating it had been neutralized.After this,10 mL 5%(w/v)solution of ammonium molybdate was added,followed by distilled water to make up a final volume of 50 mL.The solution absorbance was read at 450 nm with a spectrophotometer(Model 48000,HACH Company,Colorado,USA).K was measured using flame photometry(Birhane et al.2012).
Statistical analysis
SPSS 13.0 software was used for the one-way analysis of variance(ANOVA)at P=0.05 of differences and twoway ANOVA testing of the seedling height,collar diameter,biomass,physiological parameters,and enzyme activity between the different treatments.The figures were drawn by origin 8.5 and principal component analysis(PCA)by Canoco 5.0.
Results
Ectomycorrhizal fungus colonization rate
In this work,two S.luteus inoculation treatments were used and the mycorrhizal fungus colonization rates reached more than 65%(Fig.1).
P.sylvestris var.mongolica seedling growth
The influence of ECMF and water stress on Mongolian pine seedlings was evaluated by measuring four plant growth parameters 3 months after the inoculation(Fig.2,Table 1).Seedlings that were inoculated with S.luteus displayed the highest increase in plant height(W+S and D+S)and the highest collar diameter(W+S and D+S)compared to the control(without S.luteus).Under well watered conditions,the plant height of W+S was 26.3%higher than W-S(i.e.without S.luteus).There were significant differences between the two treatments(P≤0.05).Under water stress,the plant height with S.luteus(D+S)was 22.4%higher than it was without S.luteus(D-S).There were no significant differences between the two treatments(P>0.05),however,mycorrhizal seedlings displayed higher growth than non-mycorrhizal seedlings regardless of whether they were under drought or well-watered conditions.Similar results were observed for the biomass of the seedlings inoculated with S.luteus.The fresh weight with S.luteus of under wellwatered and water stress were 27.8 and 27.3%higher,respectively,than those without S.luteus,and there were significant differences between the two treatments(P≤0.05).The dry weight with S.luteus of under well watered and water stress were 36.4 and 33.3%higher,respectively,than those without S.luteus,there were significant differences among the two treatments(P≤0.05).
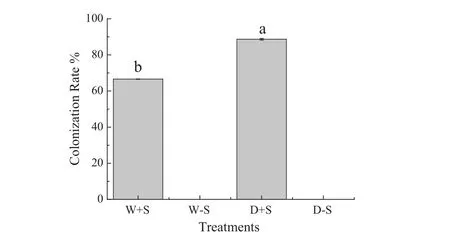
Fig.1 The colonization rates of the different treatments.Total colonization rates(mean±SD,n=20)as affected by inoculation with S.luteus and under water stress.Across all data,values with the different letter are significantly different for each treatment(P≤0.05)
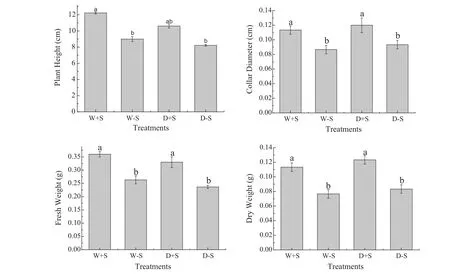
Fig.2 Effect of all treatments on seedlings growth.Growth indices(mean±SD,n=30)as affected by inoculation with S.luteus and under water stress.Across all data,values with the same letter are not significantly different for each treatment(P>0.05).Values with the different letter are significantly different for each treatment(P<0.05)
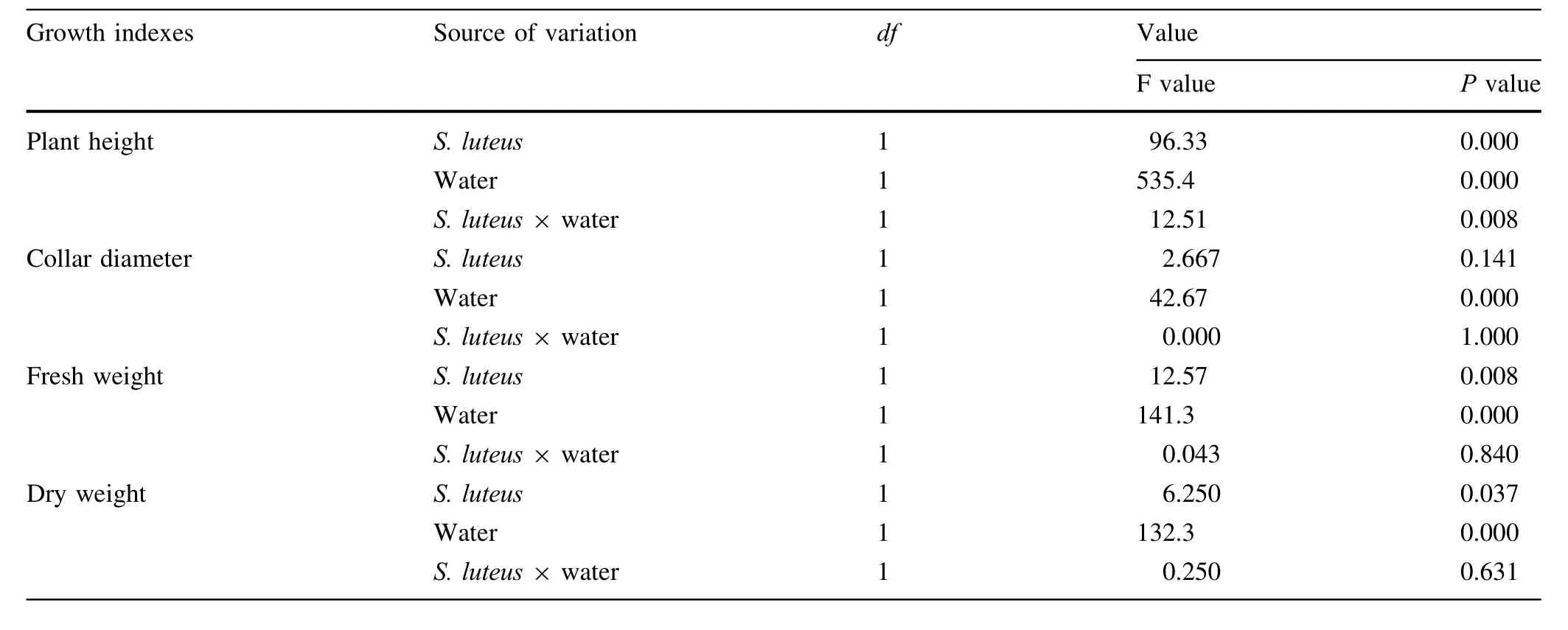
Table 1 Results of two-way ANOVA testing the effect of S.luteus and water treatment on growth
Photosynthetic pigment content of seedlings
The seedlings inoculated with S.luteus displayed the highest increase in chlorophyll a,and chlorophyll b(a+b),compared to the treatments without S.luteus,especially in the treatments under water stress(Fig.3,Table 2).In well watered conditions,the chlorophyll a and b content was 52.2 and 27.6%higher,respectively,than those of the treatments without S.luteus.Similarly,under water stress seedlings that were inoculated with S.luteus displayed the highest increase in chlorophyll a,and chlorophyll(a+b),61.6 and 37.7%higher respectively,compared to those without S.luteus.There were significant differences between all treatments(P≤0.05).
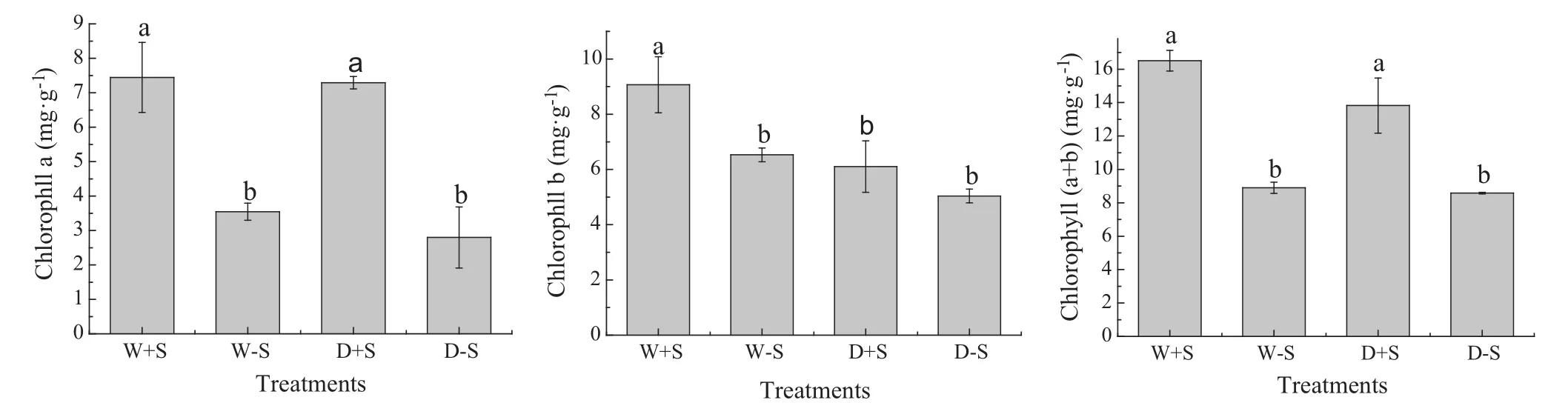
Fig.3 Photosynthetic pigment content of seedlings.Photosynthetic pigment content(mean±SD,n=10)as affected by inoculation with S.luteus and under water stress.Across all data,values with the same letter are not significantly different for each treatment(P>0.05).Values with the different letter are significantly different for each treatment(P<0.05)
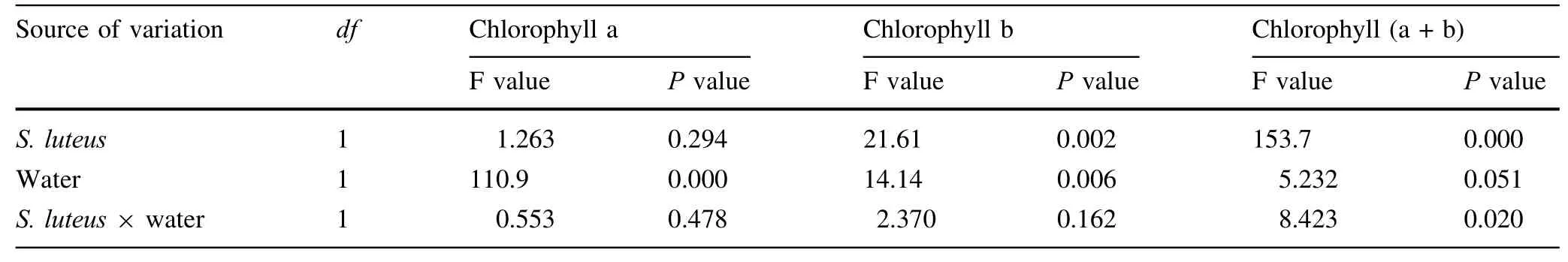
Table 2 Results of two-way ANOVA testing the effect of S.luteus and water treatment on photosynthetic pigment content
Antioxidant enzyme activities of seedlings
The influence of fungal inoculation on the antioxidant enzyme activities was evaluated by determining the activities of CAT,POD,and SOD(Fig.4,Table 3).Seedlings inoculated with S.luteus in well-watered conditions displayed the highest CAT enzyme activity compared to other treatments,and the highest POD enzyme activities under water stress compared to the treatment without S.luteus.Under water stress,the activities of POD in stems and leaves showed significant differences between treatments with S.luteus and without S.luteus(P≤0.05).The POD activity in the stem was 40%higher than that without S.luteus under water stress.In the leaf,POD activity was 35.7%higher under water stress.
Under water stress,seedlings inoculated with S.luteus displayed the highest activity in roots and leaves.However,there was no significant difference(P>0.05)on SOD enzyme activities between the two treatments D+S and D-S in the root.Similar results were observed for those in the stem and leaf.There were no significant differences(P>0.05)on SOD enzyme activity in the stem and leaf in well-watered conditions.Seedlings inoculated with S.luteus displayed higher SOD enzyme activity under water stress compared to the treatments in well-watered conditions,except in the stem.The SOD enzyme activity of D+S in the root was 46.7%higher than the treatment of W+S.There was a significant difference(P≤0.05)between the two treatments.
Content of osmotic adjustment substances of seedlings
Most of the treatments affected the soluble protein content of the seedlings.There were no significant differences(P>0.05)between the treatments with S.luteus and the non-inoculated seedlings(Fig.5,Tables 4,5).In well watered conditions,the root had the highest increment in soluble protein content compared to the treatment without S.luteus.In response to water stress,the seedlings increased the soluble protein content in stems and leaves at first.The soluble protein content in treatments D+S in the stem and leaf were 5.7 and 4.9%higher than the treatments of D-S,although there were no significant differences(P>0.05)between the treatments.
The change in soluble sugar content was mainly manifested in the roots and stems(Fig.5).The function was the same as for soluble protein.In the leaves,the content of soluble sugar increased when the seedlings were under water stress.
Good place for a subhead—the subject shifts.

Fig.4 Antioxidant enzyme activities of seedlings.Antioxidant enzyme activities(mean±SD,n=10)as affected by inoculation with S.luteus and under water stress.Across all data,values with the same letter are not significantly different for each treatment(P>0.05).Values with the different letter are significantly different for each treatment(P<0.05)
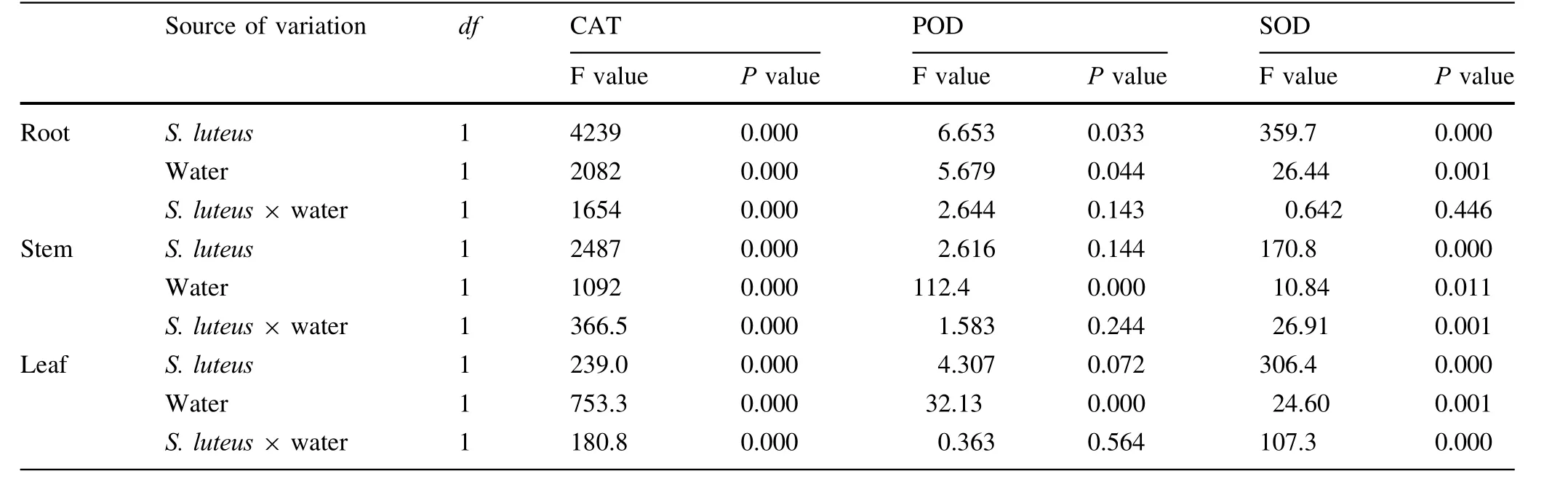
Table 3 Results of two-way ANOVA testing the effect of S.luteus and water treatment on antioxidant enzyme activities
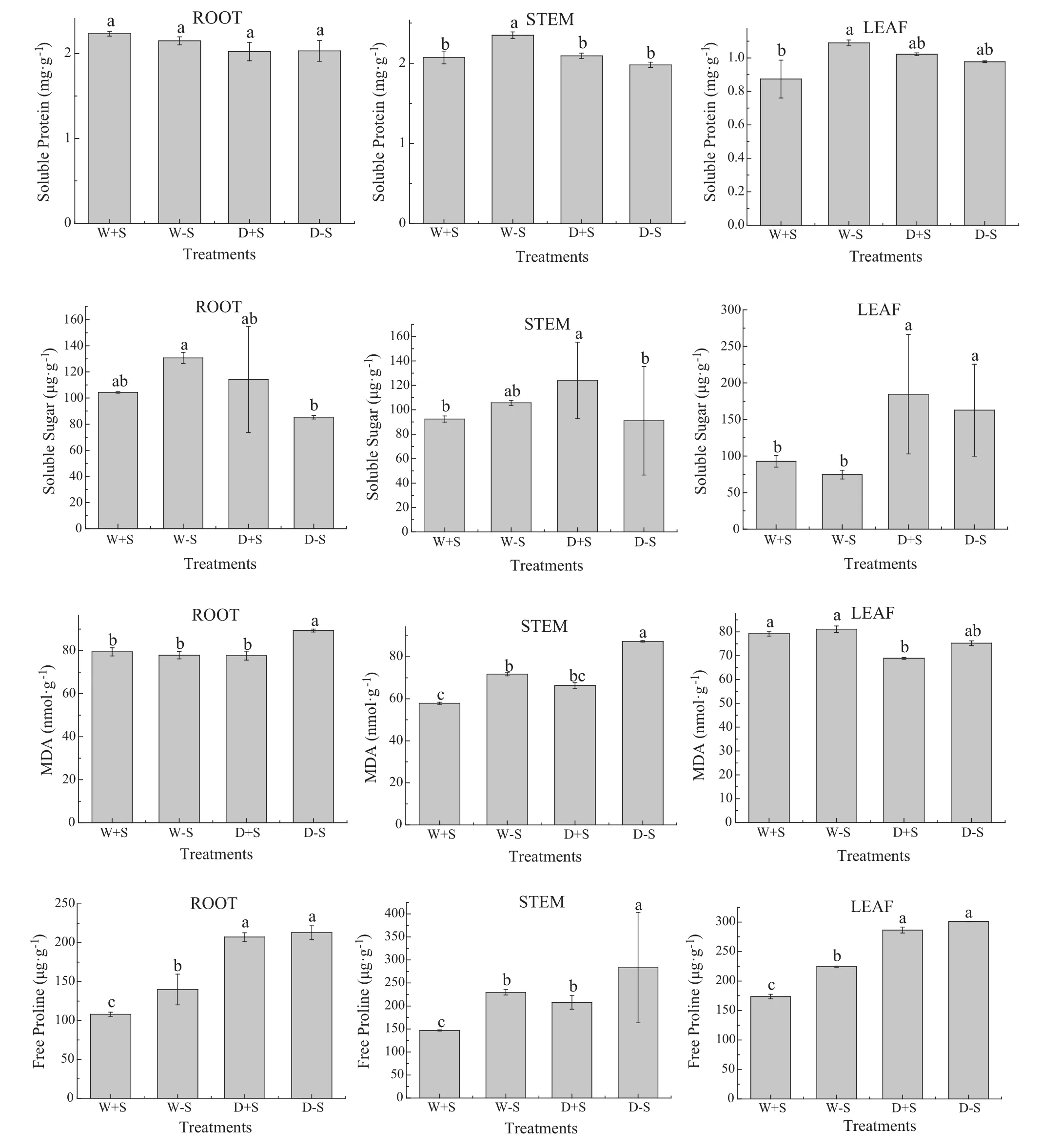
Fig.5 Content of osmotic adjustment substances of seedlings.Content of osmotic adjustment substances(mean+SD,n=10)as affected by inoculation with S.luteus and under water stress.Across all data,values with the same letter are not significantly different for each treatment(P>0.05).Values with the different letter are significantly different for each treatment(P<0.05)
In this research,the levels of MDA in the treatments with S.luteus were lower than those in the treatments without S.luteus.The D+S treatment in the root was 13.5%lower than the D-S treatments.There was a significant difference(P≤0.05)between the D+S and D-S treatments in the root.This indicates that S.luteus can reduce the level of MDA in the root when the plant is under water stress as the root was the first area to be affected by the water stress.A similar result is obtained for the level of MDA in the leaf when the plant is under waterstress.The level of MDA in the leaf was the lowest in the D+S treatment.
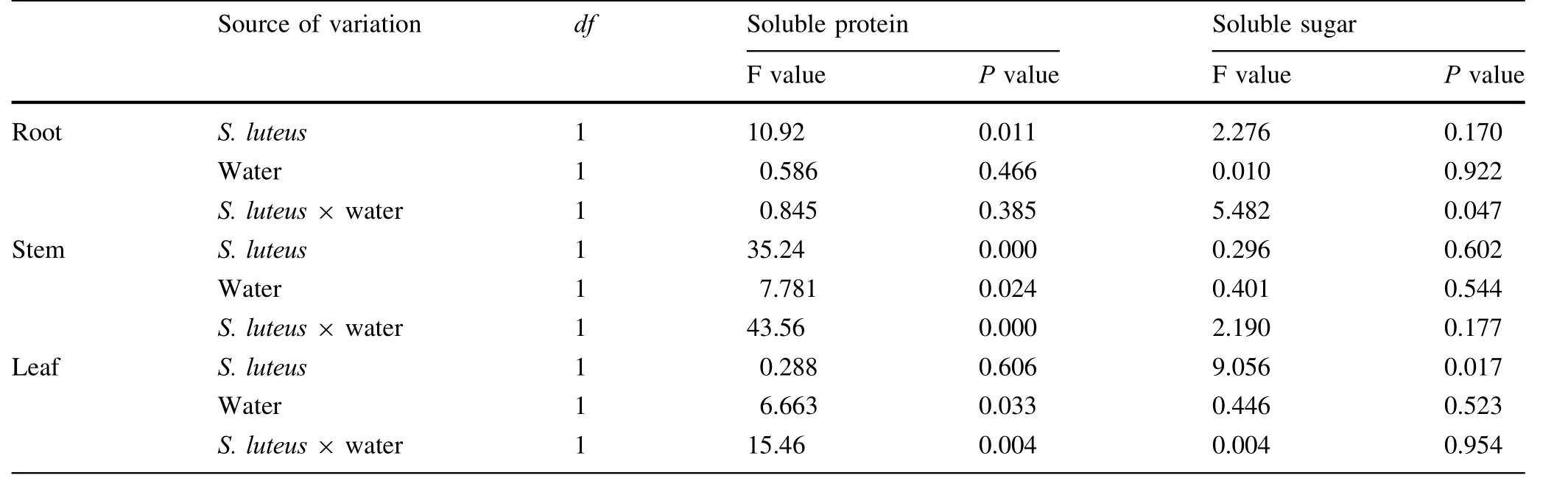
Table 4 Results of two-way ANOVA testing the effect of S.luteus and water treatment on soluble protein and sugar
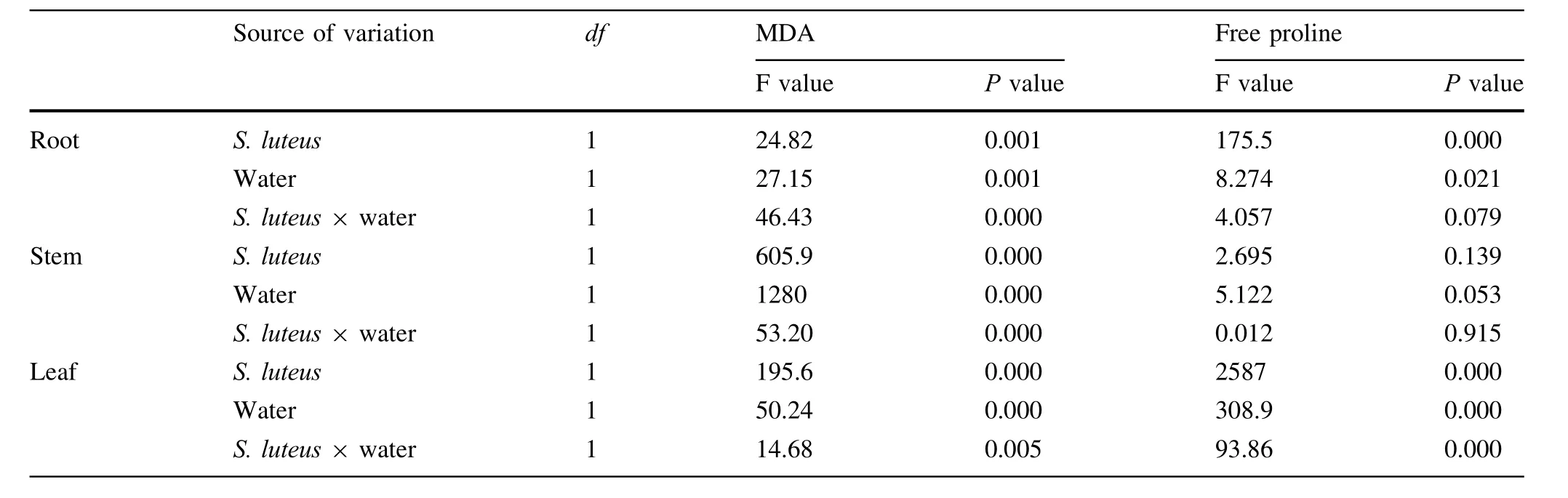
Table 5 Results of two-way ANOVA testing the effect of S.luteus and water treatment on MDA and free proline
With 1 month of water shortage,the level of free proline began to rise(Fig.5,Table 5).In the leaf,the non-inoculated seedlings under water stress reached a maximum value of 301 μg/g.The water-stressed seedlings treated with S.luteus reached a maximum value of 286 μg/g in the leaf over the same period.There were no significant differences(P>0.05)between the two treatments.
Rhizosphere soil enzymes activities
The influence of S.luteus inoculation on the rhizosphere soil-enzyme activities was evaluated by measuring catalase,urease and phosphatase activities(Table 6).Well watered,seedlings inoculated with S.luteus displayed the highest rhizosphere soil catalase activities compared to the treatment without S.luteus—about 5.3%higher.Under water stress,the seedlings inoculated with S.luteus displayed the second highest rhizosphere soil catalase activities,about 10.9%higher rhizosphere compared to the treatment without S.luteus.However,the value of W+S was higher than D+S.
In well-watered conditions,seedlings inoculated with S.luteus displayed the highest rhizosphere soil phosphatase enzyme activity,about 16.2%higher than the treatment without S.luteus.There was a significant difference(P≤0.05)between the two treatments.Under water stress,seedlings inoculated with S.luteus displayed the second highest rhizosphere soil phosphatase enzyme activity,about 11.8%higher compared to the treatment without S.luteus.There was no significant difference(P>0.05)between the two treatments.
Similar results were observed for the urease of the seedlings inoculated with S.luteus.The urease activities were higher with S.luteus than those without in both well watered and water stress conditions(Table 7).In well watered conditions,seedlings inoculated with S.luteus displayed the highest rhizosphere soil urease enzyme activity,about 21.4%higher than without S.luteus.There was a significant difference(P≤0.05)between the two treatments.Under water stress,the inoculated seedlings were about 17.5%higher than without S.luteus.There wasa significant difference(P≤0.05)between the two treatments.
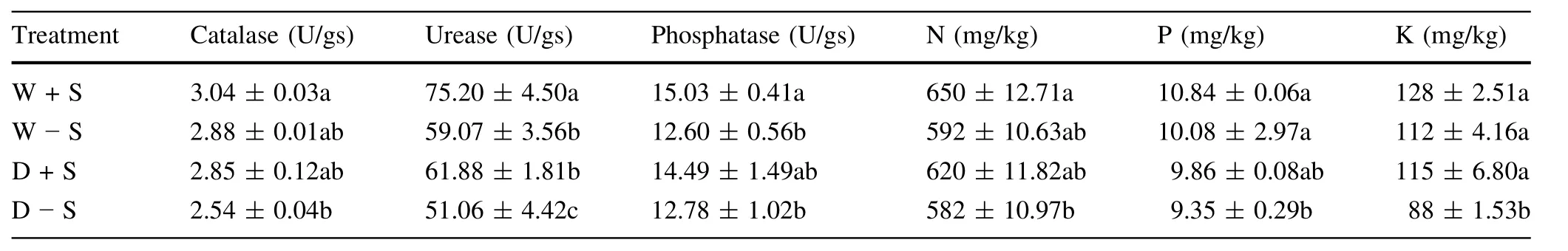
Table 6 Effect of fungal inoculation on rhizosphere soil enzymes activities and soil nutrition
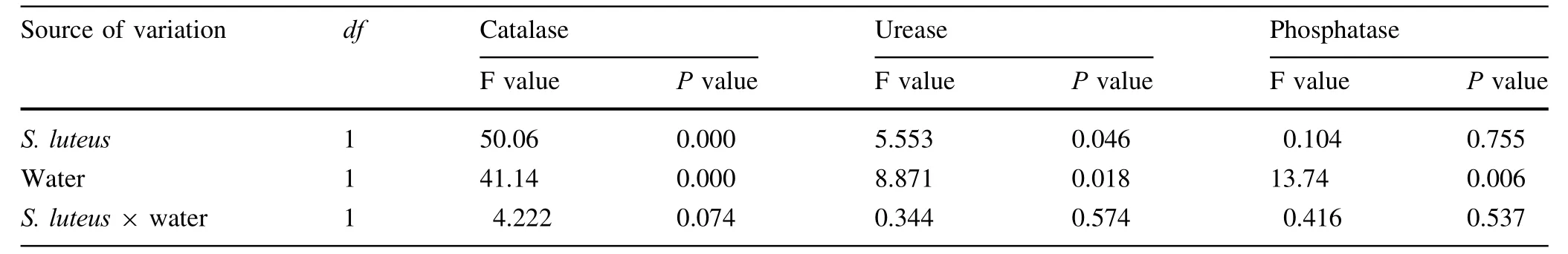
Table 7 Results of two-way ANOVA testing the effect of S.luteus and water treatment on soil enzymes activities
Rhizosphere soil nutrition
In this study,water-stress treatment resulted in a significant decrease in the nitrogen(N),phosphorus(P),and potassium(K)content of S.luteus-inoculated plant rhizosphere soil(Table 8).Under the two different water regimes,the inoculated seedlings in rhizosphere soil had higher nutrient(N,P,and K)content compared to non-inoculated seedling rhizosphere soil.Under water-stress conditions,K concentration of inoculated seedlings was 30%higher than that of non-inoculated ones;N concentration of inoculated seedlings was 6.1%higher than that of non-inoculated ones,and N concentration of inoculated seedlings was 5.2%higher than that of non-inoculated ones.Similarly,in well-watered conditions,the N,P,and K content of S.luteus-inoculated seedlings in the rhizosphere soil was higher than that of the non-inoculated seedlings in rhizosphere soil,being 8.9,7.0,and 12.5%,respectively.
Discussion
Our research showed that the roots of P.sylvestris var.mongolica form good symbiotic relationships with S.luteus.The colonization rates of the D+S treatment was higher than that of the W+S treatment,although there is no significant difference between the two treatments.It could be that there are less nutrients when the seedlings under water stress,increasing the chance of mycorrhizal colonization.However,some studies showed that the colonization rate was reduced by water stress.This phenomenon may be due to spore germination and the spread of hyphae being inhibited by drought stress(Huang et al.2011;Gong et al.2013).Our results are contrary to theirs.Perhaps more likely to be due to changes in plant exudates or in root length in response to treatment.
Previous studies have demonstrated that plant growth can be improved by mycorrhizal and other beneficial fungi(Cairney and Chambers 1999;Martins et al.1997;Martins 2004;Thomson et al.1994;Turjaman et al.2005).Inoculation with S.luteus can improve the growth of the P.sylvestris var.mongolica seedlings,affecting factors such as plant height,collar diameter,dry weight and freshweight when compared to the seedlings without S.luteus,particularly when the seedlings are under water stress.Vigorous growth was observed after inoculation with S.luteus when compared with the non-mycorrhizal seedlings.This showed that treatment with S.luteus display highest potential under water stress.
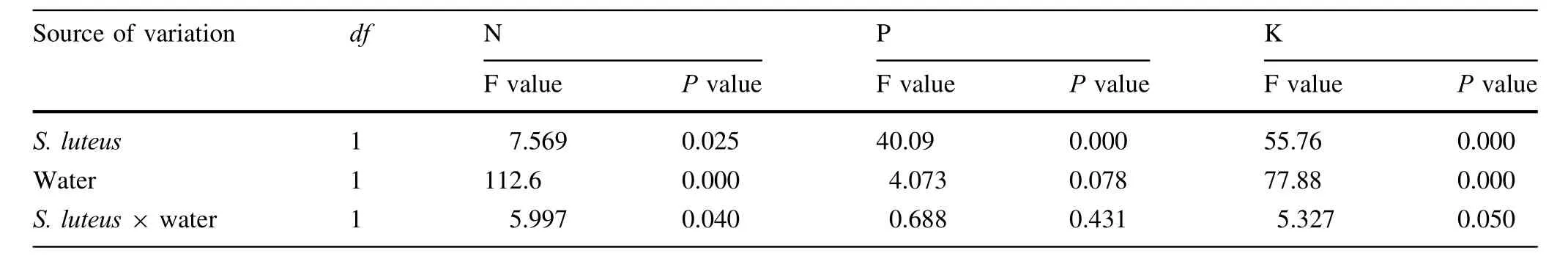
Table 8 Results of two-way ANOVA testing the effect of ECMF and water treatment on soil nutrition
In general,water stress decreases the chlorophyll content of plants.However,S.luteus inoculation may inhibit this reduction of chlorophyll content in plant leaves.In this study,the total chlorophyll content of leaves was not affected by water stress conditions in S.luteus-inoculated seedlings.There was no significant difference(P>0.05)between the W+S and D+S treatments.This result is consistent with the results of previous studies(Wu et al.2006).Maintenance of high chlorophyll content is favorable for plant photosynthesis,with S.luteus inoculated reducing the damage to photosynthesis that was caused by water stress.Thus,promoting the growth of seedlings.
Water deficiency usually increases free radicals in plant cells,resulting in oxidative stress(Ruiz-Lozano 2003).It has been well documented that increases in plant drought stress tolerance due to mycorrhizal fungi infection are related to the enhancement of antioxidant activity by mycorrhiza(Wu et al.2008;Zhang et al.2010).S.luteus colonization may reduce the concentration of the ROS in plants under water stress by improving the activity of various antioxidant enzymes,such as CAT,POD,SOD(Zhu et al.2010).In northern China,P.sylvestris var.mongolica is used as the main forest culture species,and is often planted in areas of low rainfall(i.e.arid and semi-arid areas).This low rainfall can produce ROS in plants,so seedling quality and the ability to get rid of ROS are very important at planting time(Yin et al.2014).
As Fig.6 showed,S.luteus mainly affects the change of CAT and SOD when the seedlings under water stress(root,stem,and leaf)according to the PCA.This result is consistent with the results of previous studies(Yin et al.2017).In addition,we added treatments of seedlings under water stress.However,in our study according to the PCA,POD activity was not affected significantly(Fig.6).This result indicates that the activation of antioxidant enzymatic activity by S.luteus does not always occur under adverse conditions.This result is similar to that in Zhang et al.(2014).In this work,all seedlings inoculated with S.luteus showed higher antioxidant enzyme activities(particularly CAT and SOD).This indicates that higher antioxidant enzyme activity was an important factor in mitigating environmental stress.In conclusion,it is advantageous to improve mycorrhizal fungus colonization rates when the plant under water stress.At the same time,S.luteus can activate plant antioxidant enzymatic systems and provide protection against oxidation damage in adverse conditions.
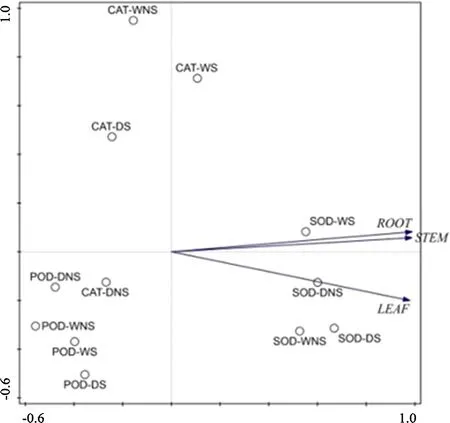
Fig.6 PCA of antioxidant enzyme activities
Previous reports indicate that to improve water absorption under drought-stress conditions,plants accumulate high amounts of soluble sugar,free proline,and soluble protein to regulate osmotic potential(Abbaspour et al.2012).Of these metabolites,soluble protein is probably the most widespread among plants(Yin et al.2017).The adjustment of the seedlings’osmotic potential by S.luteus may be the major factor enabling host plants to grow under water stress.
In this study,the soluble protein content of the root,stem and leaves of S.luteus-inoculated seedlings and noninoculated seedlings increased under water stress.As Fig.7 showed,the S.luteus mainly affects the change of soluble protein when the seedlings are under water stress(root,stem,and leaf)according to the PCA.The soluble sugar content of S.luteus-inoculated seedlings is higher than that of the non-inoculated seedlings,under water stress.This result indicates that under water stress conditions,S.luteus colonization contributes to the accumulation of carbohydrates and the reduction of the osmotic potential of the seedlings.
Higher MDA levels,measured as an index of damage to cell membrane,indicate that cell damage is greater.In the present study,the MDA levels of seedlings under water stress are higher than in the watered control indicating that the cell membranes were damaged by the drought environment.At all times,the MDA levels of seedlings inoculated with S.luteus under water stress are lower than those of the treatments of well-watered seedlings,suggesting that this inoculation is very effective at improving the drought tolerance of seedlings and reducing the MDA levels.
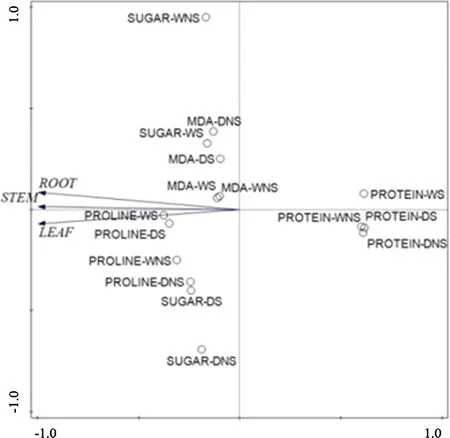
Fig.7 PCA of osmotic adjustment substances
As with MDA,free proline plays a key role in maintaining optimal growth in plant cells under potential low water conditions(Sharma et al.2011).The free proline levels increase when the plants are under water stress.In this study,free proline was higher in water-stressed seedlings than in the well-watered but was lower in water stressed seedlings treated with S.luteus treatment.The drought tolerance was thus improved by this inoculation.This phenomenon may show that mycorrhization protects seedlings from dehydration stress.Consequently,S.luteusinoculated seedlings require less proline compared to noninoculated seedlings.
Previous studies have demonstrated that mycorrhizal plants can significantly improve phosphorus content(Yin et al.2014).The insoluble phosphate in soil is dissolved by organic acids secreted by the mycorrhizal fungi and absorbed by plants.Moreover,the hyphae can expand root contact with the soil to absorb more nutrients,thereby improving the soil microenvironment(Xu et al.2012).Other studies have showed that S.luteus can also secrete enzymes to break down organic phosphorus that comes from deadwood and animal remains,to enable direct transport to plants(Ho and Zak 1979;Nannipieri et al.1980).However,there is no uni fied conclusion about this mechanism.
In this study,all treatments inoculated with S.luteus improved the rhizosphere soil enzymes of the seedlings(including catalase,urease and phosphatase).These results demonstrate that the soil enzyme activity of the seedlings was significantly improved by treatment with S.luteus,particularly those seedlings inoculated with S.luteus under water stress.Here,the rhizosphere soil enzymes inoculated with S.luteus were all higher than the treatments without S.luteus.It was shown that S.luteus played a major role in improving rhizosphere soil enzymes activities.In conclusion,S.luteus improved the soil mainly by improving the rhizosphere soil enzyme activity and improving the content of rhizosphere soil nutrition.
Additionally,S.luteus symbiosis increased P.sylvestris var.mongolica drought tolerance through the enhancement of antioxidant enzyme activity,adjustment of osmotic solutes,improvement of soil to improve nutrient acquisition,and maintenance of chlorophyll content in plants under water stress.In the arid and semi-arid areas of northeast China,the forest soil contains sufficient S.luteus loads.However,in the Horqin Sandy Land,vegetation is sparse,leading to soil degradation.In this area,we should consider using mycorrhized P.sylvestris var.mongolica seedlings for the afforestation of degraded areas to help seedlings survive the arid environment.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
- Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
