Reaction Mechanisms in the Thermal Decomposition of CL-20 Revealed by Reax FF Molecular Dynamics Simulations
REN Chunxing , LI Xiaoxia ,*, GUO Li
1 Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.2 University of Chinese Academy of Sciences, Beijing 100049, P. R. China.
Abstract: The thermal decomposition of condensed CL-20 was investigated using reactive force field molecular dynamics (ReaxFF MD)simulations of a super cell containing 128 CL-20 molecules at 800–3000 K. The VARxMD code previously developed by our group is used for detailed reaction analysis. Various intermediates and comprehensive reaction pathways in the thermal decomposition of CL-20 were obtained.Nitrogen oxides are the major initial decomposition products, generated in a sequence of NO2, NO3, NO, and N2O. NO2 is the most abundant primary product and is gradually consumed in subsequent secondary reactions to form other nitrogen oxides. NO3 is the second most abundant intermediate in the early stages of CL-20 thermolysis. However, it is unstable and quickly decomposes at high temperatures, while other nitrogen oxides remain. At all temperatures, the unimolecular pathways of N―NO2 bond cleavage and ring-opening C―N bond scission dominate the initial decomposition of condensed CL-20. The cleavage of the N―NO2 bond is greatly enhanced at high temperatures, but scission of the C―N bond is not as favorable. A bimolecular pathway of oxygen-abstraction by NO2 to generate NO3 is observed in the initial decomposition steps of CL-20, which should be considered as one of the major pathways for CL-20 decomposition at low temperatures. After the initiation of CL-20 decomposition, fragments with different ring structures are formed from a series of bond-breaking reactions. Analysis of the ring structure evolution indicates that the pyrazine derivatives of fused tricycles and bicycles are early intermediates in the decomposition process, which further decompose to single ring pyrazine. Pyrazine is the most stable ring structure obtained in the simulations of CL-20 thermolysis, supporting the proposed existence of pyrazine in Py-GC/MS experiments.The single imidazole ring is unstable and decomposes quickly in the early stages of CL-20 thermolysis. Many C4 and C2 intermediates are observed after the initial fragmentation, but eventually convert into stable products. The distribution of the final products (N2, H2O, CO2, and H2) obtained in ReaxFF MD simulation of CL-20 thermolysis at 3000 K quantitatively agrees with the results of the CL-20 detonation experiment. The comprehensive understanding of CL-20 thermolysis obtained through this study suggests that ReaxFF MD simulation, combined with the reaction analysis capability of VARxMD, would be a promising method for obtaining deeper insight into the complex chemistry of energetic materials exposed to thermal stimuli.
Key Words: CL-20; Thermal decomposition; Reaction mechanism; ReaxFF MD; Evolution of ring structure
1 Introduction
Energetic materials (EMs) are widely used in military,aerospace and civilian. 2,4,5,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20)1is one of the most promising third-generation energetic compounds due to its high density and excellent detonation performance. However, CL-20 is sensitive to external mechanical and thermal stimuli that are unavoidable during its preparation, storage and usage. It is prone to decompose and release energy that may further result in accidental fire or even explosion. Thermal decomposition is a fundamental process for any energetic materials exposed to external stimuli. It is related not only to the ignition of explosives and the subsequent detonation performance, but also to their sensitivity to various stimuli from mechanical input to direct heating2. A comprehensive understanding of thermal decomposition mechanisms is extremely important for performance and safety of EMs.
Decomposition mechanisms of CL-20 have been widely investigated in abundance by experiments and computational simulations. By isothermal thermogravimetry (TG) experiment and FTIR spectrum, Patil and Brill3proposed that the N―NO2cleavage to form NO2is one dominant pathway of CL-20 decomposition at relatively low temperatures and found that there is a dark residue with an average formula C4H4N4O2after exothermic decomposition. They further investigated the characteristics and decomposition of the residue at 700 °C and pointed out the existence of amide and azine linkages in the solid products4. Korsounskii and Nedelko5also observed the formation of condensed residue in both thermolysis and burning of CL-20 and concluded that this solid product is mainly composed of carbon and nitrogen in somewhat a form of network structure. However, the formation mechanisms of this solid products is not clear yet. Naik et al.6investigated the thermolysis of CL-20 at 800 °C by Py-GC/MS technique and observed C4products as well as a lot of small fragments from cleavage of C―N bond. Although lots of efforts are made to understand the thermal decomposition of CL-20, the overall chemical mechanisms during thermolysis remain obscure due to the extremely fast and complex reactions involved, which is difficult to be fully captured experimentally.
Quantum mechanics (QM) approach has been employed in reaction mechanisms investigations of the thermal decomposition of CL-20. Okovytyy and coworkers7calculated the unimolecular decomposition reactions of CL-20 by DFT and proposed several pathways including N―NO2bond cleavage,HONO elimination and ring-open reactions. Isayev et al.8confirmed that the N―NO2bond fission is the only distinct initial reaction pathway of CL-20 dissociation in gas phase by ab initio molecular dynamics (AIMD) simulations and provided a reaction scheme of unimolecular CL-20 decomposition. But the mechanisms for condensed phase decomposition of CL-20 still needs more investigations. Xue et al.9concluded that the dissociation of CL-20 is initiated by either N―NO2bond fission or C―N bond rupture in performing the self-consistent charge density-functional tight-binding (SCC-DFTB) molecular dynamics simulations of shocked CL-20. Using ReaxFF force field, the cleavage of N―NO2bond has also been confirmed as the initial step in decomposition of CL-20 by reactive molecular dynamics simulations (ReaxFF MD) of CL-20-based cocrystals10. Yan et al.11proposed that the decomposition of CL-20 is triggered by releasing O radicals of nitro groups to form oxygen. The ReaxFF MD approach has been widely used in investigating the complex physical and chemical behaviors of condensed EMs induced by impact12, shock13, electric field14and heat11,15.
Despite of the many discussions about the decomposition of CL-20, comprehensive reaction mechanisms about the initial decomposition and the subsequent fragmentation process in thermolysis of condensed CL-20 are rarely reported in details,which is essential for the handling and application of CL-20 in practice. This paper focuses on the identification of the varied intermediate structures and investigation of the comprehensive chemical reaction pathways of initiation and fragmentation in thermal decomposition of condensed CL-20. A series of ReaxFF MD simulations of a large-scale CL-20 super cell were performed at isothermal conditions in a wide temperature range from 800 K to 3000 K. The important intermediates and overall reaction pathways were obtained by taking advantage of the unique code VARxMD16for reaction analysis developed in the authors’ group. Simulation details including model construction,simulation strategies and reaction analysis methods are presented in Section 2. Discussions about the evolutions of intermediates, products and reaction pathways are presented in Section 3. The conclusions are provided in Section 4.
2 Simulation methods
2.1 Model construction and simulation strategies
CL-20 has a polycyclic structure with the chemical formula of C6H6N12O12as shown in Fig. 1. There are two five-membered rings and a six-membered ring in the isowurtzitane cage of CL-20. It consists of six N―NO2bonds totally, with four within the two five-number rings and the other two belonging to the sixnumber ring. Under ambient temperatures and pressure, there are four polymorphs of CL-20 crystal (α-, β-, γ-, ε-)17. Among these polymorphs, the most stable crystal is ε-CL-20 that has been widely used and well investigated6,7,9,18. Very limited work on the decomposition of the less stable β-form was reported3,4. It worth to be noted that the molecular conformation of β-CL-20 can be found in many of the CL-20 based co-crystals including CL-20/TNT, CL-20/HMX, CL-20/DNB and CL-20/BTF19.Understanding the decomposition of β-CL-20 is of interest.Therefore, a model of β-CL-20 was constructed to investigate the thermal decomposition mechanisms of CL-20 using Materials Studio20. The model contains 128 CL-20 molecules.The initial unit cell of β-CL-20 was obtained from the Cambridge Crystallographic Data Centre21. A super cell of 4 ×4 × 2 was built by expanding along with the corresponding directions respectively as shown in Fig. 1. After energy minimization with conjugate gradient algorithm, the atom coordinates and cell parameters of super cell were relaxed at 300 K and 0 GPa with ReaxFF molecular dynamics, using NPT ensemble with a time step of 0.25 fs to relieve the internal stress.The force field version of ReaxFF-lg22optimized for better description of lattice parameters and density of EMs molecule crystals was used. The Nose-Hoover thermostat and barostat were used with damping constants of 25 fs for temperature and 250 fs for pressure. After relaxation of 100 ps, an equilibrium model was obtained with an average density of 1.938 g·cm−3calculated from the last 10 ps sampling. The density value predicted by ReaxFF-lg at 300 K is in good agreement with the experimental value 1.989 g·cm−321, which suggests that the ReaxFF molecular dynamics simulation can reasonably describe the physical properties and crystal structures of energetic materials.
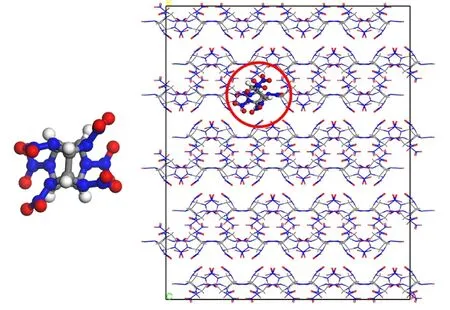
Fig. 1 Initial super cell of CL-20 with 4608 atoms.
To understand the thermal decomposition of CL-20, NVT simulations by ReaxFF MD were performed using ReaxFF-lg for 500 ps. The simulation temperatures are 800 K, 1000–2000 K with an internal of 250 K, and 3000 K. Before each of the isothermal simulations, the system was preheated from room temperature 300 K to a targeted temperature at a fast heating rate of 500 K·ps−1. During these simulations, a time step of 0.1 fs was chosen. Berendsen thermostat with a damping constant of 100 fs was used to control the temperature. Three parallel simulations were performed at each conditions. All the molecular dynamics simulations were performed using the LAMMPS package24.
2.2 Simulation analysis
It is challenging to obtain a full picture and detailed chemical mechanisms for large molecular systems with complicated and ultrafast reactions during the condensed-phase thermal decompositions of CL-20. VARxMD was employed to identify intermediates, generate the whole reaction list with reaction site details, and investigate the bimolecular reaction pathways. The trajectory internal for analysis is 500 fs. VARxMD was developed to analyze the trajectories from ReaxFF MD simulations automatically by Liu et al.25, which has played unique roles in investigating the reaction mechanisms of large scale reactive system with complex chemistry, including pyrolysis of coal26, polyethylene27, lignin28, cellulose29, pnitrophenol30as well as combustion of RP331and bio-oil32and soot formation33. Therefore, VARxMD was used to obtain the evolutions of different species and a dynamic description of CL-20 decomposition.
3 Results and discussion
3.1 Product distribution in thermal decomposition of CL-20
3.1.1 Product profile in thermolysis simulation of CL-20 at 3000 K
To have an insight into the overall chemical events, the temporal evolutions of CL-20 and major intermediates and products generated during the thermal decomposition at 3000 K are depicted in Fig. 2. It should be noted that all the quantities of species mentioned in this paper are averaged over the initial number of CL-20 to facilitate comparisons of intermediates and products generation. The time for the first consumption or formation of each species is listed in Fig. 2. As shown in Fig. 2a,nitrogen dioxide (NO2) is produced at 1.3 ps just as CL-20 start to decompose, which agrees with that the homolytic fission of N―NO2bond is a trigger of the decomposition of CL-203,8.With a sharp decrease of CL-20 molecules, a boom of NO2molecules are generated extremely fast with its quantity accumulating to 2.54 per CL-20 in a short time of 5.0 ps, which dominates the initiation stage of the thermolysis of CL-20.Following the formation of NO2, nitrate molecules (NO3), nitric oxide (NO) and nitrous oxide (N2O) appear successively. After the formation of these nitrogen oxides (NOx), many intermediates of hydrogen-abstraction and hydroxide radicals(OH) are produced. Particularly, HONO appears behind NO2with small quantity, which plays less significant role than NO2in initial decomposition of CL-20. These major intermediates of nitrogen oxides and hydrogen-abstraction participate in the subsequent reactions and decrease gradually. Most of these species are quickly consumed in simulation of 50 ps at 3000 K.As shown in Fig. 2b, nitrogen (N2) molecule is one of the major products that its formation starts very early at 3.6 ps before all CL-20 molecules dissociate, followed by the formation of other major products. The moments for the first molecule generation of other products are 4.2 ps for H2O, 6.2 ps for CO, 6.3 ps for CO2and 6.6 ps for H2respectively. Among these decomposition products NO2, NO, N2O, N2, H2O, CO2, CO and H2were all observed in experiments6,34, and the formation of HONO and OH were reported by ReaxFF MD simulations9,11.
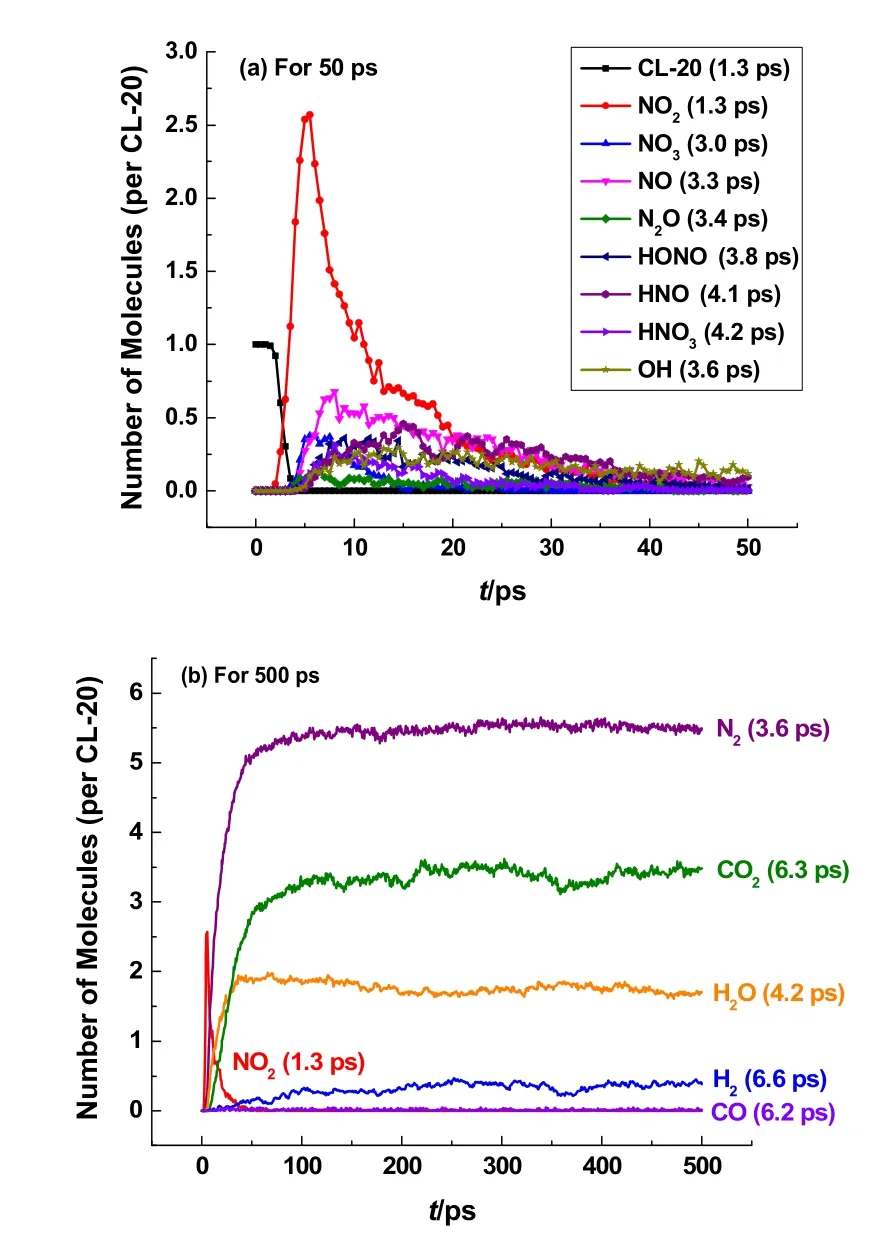
Fig. 2 Temporal evolution of CL-20 and main intermediate species and the time for their first appearing during thermolysis of CL-20 obtained in ReaxFF MD simulation at 3000 K.(a) for 50 ps, (b) for 500 ps.
It can be concluded from Fig. 2 that the 500 ps simulation at 3000 K can give a scenario close to the end of CL-20 thermolyis,because almost all the major intermediates are run out and the quantities of final products tend to equilibrium values. The numbers of these final gas products averaged over CL-20 molecules at the end of the simulation are listed in Table 1.Surprisingly, the quantities of N2, H2O, CO2and H2generated at 3000 K are in very good agreement with the product quantities of CL-20 detonation in Simpson’s experimental work34, except for the lower quantity of CO obtained in ReaxFF MD. Compared with AIMD simulation of only eight CL-20 molecules at 3000 K by Isayev8, the ReaxFF MD simulations of 128 CL-20 molecules in this work shows better agreement with experiment measurements. The quantity of H2obtained in the ReaxFF MD simulation coincides well with experiment value, while none is observed in AIMD simulations. This quantitative consistency provides solid validation for the reasonableness of the ReaxFF MD simulations performed in this work, which gives substantial support for the reaction pathways revealed in section 3.2.
3.1.2 Temperature influence on thermolysis of CL-20
To evaluate the temperature effects on CL-20 decomposition,the time for the decomposition initiation and the time for running out of all CL-20 molecules are summarized in Table 2 fortemperature ranges of 800–3000 K. The time of initiation for CL-20 decomposition is defined as the time when the consumption ratio of CL-20 molecules reaches around 2% at different temperatures. It is found that the initiation time is almost the same when temperature is above 1000 K, however the time for CL-20 exhaustion decreases as temperature increases.The total time for the consumption of all CL-20 molecules decreases sharply at temperature of 1000–1500 K, indicating that the decomposition rate of CL-20 increases quickly with temperature. All CL-20 molecules are used up in less than 5 ps when the temperature is at or over 1500 K. The condition of 800 K is a relatively low temperature for CL-20 themolysis simulation that the initiation time is the largest and there are still 29 CL-20 molecules (about 22.7%) left after the same 500 ps simulation.
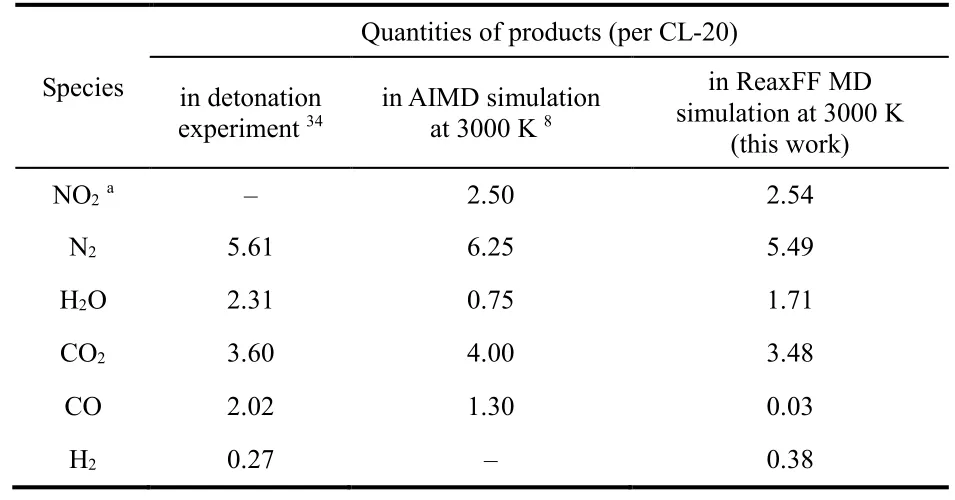
Table 1 Comparison of gas product distribution of CL-20 decomposition in detonation experiment, AIMD simulation and ReaxFF MD simulation at 3000 K.
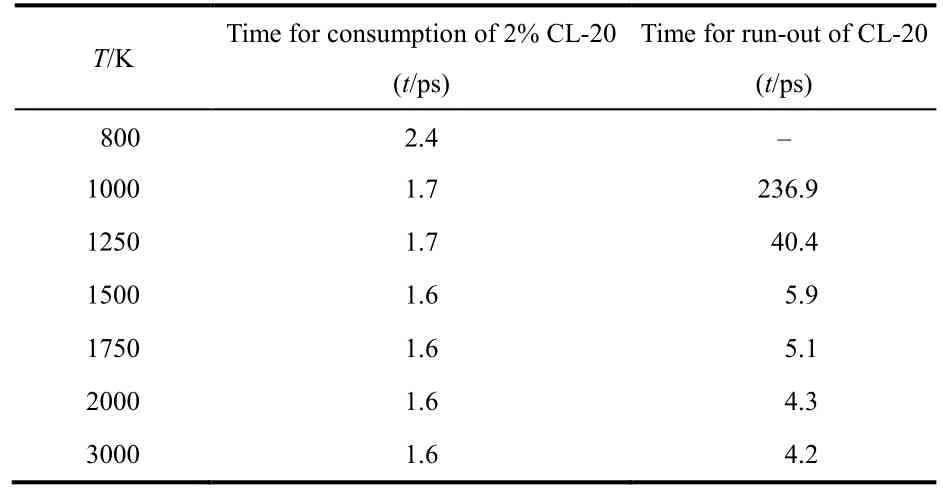
Table 2 Time for the start and end of CL-20 consumption during the thermolysis simulations at temperatures of 800–3000 K obtained by ReaxFF MD.
Temperature effects on the temporal evolutions of NOxintermediates during the CL-20 thermolysis are displayed in Fig.3. The plots for these intermediates in Fig. 3 are organized in a sequence of their generation. Thus, NO2is shown first in Fig. 3a for its very early generation when the CL-20 molecules begin to decompose. The number of NO2increases to a peak and then decreases gradually. The higher the temperature is, the earlier the peak appears. NO2is the most abundant intermediate observed in the simulations. At 3000 K, the maximal peak value of 2.54 per CL-20 agrees well with the AIMD observation at the same temperature as shown in Table 134.
The evolutions of other nitrogen oxide intermediates are similar to NO2during the themolysis simulations, as shown in Fig. 3b–d. The formation of nitrate molecules (NO3) is right behind NO2. As described in Fig. 3b, NO3is the second abundant intermediate generated during the thermolysis of CL-20.However, the maximum value of NO3decreases when temperature increases, which is different from NO2. This observation suggests that NO3is unstable at high temperatures.The generation of NO3was also observed in thermal decomposition of HMX35, and in shocked PETN36as well as HMX37. Although NO3has not been detected experimentally in decomposition of nitroamine explosives, it was proposed that the generation of NO3is both thermodynamically and kinetically possible drawn from quantum calculations of RDX decomposition18b. In our simulation, the possible reaction pathways for generation and consumption of NO3have been elucidated, which will be discussed in Section 3.2.3.
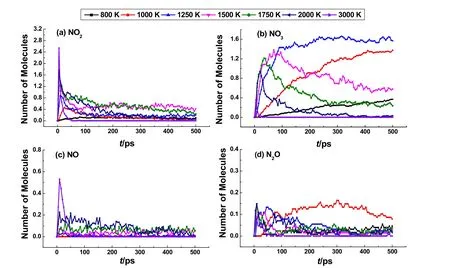
Fig. 3 Evolution of intermediates during the thermolysis simulations of CL-20 at temperatures of 800–3000 K obtained by ReaxFF MD.(a) NO2, (b) NO3, (c) NO, (d) N2O.
The formation of NO and N2O has been reported experimentally in thermal decomposition of CL-205a,6,18a. As shown in Fig. 3c, the quantity of NO is smaller compared with NO2and NO3. When the temperature increases over 2000 K, the speed of generation and the maximum quantity of NO increase a lot, implying that high temperatures favor the generation of NO.It can be observed that NO molecules still exist after the running out of NO3at 2000 K, showing high thermal stability. In addition, the generation of laughing gas (N2O) is observed during the thermolysis simulation of CL-20. As shown in Fig.3d, the maximum quantity of N2O is about 0.15 per CL-20 and varies little at temperatures of 1000–2000 K. Despite of small quantity, N2O is relatively stable and not used up during the simulation at 2000 K. The relatively lower stability of NO3compared with NO2, NO and N2O at high temperatures of 2000 K and 3000 K may explain its hard detection in experiments.
The final products of N2, H2O and CO2obtained after 500 ps simulations in the thermolysis process of CL-20 at different temperatures of 800–3000 K are described in Fig. 4. Only a few of N2molecules are generated at low temperature of 800 K. The H2O molecules appear when temperature increases to 1000 K,reaching to its maximum quantity at 2000 K and decreasing at 3000 K. The quantity of CO2molecules becomes notable until the temperature increases to 1500 K, and keeps increasing at 1500–3000 K. The product distributions obtained from isothermal simulations indicate that the simulation of 500 ps is not long enough to get an equilibrium profile of the final products when the temperature is below 3000 K. It should be noted that some H2O molecules will dissociate due to the high temperature, leading to a slight reduction of its quantity at 3000 K, as shown in Fig. 2b. The decrease of H2O quantity caused by dissociation at 3000 K may be related to the fact that no gas can escape away in NVT simulations. The quantity of H2O at 2000 K is about 2.33 per CL-20, which agrees better with Simpson’s measurement of water34than the simulation of 3000 K in this work.
3.2 Reaction pathways
3.2.1 Initial decomposition of CL-20
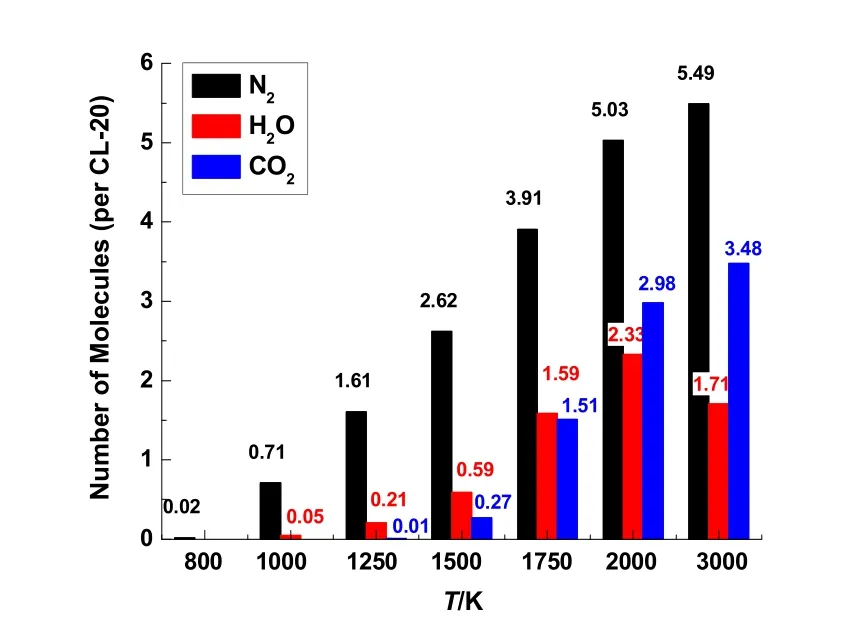
Fig. 4 Product distribution of CL-20 thermolysis at 500 ps in isothermal ReaxFF MD simulations of 800–3000 K.
To understand the whole chemical process of CL-20 thermolysis, the simulation trajectories were analyzed systematically with the aid of VARxMD. A skeleton map of initial decomposition pathways for the thermolysis of CL-20 was obtained, which is shown in Fig. 5. The detailed species and chemical structures of these intermediates in Fig. 5 are presented in Table 3. The thermal decomposition of CL-20 starts with the cleavage of N―NO2bond to form NO2and CL-20-INT I radicals (P I-1), which is a unimolecular pathway of many CL-20 molecules dissociating. CL-20-INT I radicals are generated after the departure of nitro groups either in five-membered ring or six-membered ring as shown in Table 3, which is consistent with the QM calculations by Okovytyy7. The quantity of N―NO2rupture from five-membered rings is more than that from six-membered ring at all temperatures. This agrees with the conclusion that the N―NO2bonds in five-membered ring was a little bit weaker than that in six-membered ring by DFT calculation of gas phase CL-207. Subsequently, CL-20-INT I species will undergo β-scission of C―N bond and H-shift to form CL-20-INT IV (P II-1). Alternatively, CL-20-INT I will undergo direct fragmentation (P III-1) by cleavage of N―NO2bonds, C―N bonds and C―C bonds into C4 (CL-20-INT V) or C2 intermediates (CL-20-INT VI) and release NOx(NO2, NO,NO3and N2O), which is the similar pathway for further conversion of CL-20-INT IV into CL-20-INT V or CL-20-INT VI (P III-2).
Except for the cleavage of N―NO2to form CL-20-INT I,some of the CL-20 molecules will dissociate in an alternative unimolecular pathway with scission of C―N bonds, leading to the ring-opening of the CL-20 skeleton and the formation of CL-20-INT II radicals (P I-2). Since the bond dissociation energy of C―N is larger than that of N―NO2bond7, it is less likely to initiate the dissociation of CL-20 molecule through direct ringopening by cleavage of C―N bonds. However, experimental results indicate that the products in condensed decomposition of nitroamine explosives RDX, HMX and CL-20 are mainly formed from C―N bond cleavage6,38, which suggests that the ring-opening reaction by C―N bond cleavage is a dominant pathway in CL-20 thermolysis. More interestingly, ring opening by C―N bond rupture was also observed to trigger the dissociation of CL-20 in shocked CL-20 at all shock velocities of 8 to 11 km·s−1from SCC-DFTB MD simulations9. Therefore,the initial pathway of ring-opening by C―N bond cleavage obtained in the ReaxFF MD simulations is reasonable in condensed thermal decomposition of CL-20. Within CL-20-INT II, hydrogen of β-carbon tends to transfer to the unsaturated nitrogen atom. Eventually CL-20-INT II intermediates convert to CL-20-INT V or CL-20-INT VI by fragmentation (P III-3),which is similar to pathway P III-1. Alternatively, the N―NO2bond in CL-20-INT II breaks up to form NO2and CL-20-INT IV(P II-2) that further undergoes pathway P III-2.
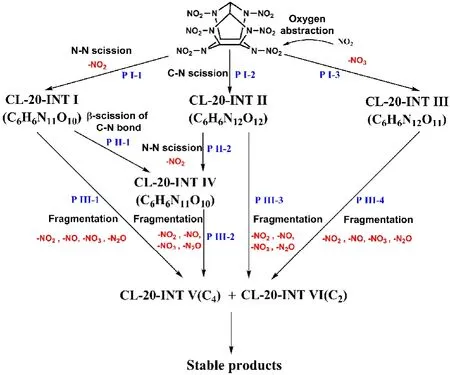
Fig. 5 Initial reaction pathways in decomposition of CL-20 molecules.
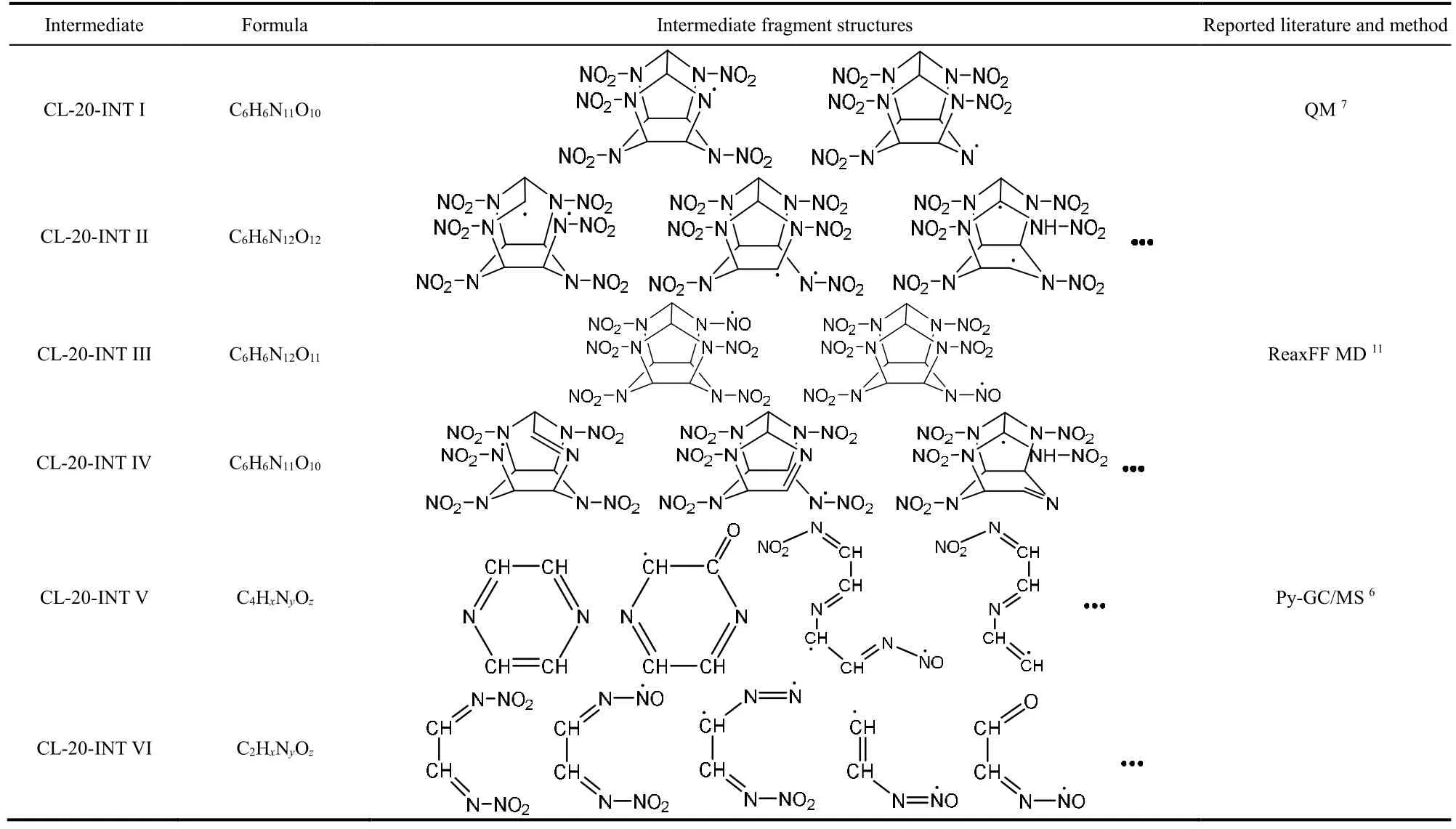
Table 3 Examples of intermediates and their chemical structures in Fig. 5 for initial pathways of CL-20 thermolysis obtained in ReaxFF MD simulations.
In addition to the two unimolecular dissociation pathways (P I-1 and P I-2) described above, a bimolecular pathway is observed to destroy the CL-20 molecules. At low temperatures,some of the early generated NO2will attack the oxygen in the nitro groups of adjacent CL-20 molecules, followed by the break-up of N-O bond in nitro groups to form NO3and CL-20-INT III (P I-3). The pathway of oxygen abstraction to form NO3observed in this work has not been reported in previous investigations of CL-20 thermolysis and CL-20 involved thermal decomposition. Interestingly, the pathway was proposed in quantum chemistry calculations of RDX thermal decomposition by Irikura et al.18band in ReaxFF MD simulations of shocked HMX37. The CL-20-INT III intermediates lack of an oxygen atom were observed in Yan’s work11. The CL-20-INT III intermediates will break down into C4 and C2 fragments,together with the formation of a lot of small molecules (P III-4).
To investigate the temperature effects on the initial reaction pathways of CL-20 thermolysis, the numbers of the initial decomposition reactions of CL-20 (P I-1 to P I-3) at different temperatures are summarized in Fig. 6. It should be mentioned that multiple bond cleavage will occur simultaneously during the time internal of 500 fs for reaction analysis due to the enhanced reactivity of CL-20 dissociation at high temperatures. A single decomposition reaction of CL-20 with simultaneous cleavage of N―NO2bond and C―N bond may be counted into the number of both P I-1 and P I-2. Thus the total number of the reaction of CL-20 decomposition in Fig. 6 is larger than the initial number of CL-20. As shown in Fig. 6, the unimolecular decomposition pathways of P I-1 and P I-2 govern the initial dissociation at all conditions. The cleavage of N―NO2bond (P I-1) dominates at high temperatures. Especially, serial scission of two to four N―NO2bonds in a single CL-20 molecule can be observed from the ReaxFF MD simulation at and over 1500 K, which agrees with the reaction pathways proposed in AIMD simulations8. The serial cleavage of more than one N―NO2bonds takes place more frequently at elevated temperatures. However, the ringopening reactions by C―N bond cleavage (P I-2) are not as sensitive to temperature as the N―NO2bond cleavage in triggering the dissociation of CL-20. It can be found that C―N bond scission is dominant at 1000 K. About 17% of CL-20 molecules are destroyed in the bimolecular pathway P I-3 by oxygen-abstraction at 1000 K. But high temperatures are disadvantage to this pathway P I-3.
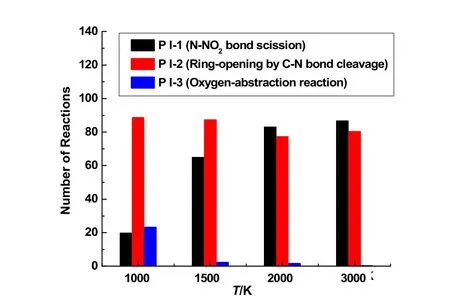
Fig. 6 Numbers of the initial decomposition reactions of CL-20 molecules obtained in three parallel ReaxFF MD simulations of CL-20 thermolysis at 1000–3000 K.
3.2.2 Mechanisms of fragmentation
To understand the fragmentation mechanism, the evolution of product in fragmentation process and various ring structures at 1000 K were investigated. The temporal evolutions of CL-20-INT V (C4), CL-20-INT VI (C2), fragments with six carbons, as well as CL-20 in the thermolysis simulation at 1000 K are analyzed and shown in Fig. 7. Due to the decomposition of CL-20 molecules, C6fragments first appear through the pathways P I-1 to P I-3. Subsequently C4(CL-20-INT V) or C2 (CL-20-INT VI) are generated mainly from the consumption of C6. At the end of 500 ps simulation, the amounts of C4and C2fragments are still increasing and about 40% of C6remains. The simulation results may suggest that C4(CL-20-INT V) fragments can be stable pyrolyzates of CL-20 at 1000 K, which roughly agrees with the detection of C4species in CL-20 thermolysis at 800 °C by Py-GC/MS6. As listed in Table 3, the chemical structures of CL-20-INT V and CL-20-INT VI intermediates obtained in the simulations mainly consist of single and double bonds of NO,CN and CC, which supports the existence of NO, CN and CC bonds of single or double in solid residues formed in thermolysis of CL-20 at low temperature3. More interestingly, the pyrazine(C4N2H4) listed as the first in CL-20-INT V of Table 3 should be the prominent fragment product of C4H5N2+detected in thermolysis of CL-20 at 800 °C by Py-GC/MS technique6.
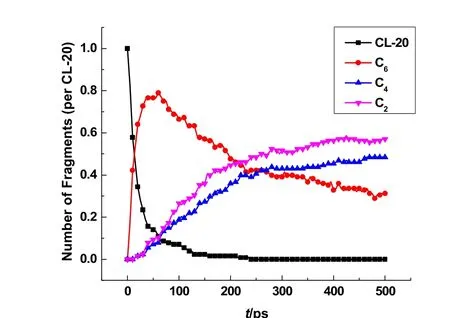
Fig. 7 Temporal evolution of CL-20 molecules and different fragments in the ReaxFF MD simulation of CL-20 thermolysis at 1000 K.
To have a deeper understanding of the fragmentation mechanism, the intermediate fragments with various ring structures are categorized into four groups with the aid of the structure analysis function in VARxMD. The evolutions of ring structures and CL-20 molecules in the thermolysis simulation at 1000 K are summarized in Fig. 8. As shown in Fig. 8, the fragments F1 are fused tricycles pyrazine derivatives with the same skeleton ring structure of CL-20. F1 can be observed very early during the simulations, and its maximal amount accounts for about 10 percent of CL-20 molecules. The F1 fragments can exist for 400 ps and will decompose almost completely at the end of 500 ps simulation. The observation suggests that F1 is an initial intermediate for CL-20 themolysis, which supports the proposed pathway to form an aromatic fragments of 1,5-dihydrodiimidazo[4,5-b:4′,5′-e]pyrazine in the unimolecular decomposition of CL-20 by DFT calculations7and the explanation for the fast thermolysis experiments of CL-20 at 800 °C6.
As shown in Fig. 8, more than half of the CL-20 molecules decompose to form fused bicycles pyrazine derivatives (F2) by C―N bond cleavage of one five-membered ring in the CL-20 structure, which dominates the initial products of fragmentation process. Subsequently, another five-membered ring in the CL-20 structure dissociates to form lots of pyrazine ring fragments (F3),leading to the quantity increase of F3. Such decomposition pathway of CL-20 into fragments F2 and further to fragments F3 roughly agrees with the proposed pathway for CL-20 themolysis in Naik’s work6. As presented in Fig. 8, the number of fragments F3 with single pyrazine ring keeps increasing to 0.4 per CL-20,indicating the relatively high stability of pyrazine structure at 1000 K that allows of its detection in Py-GC/MS at 800 °C6.Additionally, the fragments with substructure of single imidazole ring (F4) can be generated at the early stage in CL-20 decomposition. But the formed fragments F4 with small amount are run out quickly by ring-opening reactions, which explains the fact that five-membered ring species are not detected in Py-GC/MS experiment at 800 °C6. Among these pyrazine derivatives (F1, F2 and F3), the fragments with substructure of single pyrazine ring (F3) are found more stable than the fragments with fused ring structure (F1 and F2).
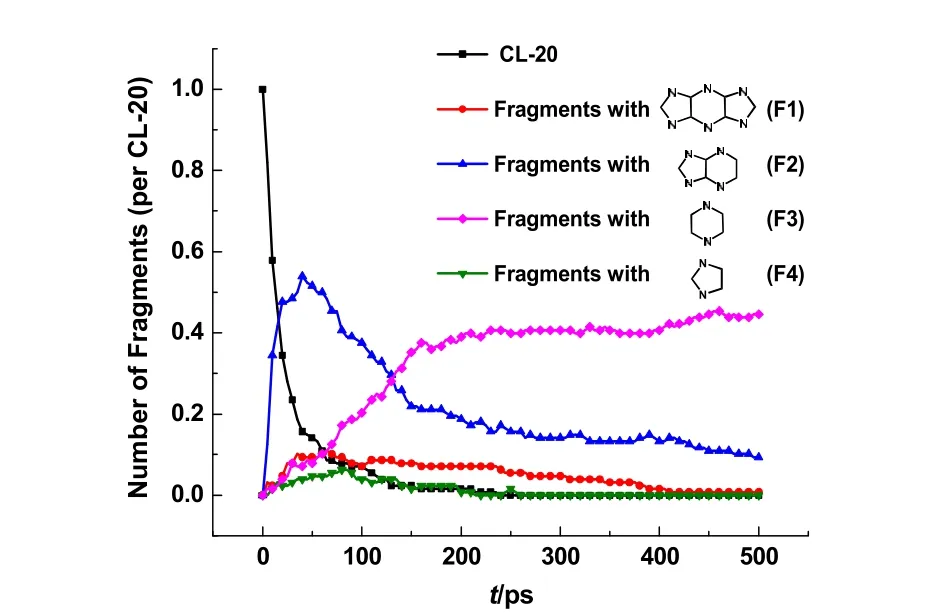
Fig. 8 Temporal evolution of CL-20 molecules and fragments with different ring structures obtained during the ReaxFF MD simulation of CL-20 thermolysis at 1000 K.
To investigate the stability of the single pyrazine ring (F3)further, the temperature effects on its stability were evaluated.The evolutions of fragments F3 in the thermolysis simulation of CL-20 at 1000–3000 K are shown in Fig. 9. At 1000 K and 1250 K, the single pyrazine ring is stable in a state of continuous accumulation within 500 ps simulation period. It can be observed that some single pyrazine ring will dissociate at the early stage around 1500 K, leading to a quick and slight reduction of quantity. Then the decrease becomes very slow and the amount of F3 tends to a plateau till the end of 500 ps simulation. Similar evolution of single pyrazine ring can be observed at 1750 K and 2000 K, except for 3000 K. These simulation results indicate that the single pyrazine ring structure is very stable. The high stability of fragments F3 may be likely responsible for the formation of the solid residue at low temperature. These simulation results on ring structures of pyrazine derivatives (F1–F3) obtained in the ReaxFF MD simulations of CL-20 themolysis at 1000 K, broadly agree with the proposed intermediate pyrazine structures and pathways in the CL-20 thermolysis at 800 °C by Py-GC/MS6. The pyrazine structure evolution obtained in the ReaxFF MD simulations can provide additional insight on the fragmentation mechanism in CL-20 thermolysis.
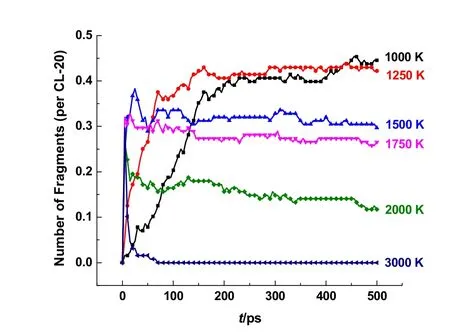
Fig. 9 Temporal evolution of fragments F3 with pyrazine ring obtained in the ReaxFF MD simulations of CL-20 thermolysis at 1000–3000 K.
3.2.3 Reaction pathways to the formation and interactions of nitrogen oxides
In addition to the formation of fragments F1–F4 by a series of bond-breaking reactions during the fragmentation process of CL-20, the initial intermediate fragments surrounded by NO2radicals prefer to produce NO3, NO, N2O, HONO, HNO3, HNO and OH radicals. By employing VARxMD, the bimolecular reaction pathways related to the formation and interactions of nitrogen oxides and OH radicals were analyzed.
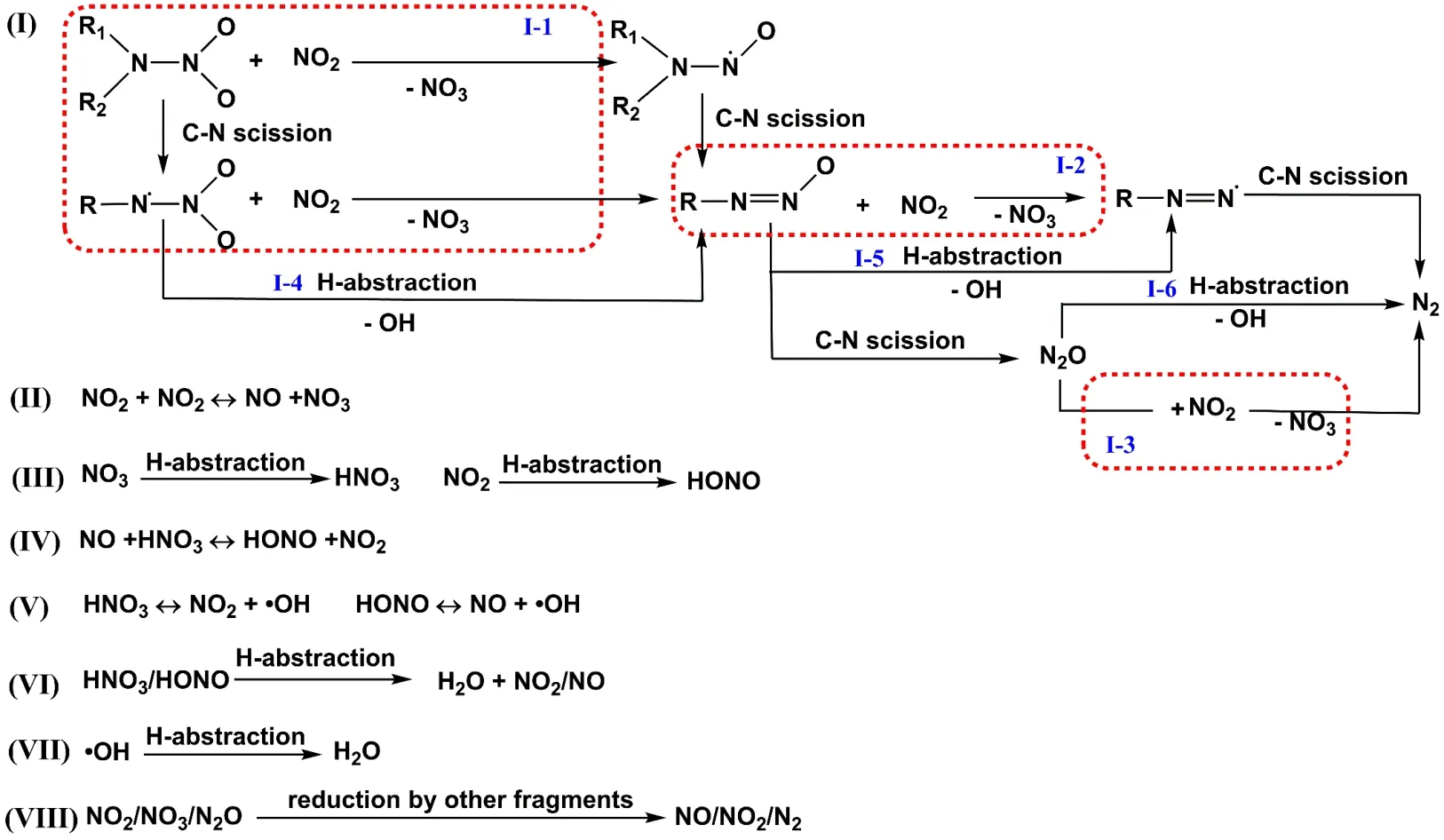
Fig. 10 Reaction pathways on the formation and interactions of nitrogen oxides and OH radicals involved in the fragmentation stage of CL-20 thermolysis obtained in ReaxFF MD simulations.
As shown in Fig. 10, four major reaction pathways of I-1 to I-3 and II to produce the important intermediate NO3can be found.As shown in the I-1 pathway, fragments with initial structure of R1R2NNO2or RNNO2formed by the C―N bond cleavage of R1R2NNO2, tend to interact with NO2to produce R1R2NNO or RNNO and release NO3, where the NO2radical will serve as reductant by abstracting an oxygen atom from the nitro groups of R1R2NNO2or RNNO2. The pathway I-1 for generating NO3was referred in the decomposition of RDX by DFT calculation18band HMX by ReaxFF MD simulations37. In addition to the conversion to RNNO by direct interaction with NO2(pathway I-1), RNNO2may convert to RRNO in pathway I-4 of hydrogenabstraction and N―O bond scission to form OH radical. The formed RNNO may further convert to RNN in pathway I-2 of oxygen-abstraction by NO2forming another NO3or in pathway I-5 of hydrogen-abstraction and form OH radical. Finally, RNN may produce N2by C―N bond scission. Besides, some RNNO can break down directly by the C―N bond rupture to form N2O.This pathway to form N2O may provide an explanation for the phenomena that the source of the two nitrogen atoms in N2O is the same RDX molecule observed in the isotope experiments of RDX39. Subsequently, the oxygen atom in N2O will be abstracted by NO2or H atoms to form NO3(pathway I-3) or OH radicals (pathway I-6) as well as N2. It is observed that the N2can come from the initial N―N structure of CL-20, which explains the early appearance of N2during the thermolysis of CL-20. In addition to these three pathways, combination of two NO2radicals will produce a lot of NO and NO3(pathway II),which was also proposed in the decomposition of single CL-20 by DFT molecular dynamics8and in the decomposition of RDX by DFT calculations18b. But recombination reactions of NO and NO3to form NO2are so easy that makes a short lifetime of many NO3molecules. NO3is an active intermediate and will consumed mainly in pathway III and VIII, as shown in Fig. 10.NO3will convert to HNO3by hydrogen abstraction (pathway III). The pathway for HNO3generation was also found in the ReaxFF MD simulation of HMX decomposition35. Besides,NO3will convert to NO2(pathway VIII) by oxidizing the carbon atoms in other early intermediates of CL-20 decomposition.
It should be noted that the pathways on the formation and consumption of NO3presented in Fig. 10 can be observed at varied temperatures. To depict the temperature effects, the reaction numbers for the pathways to generate and consume NO3at 1000 K, 1500 K and 2000 K are summarized in Table 4. As shown in Table 4, the number of reactions for pathway I-1 decreases as temperature increases, while the reaction numbers for pathways I-2 and I-3 increase at high temperatures. High temperature will favor the pathway II, though the high temperature will notably promote both the forward and backward reactions in pathway II. However, the reaction numbers for NO3conversion to HNO3(pathway III) increases very rapidly when temperature increases to 1500 K, indicating that high temperature favors the formation of HNO3. The net number for the reaction of NO3oxidizing other intermediates(pathway VIII) increases significantly when temperature increases. The two reaction pathways to consume NO3are greatly enhanced at high temperatures, explaining the quick decrease of NO3at high temperatures. Considering the relativelylarge amount of NO3at low temperature as shown in Fig. 3b, it may be detectable experimentally that will be useful to validate the pathways on the formation and consumption of NO3revealed in this work.
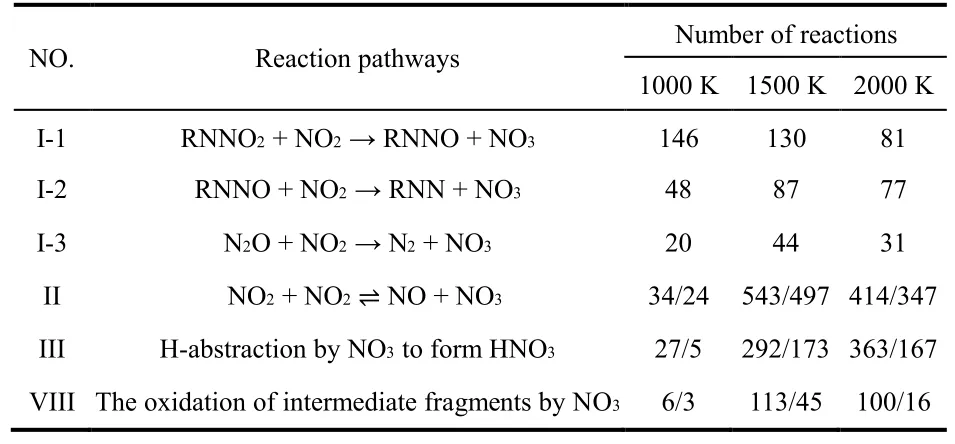
Table 4 Number of reactions on the formation and consumption of nitrate radicals (NO3) in thermal decomposition of CL-20 at 1000–2000 K obtained in ReaxFF MD simulations.
Similar to the generation of HNO3, most HONO molecules are produced by hydrogen abstraction of NO2(pathway III), which was proposed in the AIMD simulations of CL-20 decomposition8.This is different from thermolysis of RDX and HMX, where the intramolecular successive elimination of HONO is considered as a competitive pathway of initial N―NO2scission to form NO240.Moreover, HNO3and NO will produce HONO and NO2, which is a frequent back-and-forth reaction as shown in pathway IV.These generated HONO and HNO3can further decompose into hydroxyl radicals (pathway V), both of which are reversible reactions. HONO or HNO3can also convert to H2O directly by hydrogen abstraction and released NO or NO2(pathway VI). The OH formed in pathways I (I-4 to I-6) and V will convert to H2O by hydrogen abstraction (pathway VII). At later stage of thermolysis simulation, the oxidation of carbon atoms in other intermediates by generated nitrogen oxides (NO2, NO3and N2O)will produce stable gas products of NO, NO2and N2(pathway VIII) step by step and form CO2eventually.
4 Conclusions
The overall chemical events in thermal decomposition of condensed CL-20 were obtained by ReaxFF MD simulations of a super cell containing 128 CL-20 molecules at different temperatures of 800–3000 K. Comprehensive reaction mechanisms on the initial decomposition, the subsequent fragmentation process in the thermal decomposition of condensed CL-20 were revealed using VARxMD. The reaction pathways about the formation and consumption of nitrogen oxides were analyzed systematically. Nitrogen oxides are the major initial decomposition products formed in a sequence of NO2, NO3, NO and N2O. NO2is the most abundant among these nitrogen oxides, while NO3is the second abundant. However,NO3is unstable that it will be run out completely at 2000 K within the 500 ps simulation while certain amounts of other nitrogen oxides still remain. The major reaction pathway for the generation of NO3is oxygen abstraction by NO2. The final quantities of products N2, H2O, CO2and H2obtained in ReaxFF MD simulation of CL-20 thermolysis at 3000 K are quantitatively consistent with the product distribution in detonation experiment of CL-20. The pathways for the formation and interaction of N2O, NO, HONO, HNO3and OH during the fragmentation process were depicted.
The initial decomposition of condensed CL-20 at all temperatures is dominated by unimolecular pathways of the N―NO2bonds cleavage to form NO2and ring-opening by C―N bonds scission. The bimolecular pathway of oxygen-abstraction by NO2from nitro groups in CL-20 was observed. High temperature favors the cleavage of N―NO2bond but the oxygen-abstraction. The evolutions of intermediate fragments with ring structures were analyzed at 1000 K. The early generated pyrazine derivatives of the fused tricycles and bicycles will decompose into fragments of single pyrazine ring. The observed high stability of the pyrazine ring structure supports the intermediates detected in fast pyrolysis experiment of CL-20.
The comprehensive reaction pathways and intermediate structures of CL-20 thermolysis obtained in the ReaxFF MD simulations demonstrate that ReaxFF MD simulations together with VARxMD provides an effective approach to investigate the scenario of the complex chemistry in the thermolysis of condensed energy materials.
- 物理化学学报的其它文章
- On the Simulation of Complex Reactions Using Replica Exchange Molecular Dynamics (REMD)
- Influence of Photoisomerization on Binding Energy and Conformation of Azobenzene-Containing Host-Guest Complex
- Free Energy Change of Micelle Formation for Sodium Dodecyl Sulfate from a Dispersed State in Solution to Complete Micelles along Its Aggregation Pathways Evaluated by Chemical Species Model Combined with Molecular Dynamics Calculations
- Deformation of Polymer-Grafted Janus Nanosheet: A Dissipative Particle Dynamic Simulations Study
- Selective Permeation of Gas Molecules through a Two-Dimensional Graphene Nanopore
- Microscopic Investigation of Ethylene Carbonate Interface:A Molecular Dynamics and Vibrational Spectroscopic Study

