Rapid-responsive Fluorescent Probes Based on Coumarin Dye for Sensitive Detection of Hypochlorite
CHENG Xiao-hong,RUAN Zhi-jun,ZHONG Zhi-cheng,LI Wang-nan
(1.Hubei Key Laboratory of Low Dimensional Optoelectronic Materials and Devices,Hubei University of Arts and Science,Xiangyang 441053,China;2.Hubei Key Laboratory of Processing and Application of Catalytic Materials,College of Chemistry and Chemical Engineering,Huanggang Normal University,Huanggang 438000,China)
*Corresponding Author,E-mail:chengxiaohong0807@126.com
Abstract:Taking advantage of the special oxidation property of hypochlorite,two novel coumarin-type fluorescent probes(compound C2 and C4)were synthesized for the rapid detection of ClO-.By virtue of its special oxidation property,both probes displayed high selectivity for ClO-over other anions.Upon the addition of ClO-anion,probe C2 displays extraordinary fluorescence quenching,which is beneficial to the production of a high signal output during detection process.Differently,probe C4 displays apparent enhancement in fluorescence emission spectra.
Key words:probe;hypochlorite;fluorescence;selectivity;rapid detection
1 Introduction
Currently,the development of molecular probes for anions such as ashypochlorite anion,has been a subject of intense research interest,since anions play important roles in biological systems and also constitute some pollutants in our environment[1-3].Hypochlorite anion(ClO-)and its conjugate acid(HOCl)are implicated in a variety of pathological diseases including atherosclerosis,neuron degeneration,cystic fibrosis,arthritis,and cancers[4-7].Biologically,hypochlorous acid is known to be one of the important reactive oxygen species(ROSs)[8],and the uncontrolled levels of HOCl will damage DNA and proteins,consequently leading to various diseases.Nevertheless,HOCl/ClO-are extensively encountered as disinfectant of drinking water,swimming pool water and household bleach in our daily life[9-11].Therefore, the real-time detection with highly sensitive and selective for hypochlorite is of the utmost importance and has aroused extensive attention.
In particular,fluorescence detection has been widely used in enormous fields,such as analytical chemistry, environmentalbiology, biochemistry,and medical science,because of its visual simplicity,high sensitivity,inexpensive apparatus,instantaneous response,real-time detection and nondestructive properties compared to traditional analytical methods[12-14].Toward this end,a number of smallmolecule fluorescent probes through modification of common fluorophores for ClO-/HClO has been devised[15-22].As an important group of organic heterocycles,coumarin is often selected as a signaling moiety due to its high quantum yield,large Stokes shift and low cytotoxicity.It is easy to modify the coumarin on its 3-C,4-C and 7-C positions,introducing electron donating groups and electron accepting groups contributes to produce many strong fluorescent dyes with different wavelength ranges of absorption and fluorescence emission[23-26].Therefore a large number of coumarin derivatives has been used as signaling unit in sensors and sophisticated photophysical systems.Till date coumarin derivatives have been used as fluorescent probes of pH[27-28],nitrogen oxides[29]and hydrogen peroxide[30].They have been proven to be an excellent agent for accurate on-line,and economical detection of toxic heavy metal ions,anions,and enzymes with a high degree of selectivity and sensitivity[31-35].
As a potential sensing system,specific chemical reaction with high selectivity of toxic species has drawn great attention.The reaction between the sensor molecule and target analytes will give a unique spectroscopic change[36-38].In organic synthesis,aldehyde groups can be protected as oximes[39-40],which are rapidly deprotected by hypochlorite under mild conditions(i.e.,at ambient temperature).The resultant aldehyde and the previous oxime group possess different intramolecular charge transfer(ICT)efficiency and optical properties.Therefore,this deprotection reaction can be exploited as an interesting platform for the design of reaction-based chemosensors for ClO-[41-43].
With these considerations in mind,herein,we developed two chemical reaction-based fluorescent probes for hypochlorite ion,namely,compound C2 and C4.The design of these probes took advantages of both the well-known coumarin structure and the ClO--promoted deprotection reaction of oximes.After the reaction with NH2OH,the fluorescent properties of obtained oximes C2 and C4 became relatively different from its precursor aldehydes C1 and C3,respectively.Therefore,it was reasonable that novel fluorescent probes for ClO-could be developed by utilization ofthe unique spectroscopic change induced by the ClO--promoted transformation from oxime to aldehyde.Herein,we would like to describe the synthesis and the spectroscopic evaluation of the new fluorescent probes for hypochlorite anion in detail,featuring advantages such as easy-tomake,rapid fluorescence response as well as high sensitivity.
2 Experiments
2.1 Materials and Instrumentations
Ethanol was dried over and distilled from Na under an atmosphere of dry nitrogen.N,N-Dimethylfomamide(DMF)was dried over and distilled from CaH2under an atmosphere of dry nitrogen.
All reagents were of analytical reagent grade and used without further purification.Deionized water was used in all experiments.Inorganic salts including NaNO3,NaOAc·3H2O,NaNO2,KClO3,NaF,KClO4,NaHCO3,NaCl,Na3PO4,KSCN,NaIO3,Na2HPO4·12H2O,KBr,NaHSO3,NaHSO4,KI,Na2S2O3·5H2O,H2O2and NaClO were purchased from Shanghai Chemical Reagent Co.(Shanghai,China).
The1H and13C NMR spectra were measured on Varian Mercury 300 and Bruker 500 spectrometer using tetramethylsilane(TMS;δ=0)as internal standard.The ESI mass spectra were measured on a Finnigan LCQ advantage mass spectrometer.Elemental analyses were performed by a CARLOERBA-1106 microelemental analyzer.Photoluminescence spectra were performed on a Hitachi F-4600 fluorescence spectrophotometer.The pH values were determined by using a DELTA 320 PH dollar.
2.2 Synthesis of Compound C2 and C4
Compound C1 was readily synthesized according to the literature[44](766 mg,89%).1H NMR(300 MHz,CDCl3)d:1.24 -1.28(t,6H,J=6.0 Hz,—CH3),3.45 - 3.51(q,4H,J=6.0 Hz,—CH2—),6.49 -6.50(d,1H,J=3.0 Hz,Ar—H),6.63 -6.66(m,1H,Ar—H),7.40 -7.43(d,1H,J=9.0 Hz,Ar—H),8.26(s,1H,Ar—H),10.13(s,1H,—CHO).13C NMR(75 MHz,DMSO-d6)d:12.1,44.3,96.1,107.4,110.2,112.9,132.9,145.9,153.2,158.2,160.5,186.9.MS(ESI):m/z[M-H]-:243.9,calcd,244.1.
Compound C2 was prepared conveniently from compound C1 and NH2OH(148 mg,57%).1H NMR(300 MHz,DMSO-d6)d:1.08 - 1.12(t,J=12.0,6H),3.39 -3.45(q,J=6.0,4H),6.53(s,1H),6.69 - 6.72(d,J=9.0,1H),7.50 -7.53(d,J=9.0,1H),7.96(s,1H),8.13(s,1H),11.29(s,1H).13C NMR(75 MHz,DMSO-d6) δ:13.0,44.8,97.0,108.5,110.1,112.2,131.0,139.0,143.5,151.6,156.9,160.8.MS(ESI),m/z[M+H]+:261.1,calcd,261.1.C14H16N2O3(EA)(%,found/calcd):C,64.46/64.60;H,6.308/6.20;N,10.86/10.76.
Compound C3 was readily synthesized according to the literature[44].1H NMR(500 MHz,CDCl3)d:10.06(1H,s),8.06(1H,s),6.93(1H,s),3.35 - 3.39(4H,t,J=10.0 Hz),2.84 -2.87(2H,t,J=7.5 Hz),2.73 - 2.77(2H,t,J=10.0 Hz),1.96 - 1.99(4H,m).13C NMR(125 MHz,CDCl3)d:187.84,162.19,153.71,149.38,144.82,128.28,119.89,112.68,108.00,106.03,50.37,49.94,27.21,20.85,19.89.MS(ESI),m/z[M+H]+:269.8,calcd,269.1.
Compound C4 was prepared conveniently from Compound C3 and NH2OH(yellow solid,75%).1H NMR(500 MHz,DMSO-d6)d:11.04(1H,s),8.04(1H,s),7.98(1H,s),7.14(1H,s),3.28 -3.35(4H,m),2.70 -2.78(4H,m),1.85-1.90(4H,m).13C NMR(125 MHz,DMSO-d6)d:160.79,151.61,146.89,143.44,138.94,126.59,119.20,110.80,108.05,105.70,49.85,49.34,27.28,21.21,20.31,20.15.MS(ESI),m/z[M+H]+:282.8,calcd,283.1.C16H16N2O3(EA)(%,found/calcd):C,67.64/67.59;H,5.29/5.67;N,9.99/9.85.
2.3 Preparation of Solutions of Anions
1 mmol of each inorganic salt(NaNO3,NaOAc·3H2O,NaNO2,KClO3,NaF,KClO4,NaHCO3,NaCl,Na3PO4,KSCN,NaIO3,Na2HPO4·12H2O,KBr,NaHSO3,NaHSO4,KI,Na2S2O3·5H2O,H2O2and NaClO)was dissolved in distilled water(10 mL)to afford 1 ×10-1mol/L aqueous solution.The stock solutions were diluted to desired concentrations with water when needed.
2.4 Fluorescence Titration of C2/C4 with ClO-
A solution of C2(1 ×10-5mol/L)was prepared in HEPES∶DMSO=1∶9(v/v,10 mmol/L,pH=7.4).A solution of C4(1 ×10-5mol/L)was prepared in HEPES∶DMSO=9∶1(v/v,10 mmol/L,pH=7.4).Then 3.0 mL of the solution of C2 or C4 was placed in a quartz cell(10.0 mm width)and the fluorescence spectrum was recorded.The NaClO solution was introduced in portions and fluorescence intensity changes were recorded at room temperature each time.
2.5 Fluorescence Intensity Changes of C2/C4 with Other Anions
A solution of C2(1 ×10-5mol/L)was prepared in HEPES∶DMSO=1∶9(v/v,10 mmol/L,pH=7.4).A solution of C4(1 ×10-5mol/L)was prepared in HEPES∶DMSO=9∶1(v/v,10 mmol/L,pH=7.4).Then 3.0 mL of the solution of C2 or C4 was placed in a quartz cell(10.0 mm width)and the fluorescence spectrum was recorded.Different anion and oxidant solutions were introduced and the changes of the fluorescence intensity were recorded at room temperature each time.
2.6 Quantum Yield Calculation
Quantum yield was determined according to the equation as follows:

ΦFwas the fluorescence quantum yield,A was the absorbance,F was the area under the corrected emission curve.Here,fluorescein was used as the standard,the quantum yield of fluorescein in 0.1 mol/L M NaOH was 0.90[45].
3 Results and Discussion
3.1 Synthesis and Structural Characterization
The target compounds C2 and C4 were prepared conveniently through the general nucleophilic addition between aldehyde and hydroxylamine,following the similar procedure in the reported literature.The whole synthetic route was simple and the purification was easy.Target compounds C2 and C4 exhibited good solubility in common organic solvents,such as acetone,DMF,DMSO,CH3CN,and THF.Their structures were well characterized by1H,13C NMR,ESI-MS and elemental analyses,and all gave satisfactory spectral data.
3.2 Optical Properties of Compound C2 and C4
Before the sensing experiments,we firstly examined the photoluminescence behaviors of compound C1-C4.Both in DMSO and acetonitrile,the emission intensity of compound C2 decreased gradually with the increasing of H2O(with final concentrations kept unchanged at 10 μmol/L),which was similar to the conventional ACQ(aggregation-caused quenching)chromophores[46-47].However,in terms of compound C1,the emission intensity decreased dramatically even with a little portion of H2O,while the intensity kept almost stable with the continued increasing of H2O.The above result was consistent with the literature[48]:if an aldehyde group was introduced to coumarin,the luminescence of the resultantcompound would be nearly completely quenched in polar solvents.That was to say,the emission spectra of C1 and C2 displayed different changes with the addition of some portion of H2O.It the dyes slowed down the intramolecular C N isomerization and induced strong emission intensity[49-52].The similar results could be obtained when water was added into the dye solutions in CH3CN.In terms of aldehyde C3,the emission spectra shifted from 507 nm to 510 nm with the addition of 10%water;however,the intensity kept almost stable with the continued increasing of H2O.We thus plotted the changes in their emission peak intensities vs.water contents in the aqueous mixtures.It was apparent that in wide ratio range of DMSO and H2O(the fraction of H2O changing from 0-90%),the transformation from compound C4 to aldehyde C3 would induce obvious enhancement on emission intensity,which was beneficial to the design of turn-on fluorescence probe.To the end,reaction medium of 10 mmol/L HEPES buffer(pH 7.4)containing 10%(v/v)DMSO was chosen for the purpose of physiological application.
The different photoluminescence behaviors between aldehydes C1&C3 and oximes C2&C4 might be ascribed to their different structural planarity and solubility,which was consistent with the reported literature[53].In summary,both compounds C2 and C4 could sense ClO-with respective fluorescence“turn-off”and“turn-on”approach,in different experimental conditions.
3.3 Sensing Properties of C2 Toward ClO-
Compound C2 exhibited very strong green was apparent that with the fraction of H2O of 10%,the largest fluorescence difference could be achieved.As a result,a reaction medium of DMSO containing 10%HEPES buffer was chosen in the titration experiment in order to detect hypochlorite ion with remarkable“on-off”fluorescent response.
DMSO solution of C4 was relatively emissive with the maximum emission wavelength centered at about 510 nm.Excitingly,with the addition of water into its DMSO solution(with final concentrations kept unchanged at 10 μmol/L),the fluorescent intensity was greatly enhanced.As we know,with the increasing amount of water,the solubility of organic compound C4 became poor,and more molecules existed as aggregate state.Evidently,the aggregates of luminescence with the maximum wavelength centered at about 503 nm,which could be observed by naked eyes under normal UV lamp;however,the fluorescent intensity of compound C1 was much weaker with the quantum yield of 0.03 and nearly no photoluminescence could be seen upon excitation.Then,it was expected that the ClO--promoted deprotection reaction of oxime C2 occurred to generate the aldehyde C1. As a result,the electronic structures changed which would give detectable fluorescent signal.If hypochlorite anion was the only analyte capable of inducing these changes,it seemed that compound C2 could act as a hypochlorite anion-selective optical chemosensor.
Then,we added hypochlorite anion into the diluted solution of compound C2 and investigated its response toward ClO-in DMSO-HEPES(v/v,9/1),in detail.As shown in Fig.1,the emission intensity at about 503 nm decreased apparently compared to the original one even at the concentration of hypochlorite anion as low as 0.5 ×10-6mol/L.Excitingly,the fluorescence intensity decreased immediately to about 85%of the original one at the concentration of ClO-anion as low as 1.0 ×10-6mol/L.When 2 equiv.of ClO-was added,the emission intensity reached the minimum with about 60-fold reduction(I0/I -1).With the increasing of the concentration of ClO-in the test system,the intensity centered at about 503 nm decreased correspondingly and the emission profile became gradually similar to that of its precursor,aldehyde C1.Thus,these results indicated that upon the addition of ClO-anion,the ClO--promoted deprotection reaction of oxime C2 really occurred as expected,and aldehyde C1 was formed step-by-step.To see the results more visually,we summarized the intensity change as a function of the ClO-concentration.As demonstrated in the inset of Fig.1,in the range of 0 - 10 μmol/L,there was a nearly linear relationship between the intensity change and the concentration of ClO-.A linear regression curve could be simulated,and the point at which this line crossed the abscissa axis was taken as the detection limit and equaled approximately 2 × 10-7mol/L[54].Generally,fluorescence quenching is undesirable for analytical purposes in which variations in the environmental sample and probe distribution were problematic for quantitative measurements.However,here,in the presence of ClO-,the solution of C2 displayed extraordinary fluorescence quenching which was beneficial to the production of a high signal output during detection process.
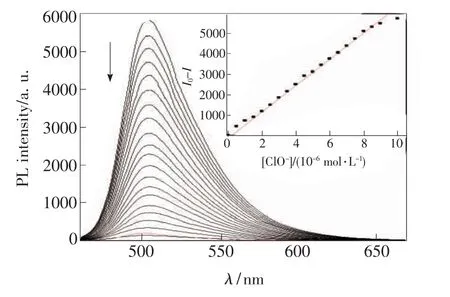
Fig.1 Fluorescent emission spectra of C2(10 μmol/L)in the presence of increasing concentration of ClO-(black lines,in DMSO/H2O=9/1,v/v,10 mmol/L HEPES,pH 7.4),with that of C1 for comparison(red line),excited at 430 nm.From top to bottom:0.0,0.5,1.0,1.5,2.0,2.5,3.0,3.5,4.0,4.5,5.0,5.5,6.0,6.5,7.0,8.0,9.0,10.0,15.0,20.0 μmol/L.Inset:plot of fluorescent intensity at 503 nm of C2 as a function of the concentration of ClO-.
To evaluate the specific nature of C2 toward hypochlorite anion,the influence of other anions was investigated.As shown in Fig.2,for all other anionsnearly no changes on the fluorescence intensity observed and the different sensing behavior could be easily seen by the naked eyes under a normal UV lamp(as displayed in the inset fluorescence photograph in Fig.2).Actually,only in the presence of hypochlorite anion,the strong green emission could be quenched,indicating that hypochlorite could be conveniently detected with the aid of a normal UV lamp visually.The above results indicated that probe C2 had selective response toward hydrogen sulfite over other anions.

Fig.2 Emission intensity changes of C2(10 μmol/L)in the presence of different anions(ClO-,20 μmol/L;H2O2,100 μmol/L;others,150 μmol/L).Inset:fluorescent photograph of C2 to various anions.a -
3.4 Sensing Properties of C4 Toward ClO-
The sensing properties of C4 in response to ClO-were investigated and a reaction medium of 10 mmol/L HEPES buffer(pH 7.4)containing 10%(v/v)DMSO was chosen.We tried to add ClO-into the diluted solution of oximes C4(Fig.3),immediately,the emission spectra displayed obvious increase even at the concentration of ClO-as low as 2 μmol/L.With the increasing of the concentration of ClO-in the test system,the intensity centered at about 510 nm increased correspondingly.When 20 μmol/L of ClO-was added,the emission intensity reached the maximum with remarkable enhancement.To see the results more visually,we summarized the intensity change as a function of the ClO-concentration.As demonstrated in the inset of Fig.3,in the range of 0 - 20 μmol/L,there was a nearly linear relationship between the intensity change and the concentration of ClO-.
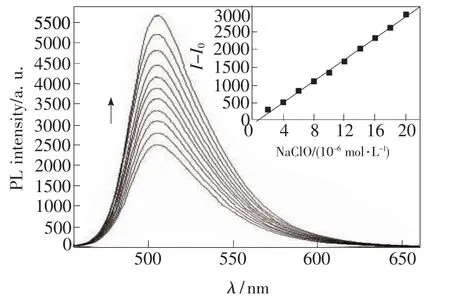
Fig.3 Fluorescent emission spectra of C4(10 μmol/L,in DMSO∶H2O=1∶9,v/v)in the presence of different concentrations of NaClO excited at 430 nm(from bottom to top:0.0,2.0,4.0,6.0,8.0,10.0,12.0,14.0,16.0,18.0,20.0 μmol/L of ClO-).Inset:plot of fluorescent intensity at 510 nm of C4 as a function of the concentration of ClO-.
Similarly,the influence of other anions and oxidant were investigated to evaluate the specific nature of C4 toward ClO-.For all other anions and H2O2,there were nearly no changes on the fluorescence spectra observed upon the addition of the anions with the concentration of 150 μmol/L.Moreover,the response of C4 to hypochlorite anion in the presence of other competitive anions was measured to evaluate its selectivity.As shown in Fig.4,except the faint interference from H2O2,the presence of other background anions did not show any obvious disturbance,indicating that C4 had selective response toward hypochlorite over other anions.
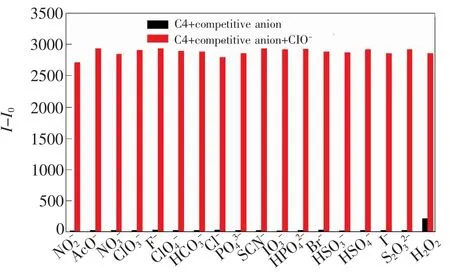
Fig.4 Fluorescent responses of C4(10 μmol/L)to ClO -(20 μmol/L)in the presence of other competitive ions(H2O2,100 μmol/L;others,150 μmol/L).
3.5 Investigation of Sensing Mechanism
The sensing mechanism was exploredby ESI-MS spectrometry and1H NMR spectroscopy characterization and it was found that the main formed product from the reaction of oximes with ClO-was aldehyde compound.Firstly,the reaction solution of compound C2 and excess ClO-was characterized by ESI-MS spectroscopy.ESI-MS spectrum of C2 revealed a main peak at 261.1 before ClO-was added to C2,exactly corresponding to the species[C2+H]+(m/zcaled=261.1).After the addition of excess ClO-into the solution of C2,a relatively weak peak at about 244.0 appeared coinciding with that for the species[C1—H]-(m/zcaled=244.1),indicating that the main formed product from the reaction of C2 with ClO-was compound C1,in which the oxime group was converted to an aldehyde group.
To further confirm the generation ofaldehyde,the reaction of C4 with 20 equiv.ClO-was carried out in CH3OH-H2O(v/v,9∶1)solution.After stirring at ambient temperature for 2.5 h,the major product was purified by chromatography on a silica gel column and underwent1H NMR analysis.For the isolated product of C4-ClO-from the reaction of C4 with ClO-(Fig.5b),the resonance signals assigned toprotons at 11.04 ×10-6disappeared;while a new peak at 10.03 ×10-6emerged corresponding to the aldehyde proton.In fact,the corresponding1H NMR spectrum of C4-ClO-was the same as that of aldehyde C3(Fig.5c).This confirmed that thegroup in C4 was indeed oxidized to an aldehyde group by ClO-,thus resulting in the formation of product C3.Accordingly,we proposed the possible sensing mechanism of the present system as demonstrated in Fig.5.

Fig.5 Proposed sensing mechanism and1H NMR spectra of C4(a,in DMSO-d6),the isolated resultant compound C4-ClO-(b,in CDCl3)and that of C3 for comparison(c,in CDCl3).The asterisk represented the signal of solvent.
4 Conclusion
In summary,two novel coumarin-type fluorescent probes(compound C2 and C4)were synthesized for the rapid detection of ClO-.Both compounds C2 and C4 could sense ClO-with respective fluorescence“turn-off”and“turn-on”approach,in different experimental conditions.Furthermore,the sensing mechanism was explored by ESI-MS and1H NMR spectroscopy and it was found that the conversion from oxime to aldehyde group with the addition of ClO-induced the remarkable fluorescence changes.The results reported in this work might provide some useful information for the design of new fluorescent probes,that was to say,the subtle adjustment on the chemical structure could dramatically affect the sensing behaviours in the present system.Further study on the design of probes for toxic anions and metal ions with better performance was still in progress in our lab.
Acknowledgement:This work was financially supported by the National Natural Science Foundation of China(No.21502047)and the Natural Science Foundation of Hubei Province(2017CFB295).

