Synthesis and Luminescence of α-Ba3Y(BO3)3∶Dy3+Phosphors
NING Hong-yu,CHENG Zhi-yuan,DONG Chao,HU Ze-qing,YU Jing-jie,ZHANG Yan-jie
(Research Institute of Photonics,Dalian Polytechnic University,Dalian 116034,China)
*Corresponding Authors,E-mail:yujingjie@dlpu.edu.cn;zhang_yj@dlpu.edu.cn
Abstract:A series of phosphors α-Ba3Y(BO3)3∶Dy3+have been synthesized via conventional solid-state method and characterized.Fluorescence spectra(FL)show that the as-synthesized phosphors mainly exhibit two dominating emission peaks at 488 and 577 nm corresponding to the transitions from4F9/2to6H15/2and6H13/2,respectively under ultraviolet(UV)or blue radiation.The best synthesis temperature is 1 100℃,leading to the most intensive luminescence,pure phase and most sufficient crystal growth ensured by FL,X-ray diffraction(XRD)and scanning electron microscope(SEM),respectively.The concentration quenching mechanism has been determined to be mainly dipoledipole interaction and exchange interaction is not negligible due to the specific unit cell structure;the critical concentration is 0.07.The second-order exponential lifetime decay curves are observed and its origin related to the specific unit cell structure is discussed.The as-synthesized phosphor has potential application for pc-WLEDs in the lighting field.
Key words:phosphors; α-Ba3Y(BO3)3∶Dy3+;crystal structure
1 Introduction
In lighting field,phosphor converted white light emitting diodes(pc-WLEDs)play an important role as solid-state light(SSL)due to their outstanding performances,such as high efficiency,energy saving,long lifetime and environment friendly.To obtain whitle light,LED chip must be coated with tricolor phosphors or white light emitting single matrix phosphors.While the development of the blue and ultraviolet(UV)chips,lots of contributions are focused on novel phosphors for LED chips[1-2],especially blue,green,red and white phosphors.
Many kinds of mineral salts could be used as host material,such as tungstates,vanadates,aluminates,phosphates,borates and silicates[3-9].Because of the low synthesis temperature,stable physical chemical property and various crystal structures of borates,theses concentrating on rare earth(RE)ions doped borate phosphor are reported very frequently[9-16].Among all sorts of borates,α-Ba3Y-(BO3)3with layered crystal structure belonging to hexagonal system has been proved to be qualified matrix for Eu3+,Ce3+and Tb3+activated phosphor with single colour luminescence[17-18].As far as we know,luminescence properties of Dy3+doped α-Ba3Y(BO3)3phosphor haven't been reported yet.Trivalent dysprosium is very promising because its emission spectrum consists of two peaks at blue and yellow region,corresponding to electronic transition from4F9/2to6H15/2and6H13/2,respectively.Therefore,doping in proper matrix,Dy3+ions could emit white light without co-doping any other activators[19-21].It's significant to scope the luminescence behavior of Dy3+doped α-Ba3Y(BO3)3.
In this work,α-Ba3Y(BO3)3∶Dy3+phosphors were synthesized at different temperature by traditional solid state method.Crystal structure,morphology,luminescence and lifetime properties were explored.The concentration quenching mechanism was supposed to be relative to the unique crystal structure.
2 Experiments
The α-Ba3Y1-x(BO3)3∶xDy3+phosphors were prepared via a conventional solid state method using Dy2O3(99.99%),BaCO3(A.R.),Y2O3(99.99%)and H3BO3(A.R.)as starting materials without any further purification.All materials were weighed stoichiometrically except H3BO3with 3%(mass fraction)overdose for charge compensation;mixed and ground thoroughly in an agate mortar for 30 min.Then the homogeneous mixture was transferred to a corundum crucible,heated at 170℃ for 60 min in furnace.After cooled down with the furnace,samples were ground and mixed again,heated at 850,1 000,1 100℃ for 400 min in atmosphere.Finally,the crystalline powders were obtained.
The phases were identified by XRD(XRD-7000S,Shimadzu,40 kV and 30 mA,Cu Kα,λ=0.154 056 nm,0.02°by step);the morphology was photographed by a SEM(JSM7800F,JOEL);the luminescence spectra were recorded by a HITACH F-4500 fluorescence spectrophotometer with a xenon lamp operated at room temperature;the lifetime decay curves(λex=350 nm,λem=577 nm)were investigated using a spectrofluorometer(HJY,TRIAX550)with a laser source(Horizon,OPO system,10 Hz).
3 Results and Discussion
3.1 XRD and Morphology
α-Ba3Y(BO3)3has a hexagonal layered structure with space group P63cm(185)and unit cell parameters a=b=0.941 9 nm,c=1.759 5 nm,V=1.351 87 nm3and Z=6.As shown in Fig.1,there are 4 and 2 lattice sites for Ba2+and Y3+ions,respectively,separated by paralleled planes formed byanion groups.Basically,this unusual structure can be described as multilayer sandwiches:anion groups are bread and cations are fillings.All Y3+ions are arranged in the same layers named as Y,then the left layer ofto Y can be named as‘bread A’and the right one as‘bread B’.The layer ofseparating Ba1 and Ba2 can be named as‘bread C’.Ba1/Ba3 sites are set between C and A while Ba2 and Ba4 sites between B and C,respectively.Meanwhile,Ba3/Ba4 are much closer to the nearest Y sites with distance about 0.387 6/0.390 9 nm than Ba1/Ba2 sites[22].
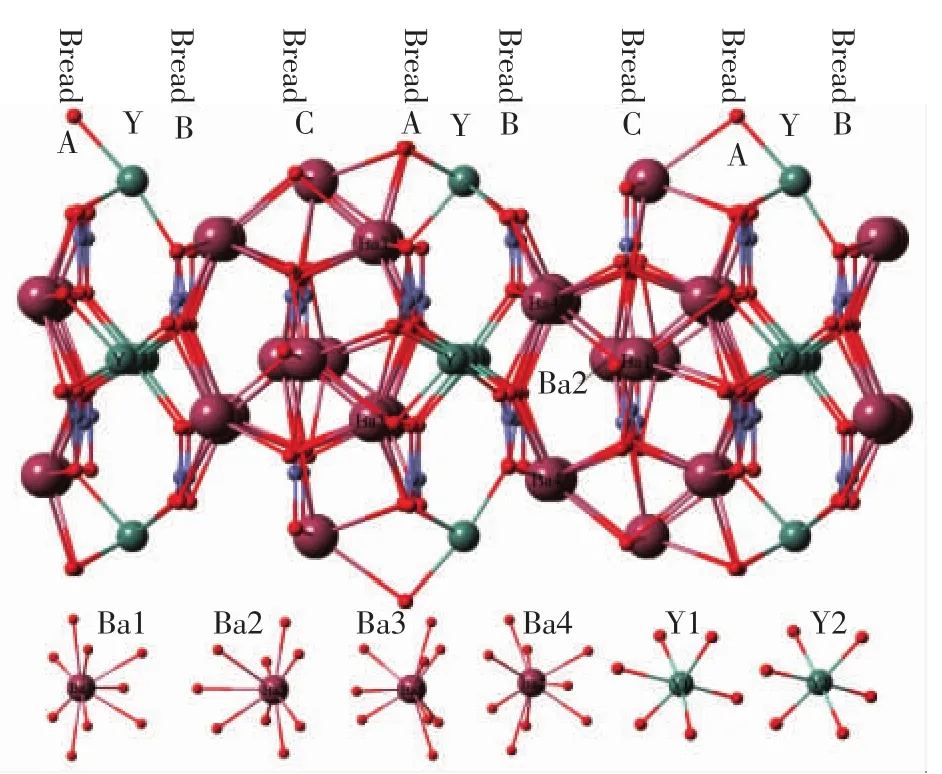
Fig.1 Unit cell structure of α-Ba3Y(BO3)3and the coordination environment of Y3+and Ba2+ions in the host lattice
Fig.2 presents the normalized XRD patterns of α-Ba3Y0.93(BO3)3∶0.07Dy3+synthesized at 850,1 000,1 100℃,respectively and the standard of α-Ba3Y(BO3)3(PDF No.51-1849).Compared with the standard patterns,sample synthesized at 850 ℃ mainly belongs to α-Ba3Y(BO3)3phase and the weak impurity belongs to YBO3marked by.However,samples synthesized at 1 000 and 1 100 ℃ only show peaks of α-Ba3Y(BO3)3without any significant impurity,indicating that pure phase of α-Ba3Y(BO3)3has been achieved.
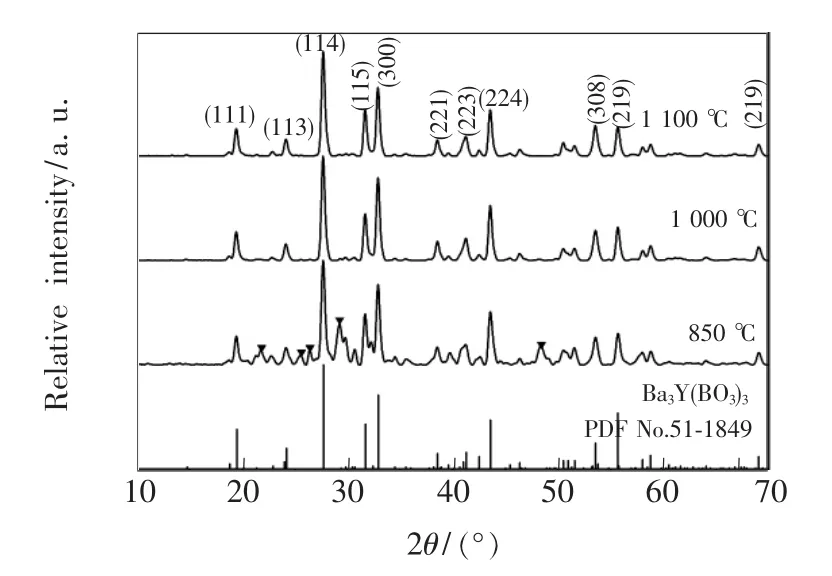
Fig.2 XRD patterns of α-Ba3Y0.93(BO3)3:0.07Dy3+synthesized at 1 100,1 000,850℃,respectively and the standard of α-Ba3Y(BO3)3(PDF No.51-1849).
Fig.3 presents the XRD patterns of α-Ba3Y1-x-(BO3)3∶xDy3+(x=0.00,0.03,0.07,0.05,0.09,0.12,0.15,respectively)synthesized at 1 100℃ and the standard.XRD patterns of all samples have the same discipline and accord with the standard,suggesting that substituting with Dy3+ions barely affects the crystal structure.No significant offset of diffraction peak is observed,which can be explained by the low doping concentration of Dy3+and the tiny difference between the radius of Dy3+and Y3+(0.091 2 nm for Dy3+and 0.090 nm for Y3+).

Fig.3 XRD patterns of α-Ba3Y1-x(BO3)3∶xDy3+synthesized at 1 100 ℃ and the standard of α-Ba3Y(BO3)3(PDF No.51-1849)
Fig.4 exhibits the morphology of α-Ba3Y0.93-(BO3)3∶0.07Dy3+synthesized at 850,1 000,1 100 ℃,respectively and α-Ba3Y(BO3)3synthesized at 1 100℃.When synthesized at 850℃,phosphor consists of many small particles without certain shape and much less number of bigger layered particles.As mentioned before,α-Ba3Y(BO3)3has a layered structure,similar with the layered particles,and XRD proves that sample basically has α-Ba3Y(BO3)3phase with some impurity,thus one can conclude that the layered particles have the isostructure with α-Ba3Y(BO3)3.When synthesized at 1 000 and 1 100℃,only layered particles with even bigger size and fragments generated from grinding are found,suggesting that pure phase of α-Ba3Y-(BO3)3with no impurity achieved,fitting with the XRD result.Based on the particle size and appearance,when synthesized at 1 100℃,the degree of crystal growth is the most sufficient;in another word,1 100℃ is the best synthesis temperature for α-Ba3Y(BO3)3.Further more,the particle size and appearance of α-Ba3Y0.93(BO3)3∶0.07Dy3+and α-Ba3Y(BO3)3obtained at 1 100℃don't differ much from each other,indicating that substituting with Dy3+ions doesn't influence the morphology,either.
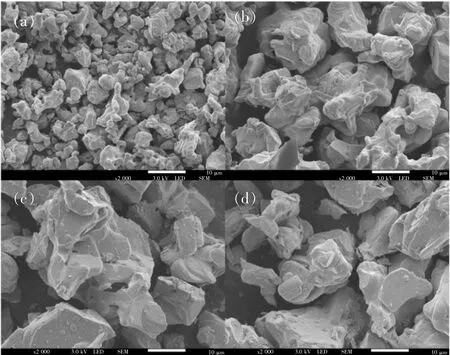
Fig.4 Morphology of α-Ba3Y0.93(BO3)3∶0.07Dy3+synthesized at 850℃(a),1 000℃(b),1 100℃(c)and α-Ba3Y(BO3)3synthesized at 1 100℃(d).
3.2 Luminescence of α-Ba3Y(BO3)3∶Dy3+
Fig.5 illustrates the excitation and emission spectra of sample α-Ba3Y(BO3)3∶Dy3+monitored at 488 and 350 nm,respectively.In the UV and blue region,a series of peaks at 326,350,365,387,425,454 nm corresponding to electron transitions within 4f9configuration from the ground state6H15/2to7F5/2,4M15/2+6P5/2,4I11/2+6P7/2,4I13/2+4F7/2,4G11/2and4I15/2are observed in the excitation spectrum,among which transition6H15/2→4M15/2(6P5/2)at 350 nm dominates.Meanwhile,the emission spectrum(λex=350 nm)mainly consists of two peaks at 488 nm due to a forced magnetic dipole transition4F9/2→6H15/2and 577 nm due to a hypersensitive forced electric dipole transition4F9/2→6H13/2strongly influenced by thedoping environment,respectively.Generally,when Dy3+occupies a high symmetry lattice site with an inverse centre,the yellow emission is weaker than the other;otherwise it exceeds.In our case,transition4F9/2→6H15/2surpasses4F9/2→6H13/2,indicating that Dy3+ions tend to occupy centrosymmetrical sites,namely Y sites according to Fig.1.In summary,Dy3+doped α-Ba3Y(BO3)3could be excited by UV and blue light(namely,350 nm and 454 nm)effectively and emit white light,promising a potential application for pc-WLEDs in the outdoor lighting and display fields.

Fig.5 Excitation(left)and emission(right)spectra of α-Ba3Y(BO3)3∶Dy3+
3.3 Influence of Synthesis Temperature and Doping Concentration of Dy3+
The emission spectra of α-Ba3Y0.93(BO3)3∶0.07Dy3+synthesized at 1 100,1 000 and 850 ℃are presented in Fig.6.As the synthesis temperature rising from 850 to 1 100℃,the luminescent intensity increases as well.It's been mentioned before that the degree of crystal growth can be strongly influenced by synthesis temperature,and samples grow most sufficiently at 1 100℃.The most intense luminescence shall be originated from the most sufficient crystal growth.

Fig.6 Emission spectra of α-Ba3Y0.93(BO3)3 ∶0.07Dy3+synthesized at 1 100,1 000 and 850℃,respectively.

Fig.7 Emission spectra of α-Ba3Y1-x(BO3)3∶xDy3+.The inset is the linear dependence of lg(I/x)on lgx.
To invest the influence of Dy3+concentration(x)on the luminescence behavior and obtain the best intensity,a series of α-Ba3Y1-x(BO3)3∶xDy3+(x=0.03,0.05,0.07,0.09,0.12,0.15)are synthesized and their emission spectra are demonstrated in Fig.7.The luminescent intensity first increases slowly while x increasing from 0.03 to 0.07,and then decreases rapidly if x increases persistently,hence x=0.07 is the optimum concentration(χc).There are lot of energy levels between6F1/2and6H11/2of Dy3+and many ways of energy transfer between different Dy3+ions to decrease the population of Dy3+ions on the4F9/2level without any photon emitted.The numerous cross relaxations are the main reason for the low critical concentration(χc)of Dy3+[23].Another interesting characteristic is the barely changeable yellow-to-blue intensity ratio(Y/B ratio)of Dy3+ions varying from 0.612 to 0.623.The corresponding CIE color coordinates(x=0.273 ~ 0.276,y=0.325 ~ 0.330 in white zone)are shown in Fig.8 marked by ▲ and the inset presents the details of the chromaticity diagram,barely influenced by the doping concentration.

Fig.8 The CIE color coordinates of α-Ba3Y1-x(BO3)3 ∶xDy3+(λex=350 nm).The inset shows the details of the chromaticity diagram.
Distance between different Dy3+ions decreases while increasing Dy3+content and the non-radiative energy migration between different activators becomes more and more often,then the concentration quenching phenomenon occurs.The concentration quenching phenomenon usually origins from exchange interaction,dipole-dipole interaction,dipole-quadrupole interaction or quadrupole-quadrupole interaction.If the energy transfer occurs between same sort of activators,the strength of the multipolar interaction can be determined from the change in the emission intensity of the semi-stable state.According to Dexter's theory[24-25],the emission intensity(I)per activator ion follows equation(1):
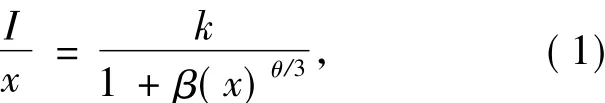
where k and β are constants for the same excitation condition for a given host,x is the concentration of the activator,I is the emission intensity,and θ=3,6,8 and 10 stands for exchange interaction,dipoledipole interaction,dipole-quadrupole interaction and quadrupole-quadrupole interaction,respectively.The linear dependence of lg(I/x)on lgx is shown in the inset of Fig.7 with an slope equaling -1.683 7.The value of θ is then calculated to be 5.051 1,close to 6,suggesting that dipole-dipole interaction is the main concentration quenching mechanism for Dy3+in α-Ba3Y(BO3)3and the exchange interaction can not be ignored,either.
In the unit cell of α-Ba3Y(BO3)3,Dy3+ions tend to occupy Y sites in series of paralleled planes due to the same valence and similar ion radius(0.091 2 nm for Dy3+and 0.090 0 nm for Y3+).However,there are 6 nearest Ba3/Ba4 sites for every Y site with an distance between 0.382 3 and 0.393 9 nm,making the exchange of Ba2+and Dy3+(Y3+)ions possible during the high temperature solid state reaction[18].In other words,the following situation exists in this case:most Dy3+ionsbetween these two luminescence centers,0.387 6/0.390 9 nm,is short enough for exchange interaction,making θ less than 6.
3.4 Lifetime Decay Curves
To investigate the essential luminescence of Dy3+,lifetime decay curves of α-Ba3Y1-x(BO3)3∶xDy3+are presented in Fig.9 as well as fitting results using the following second-order exponential function(2):where A1and A2are constants,t is the time,τ1and τ2are rapid and slow decay lifetimes for exponential components,respectively.Further more,based on parameters A1,A2,τ1and τ2in equation(3),the average lifetime(τ*)can be calculated by equation(3)[26]:

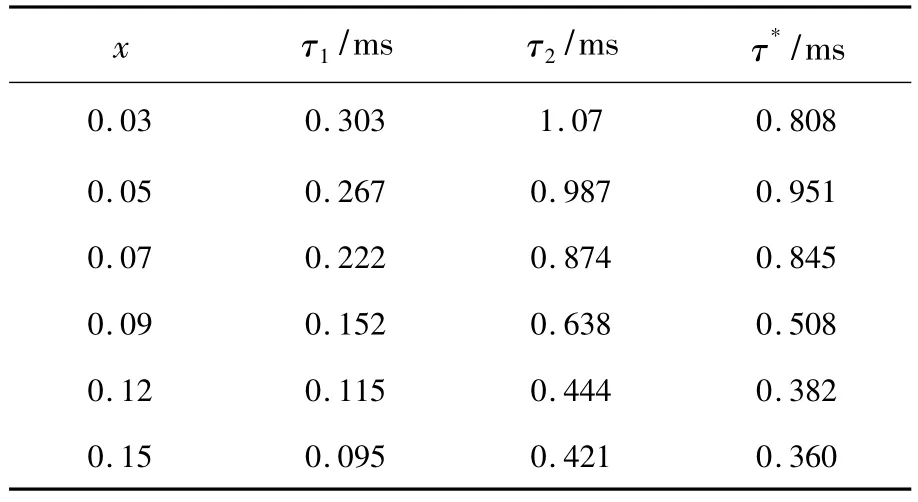
Tab.1 The values of τ1,τ2and τ*for decay curves ofα-Ba3Y1-x(BO3)3∶xDy3+.

Fig.9 Decay curves of α-Ba3Y1-x(BO3)3∶xDy3+(λex=350 nm,λem=577 nm,room temperature,x=0.03,0.05,0.07,0.09,0.12 and 0.15,respectively)and the corresponding fitting results(red lines)
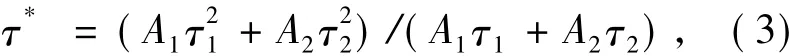
parameters τ1,τ2and τ*for decay curves of α-Ba3Y1-x(BO3)3∶xDy3+are demonstrated in Tab.1.The average distance between two Dy3+ions decreases with the increasing Dy3+concentration,which enhances the energy migration or cross-relaxation between different Dy3+ions via the dipole-dipole interaction or exchange interaction and decreases the luminescence lifetime from 0.808 ms to 0.360 ms[20,27-28]. The second-order exponential curves generate from the presence of two luminescence centers formed by Dy3+taking both Y1/Y2(CN:6)and Ba3/Ba4 sites(CN:9);the experimental behavior of α-Ba3Y1-x(BO3)3∶xDy3+can be ascribed
4 Conclusion
A series of Dy3+doped α-Ba3Y(BO3)3were synthesized by conventional solid state method at different temperature.The results indicate that phosphors synthesized at 1 100℃ have pure phase,most sufficient crystal growth and most intensive luminescence.Under 350 or 454 nm radiation,phosphors present white light emission with the CIE color coordinates around(0.273,0.327),making the phosphor suitable for pc-WLEDs.The luminescence intensity is strongly influenced by Dy3+concentration due to concentration quenching phenomenon.The concentration quenching mechanism and the secondorder exponential decay curves are discussed in detail and supposed to be related to the specific unit cell structure of α-Ba3Y(BO3)3.
Acknowledgements:This work was supported by Natural Science Foundation of Liaoning Province(20170540074).

