Research on Bipolar Membrane Electrodialysis for Ion Exchange of Molecular Sieves
Abstract: In view of the problems associated with large amount of discharged wastewater and serious pollution in the existing technology for removing sodium species from molecular sieves, this research work introduces the bipolar membrane electrodialysis into the process of removing sodium species from molecular sieves, and proposes a novel method of cleanly removing sodium from molecular sieves. The results show that the technology for removing sodium ions from the molecular sieves with an indirect electrodialysis process is feasible, and can recover NaOH solution. The bipolar membrane electrodialysis is especially suitable for treating the USY, ZSM-5 and Beta molecular sieves with high acid-resistance, and the physicochemical properties and catalytic performance of the prepared molecular sieves are roughly equivalent to those of the ammonium ion-exchange method. In comparison with the ammonium ion-exchange method, the process is clean and environmentally friendly,which consumes less water, and does not discharge wastewater to exhibit a rosy prospect of industrial application.
Key words: molecular sieve, ion exchange, bipolar membrane, electrodialysis
1 Introduction
As an important type of solid acid catalytic material,molecular sieve plays an important supporting role in petrochemical and coal chemical processes. The key molecular sieve catalysts in industry mainly comprise Y,MOR, ZSM-5, ZSM-22, ZSM-35, MCM-22, SAPO-34,TS-1, Beta, and SSZ-13 molecular sieve catalysts. The molecular sieve is generally a crystalline solid having regular pores prepared by hydrothermal crystallization of a synthetic gel consisting of a silicon source, an aluminum source, an alkali and/or organic amine template agent and water at a certain temperature. The byproducts in the production process of molecular sieves comprise a large amount of crystallization mother liquor, washing and exchanging wastewater, which contain a great deal of silicon, aluminum, sodium, ammonium salts, organic amine template agent, and the like. The concentration of pollutants far exceeds the national emission standards. The molecular sieve wastewater contains a high concentration of organic amines with bio-toxicity, and thus the wastewater cannot be treated by biochemical methods. The treatment methods such as supercritical deep oxidation,electro-flocculation, ultraviolet (UV) photocatalysis,adsorption and ion exchange have been tried, except for the incineration method, while there is no feasible route which can meet the national emission standard in the People’s Republic of China (PRC) specifying that the chemical oxygen demand (COD) should be less than 60 mg/L. The environmental protection issue has become the bottleneck for the development of the catalyst factory, and hence there is an urgent need to develop the low-cost and clean technology for production of molecular sieves.
The bipolar membrane electrodialysis can use salt as raw material to prepare acid and alkali, and has important application in the treatment of wastewater containing soluble electrolytes[1-2]. For example, the bipolar membrane electrodialysis method uses sodium silicate (i.e., water glass) as the raw material to produce silica sol and NaOH. Compared with the traditional ion exchange method for producing silica sol, the bipolar membrane electrodialysis method has the advantage of a simple process, since it does not produce wastewater,while a byproduct NaOH is produced at the same time[3].The research uses the properties of dissociating water for producing H+and OH-by means of bipolar membrane electrodialysis under the influence of an electric field in order to perform the cleaned removal of sodium from the molecular sieve and recover NaOH simultaneously.
2 Experimental
2.1 Experimental instruments and raw materials
The bipolar membrane electrodialysis device is an electrodialyzer manufactured by the ARDEX Company.The ion exchange membrane is a bipolar membrane(BPM), a cation exchange membrane (CEM) and an anion exchange membrane (AEM) produced by ASTOM Corporation in Japan.
The raw materials include ZSM-5, Beta, NaY and REUSY molecular sieves produced by the Changling Division of Sinopec Catalyst Co., Ltd.
2.2 Experimental method
Figure 1 illustrates the schematic diagram of the bipolar membrane electrodialysis used in the molecular sieve ion-exchange process. The molecular sieve and water are mixed and pulverized into slurry in a molecular sieve slurry tank, from which the slurry passes through a filter, and then the filtrate enters a bipolar membrane electrodialysis acid chamber as a reaction raw material.Under the action of a direct current (DC) electric field,the cations such as Na+and organic ammonium cations in the acid chamber pass through the cation exchange membrane and enter the alkali chamber to form alkali;the acid generated in the acid chamber enters then the molecular sieve slurry tank and performs ion exchange with the molecular sieve so that the molecular sieve is transformed into the H-type molecular sieve.
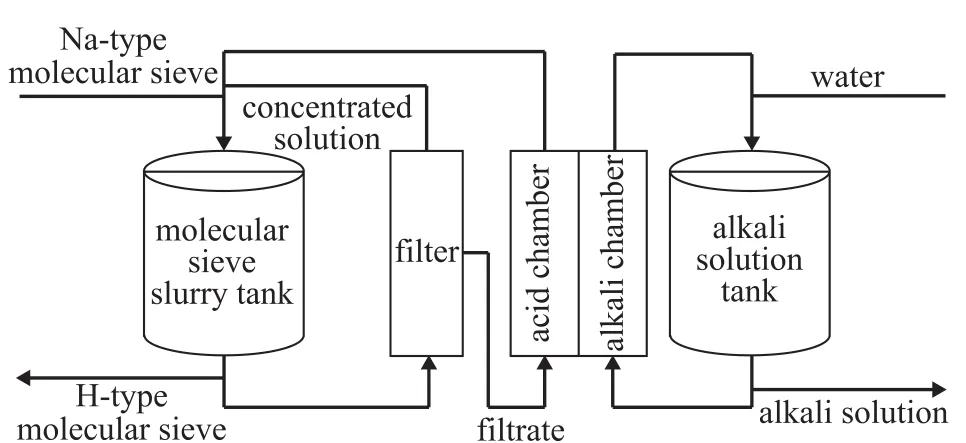
Figure 1 Schematic diagram of the bipolar membrane electrodialysis used in the molecular sieve ion-exchange process
2.3 Characterization method
The content of sodium ions in the molecular sieve is measured with a Rigaku ZSX Primus II-type X-ray fluorescence spectrometer by a label-free quantification method using a rhodium target operating at an excitation voltage of 50 kV and an excitation current of 50 mA.
The physical image and crystallinity of molecular sieve are characterized by a Philips X’Pert diffractometer using CuKα radiation operating at a tube voltage of 40 kV and a tube current of 40 mA, with the ZSM-5 slit width equating to 0.25°.The parameters of the sample pore are measured with an AS-6B physical adsorption instrument made by the Quantachrome Corporation in USA. The sample is heated to 300 °C under a vacuum of 1.3 Pa and degassed for 4 hours, and then the sample is kept in contact with nitrogen at an adsorption temperature of 77 K to reach the static adsorption equilibrium.
3 Results and Discussion
3.1 Principle of removing sodium from the molecular sieve and recovering template agent
Figure 2 illustrates the principle of removing Na+cations from the molecular sieve and recovering template agent by using the bipolar membrane electrodialysis. As shown in Figure 2, under the action of the DC electric field, the water molecules in the bipolar membrane are dissociated into H+and OH-ions, which enter the acid chamber and the alkali chamber, respectively[4]. In the acid chamber, H+cations bonded to the anions in the Na-type molecular sieve filtrate by means of bipolar membrane electrodialysis can generate acid for the ion exchange of molecular sieves, while in the alkali chamber, OH-anions bonded to Na+ions and organic ammonium cations in the Na-type molecular sieve filtrate upon passing through the cation exchange membrane enter the alkali chamber to generate a mixed solution of NaOH and organic ammonium cations, and the mixed solution is continuously concentrated during the reaction process so that sodium cations are removed from the molecular sieve along with recovery of the organic amine templating agent.
3.2 Results of sodium removal from several molecular sieves
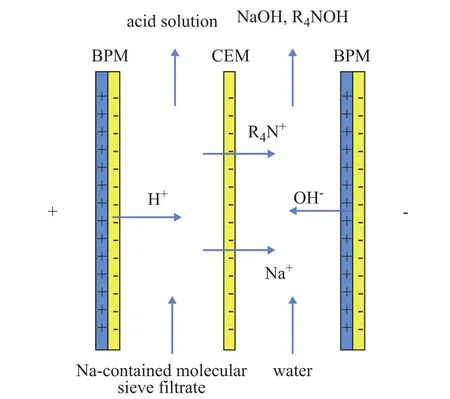
Figure 2 Principle of removing Na+ cations from the molecular sieve and recovering template agent by using the bipolar membrane electrodialysis
The results of sodium removal via the indirect method from NaY molecular sieve are shown in Figure 3. Under the action of an electric field, the bipolar membrane dissociates water to form H+cations and OH-anions, wherein the H+cations migrate into the acid chamber to gradually conduct ion exchange reaction with Na+cations in NaY molecular sieves, and then the Na+cations in the molecular sieve are replaced by H+cations, in the meantime the content of Na2O dramatically decreases from 12.9% to 3.76%so that the effect of electrodialysis on sodium removal is significant. Meanwhile, because the NaY molecular sieve has a low silica-alumina ratio and it is sensitive to pH value, the crystallinity in the sodium removal process is reduced from the initial value of 83% to 40%.

Figure 3 Effect of sodium removal from NaY molecular sieve and the variation in crystallinity
The results of sodium removal from the REUSY, Beta,and ZSM-5 molecular sieves are shown in Figure 4.As illustrated in Figure 4, when the bipolar membrane electrodialysis is used for removing sodium cations from the molecular sieve, the pH value of the molecular sieve slurry is lowered, Na+cations in the molecular sieve are gradually replaced by H+cation, and the content of Na2O is decreased. The Na2O content in the REUSY molecular sieve decreases from 3.5% to 1.1%, and the Na2O content of the Beta molecular sieve declines from 2.15% to 0.43%,while the Na2O content of the ZSM-5 molecular sieve reduces from 4.22% to 0.5%. The effect of electrodialysis on sodium removal is obvious. The decreased pH value of the slurry has a slight influence on the crystallinity of the REUSY, ZSM-5 and Beta molecular sieves. This outcome is mainly attributed to the high silica-aluminum ratio and the desirable acid resistance of the three molecular sieves.
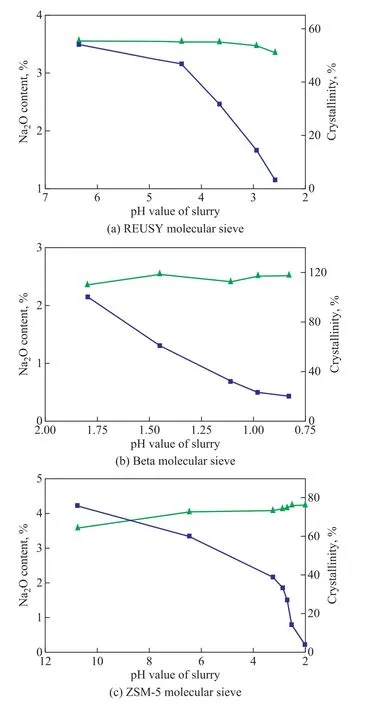
Figure 4 Effect of sodium removal from REUSY, Beta,ZSM-5 molecular sieves and variation in crystallinity
Figure 5 shows the curves related with the variation in Na content in the electrodialysis acid chamber, the alkali chamber, and the molecular sieve samples during the process of removing sodium with electrodialysis. As illustrated in Figure 5, the Na content in the molecular sieve and the electrodialysis acid chamber decreases with the elapse of reaction time, while the Na content in the alkali chamber increases gradually. The reason lies in that the bipolar membrane dissociates water to produce H+cations and OH-anions under the action of an electric field. The anions in the acid chamber are impervious due to the selectivity of the cation exchange membrane, the anions combine with H+cations to form an acid solution.After the ion exchange reaction taking place between the acid liquid and the molecular sieve, the Na+cations in the molecular sieve gradually reduce while migrating to the alkali chamber via the cation exchange membrane under the action of the electric field in order to be bonded with the OH-anions generated by the bipolar membrane to form NaOH. About 75% of Na+anions in the system can migrate and pass through the cation exchange membrane to be converted into NaOH at the end of the sodium removal reaction.

Figure 5 Equilibrium curve of sodium anions in the course of removing sodium from molecular sieve
It can be derived from the sodium removal experiments of four common molecular sieves, namely NaY, ZSM-5,Beta and REUSY molecular sieves, that the bipolar membrane electrodialysis can be used for removing Na+cations contained in molecular sieves, and the bipolar membrane electrodialysis is particularly suitable for molecular sieves having a high silica-alumina ratio and desirable acid resistance, while the process product NaOH can be recovered. The indirect method of sodium removal may be suitably adopted in order to ensure the stable operation of the equipment.
3.3 Properties of molecular sieves after sodium removal through electrodialysis
Table 1 illustrates the results of characterizing physicochemical and catalytic performance of the REUSY and ZSM-5 molecular sieves prepared by selecting the electrodialysis sodium removal method and the ammonium ion-exchange method, respectively. As shown in Table 1, the two methods for removing sodium (i.e.,electrodialysis and ammonium ion-exchange methods)are not significantly different in terms of the effect for removing sodium. The physicochemical properties such as crystallinity, pore volume, specific surface area of the fresh samples of molecular sieves prepared by the two methods for removing sodium are roughly similar.After being subject to aging with 100% water vapor at a temperature of 800 °C for 17 h, the physicochemical properties such as crystallinity, pore volume, specific surface area of the ZSM-5 molecular sieves prepared by the two methods for removing sodium are substantially identical, however, the crystallinity, pore volume and specific surface area of the REUSY molecular sieve prepared by the bipolar membrane electrodialysis after the aging process are slightly lower than those of the REUSY molecular sieve prepared by the ammonium ion-exchange method. It can occur because the process for removing sodium by bipolar membrane electrodialysis is essentially an acid ion-exchange process, which has a small influence on the physicochemical properties of ZSM-5 molecular sieves with a high silica-alumina ratio and a desirable acid resistance, but it imposes a significant influence on the physicochemical properties of REUSY molecular sieves having a low silica-alumina ratio and a poor acid resistance.
Table 2 illustrates the comparison of catalytic properties between the aged samples of molecular sieves prepared by two sodium removal methods. As shown in Table 2, compared with the molecular sieve prepared by ammonium ion-exchange, the gasoline yield of the REUSY molecular sieve prepared by bipolar membrane electrodialysis increases slightly, and the coke yielddecreases, but the catalytic properties of the molecular sieved prepared by two methods are still basically equivalent. With respect to ZSM-5 molecular sieve, the catalytic properties of the molecular sieves prepared by the electrodialysis and ammonium ion-exchange methods are fundamentally consistent.
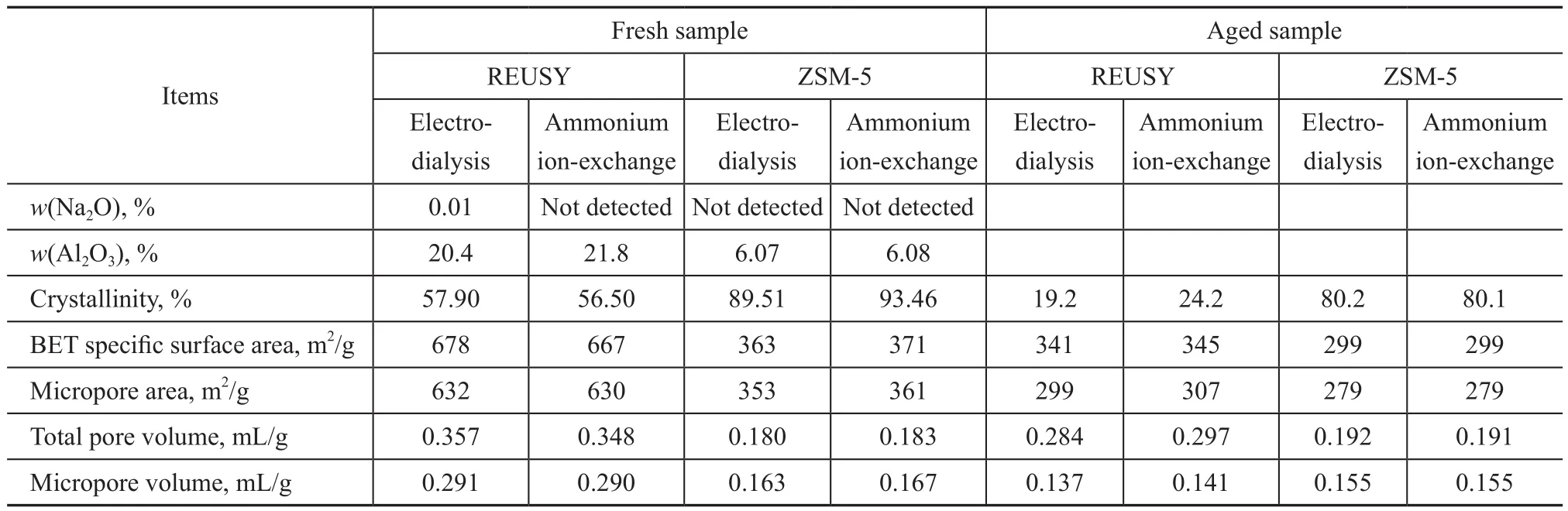
Table 1 Comparison of physicochemical properties of molecular sieves prepared by two sodium removal methods
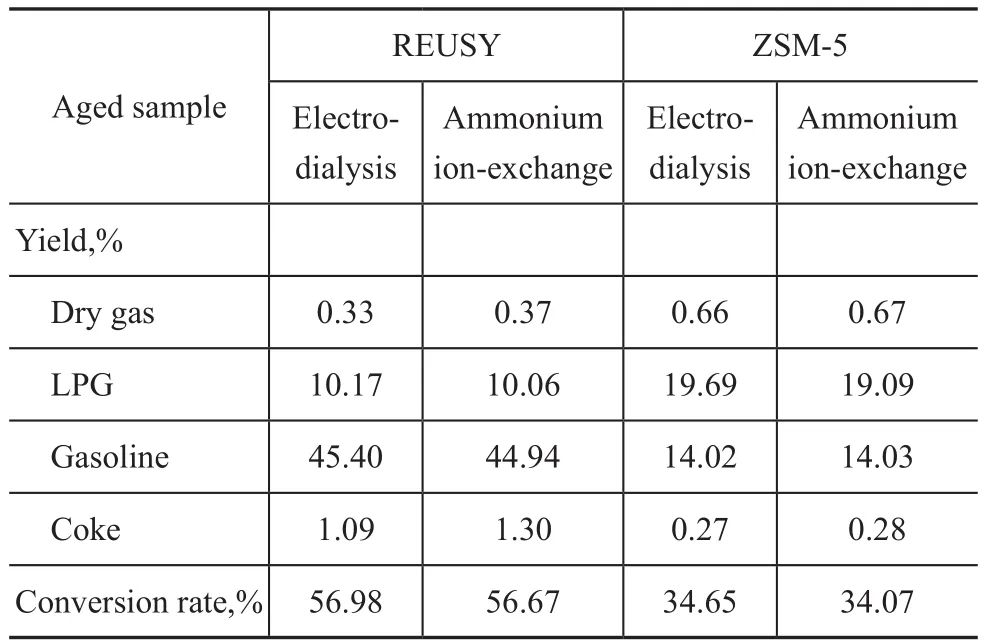
Table 2 Comparison of catalytic properties of aged samples of molecular sieves prepared by two sodium removal methods
4 Conclusions
In view of the problems of serious pollution in the existing technology for removing sodium from molecular sieves, this research work introduces for the first time the bipolar membrane electrodialysis into the process of removing sodium from molecular sieves, and proposes a novel method for removing sodium cleanly. The results show that the technology for removing sodium ions contained in the molecular sieves with an indirect bipolar membrane electrodialysis process is feasible,and can recover NaOH solution. The bipolar membrane electrodialysis is especially suitable for the USY, ZSM-5 and Beta molecular sieves with high acid-resistance, and the physicochemical properties and catalytic performance of the molecular sieves prepared by the bipolar membrane electrodialysis are roughly equivalent with those of the ammonium ion-exchange method. As compared with the ammonium ion-exchange method, the process is clean and environmentally friendly, consumes less water, and does not discharge wastewater, which can thereby exhibit a rosy prospect of industrial application.
Acknowledgments:The work is financially supported by the National Basic Research Program of China (973 Program) under the Grant No.2015AA03A061.
- 中国炼油与石油化工的其它文章
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Research on Catalytic Cracking Performance Improvement of Waste FCC Catalyst by Magnesium Modification
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield

