Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
Guo Pengfei; Yang Jingyi; Pelayo Envo Esono Maye; Xu Xinru
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology,Shanghai 200237)
Abstract: A series of highly cross-linked polymeric ionic liquids P[Ci(Vim)2][Cl]2 (i = 2, 3, 4, 5, 6) were synthesized by quaternization reaction and polymerization, and used to remove nitrogen compounds from oils. The polymeric ionic liquids P[Ci(Vim)2][H2PO4]2 (i = 2, 3, 4, 5, 6) were then obtained via ion exchange. The structures of P[C4(Vim)2][Cl]2 and P[C4(Vim)2][H2PO4]2 were characterized by Fourier transform infrared (FT-IR) spectroscopy, energy dispersive spectrometry(EDS), N2 adsorption-desorption isotherm measurements, scanning electron microscopy (SEM), thermogravimetric analysis and differential scanning calorimetry (TG/DSC). The removal of nitrogen compounds was characterized by pyridine-FTIR spectrometry. The results indicated that P[C4(Vim)2][H2PO4]2 with an average pore size of 19.23 nm and a specific surface area of 11.78 m2/g was efficient for the removal of nitrogen compounds, and exhibited good thermal stability. The adsorption rate in the simulated oil reached 93.8% when using a polymeric ionic liquid P[C4(Vim)2][H2PO4]2 to oil ratio of 0.04 and a temperature of 313 K. The nitrogen removal rate from the coal-tar diesel fraction achieved by P[C4(Vim)2][H2PO4]2 was 90.3%.
Key words: nitrogen removal; ionic liquids; polymer; immobilization
1 Introduction
Coal tar is the main by-product of the coal coking and gasification process[1]. Low-temperature coal tar is quite complicated in composition, which contains a significant amount of phenols, sulfur, and 0.48%―0.82% of nitrogen compounds[2]. Nitrogen compounds in low-temperature coal tar can be basic or non-basic nitrogen compounds,mainly in the form of heterocyclic aromatic compounds,which are difficult to remove by hydrogenation[3].These compounds not only can affect the stability of the products, but also can be adsorbed onto the catalyst surface during the hydrogenation process, leading to reductoin of the catalytic activity. The National VI standard on fuel quality has been implemented in China,specifying the sulfur content in automotive gasoline and diesel fuel to be less than 10 μg/g. Thereby the inhibiting effect of nitrogen compounds on deep desulfurization has been attracting more and more attention.
The processes for removal of nitrogen compounds from petroleum products and coal tar include hydrogenation,adsorption, extraction, and oxidation[4], etc. In recent years, the use of ionic liquids for the removal of nitrogen compounds from petroleum products and coal tar has been attracting the attention of researchers thanks to the mild reaction conditions, high selectivity and simplified operating process[5]. Ravilla[6]studied the liquid-liquid equilibrium data of three different ionic liquids, and selected imidazolium ionic liquids, which could effectively remove pyridine in the simulated oil. Studies by Huh[7]showed that an ionic liquid containing zinc could effectively remove quinoline, acridine and indole in the simulated oil, and could increase the efficiency for removal of basic nitrogen compounds when it is used in tandem with the Lewis acid ZnCl2. Lin[8]used a sulfonic acid functionalized ionic liquid as a nitrogen removing agent, and showed that it could effectively remove the basic nitrogen compounds from petroleum coke-derived diesel fuel. A study by Xie[9]showed that chlorinated imidazolium ionic liquids had a highly selective effect for removal of non-basic nitrogen compounds in a simulated coal-tar diesel fraction.However, high viscosity and secondary pollution resulted from oil treatment are barriers to the practical application of ionic liquids. If ionic liquids can be immobilized and used as adsorbents, it may be possible to avoid the above-mentioned barriers to the use of ionic liquids.One approach would be adopted to synthesizing the ionic liquid polymers, which could have the structural properties of both ionic liquids and polymers[10]. They are a kind of special polyelectrolyte, which can be solidified from ionic liquids[11]. There are two main synthesis[12]pathways, namely: polymerization of ionic liquid monomers and modification of polymer products by ionization. By means of the former approach, polymeric ionic liquids (PILs) can be gradually transformed from a liquid into a solid. Through polymerization, the porosity and specific surface area can be changed, and the solubility of the solvent is reduced[13]. Meanwhile, the adsorption performance is improved, and the secondary pollution can be greatly reduced[14]. In addition, the practical application of ionic liquids can be promoted for nitrogen removal. The second method aims to modify the polymer by quaternization reaction to create a structure which is conducive to adsorption.
In the present study, based on the simulation of a coaltar diesel fraction, the highly cross-linked polymeric ionic liquids with different anions were synthesized as the novel,green and reusable sorbents for adsorptive denitrification.FT-IR spectrometry, SEM and TG/DSC techniques were employed to characterize the properties of the synthesized samples. The mechanism for nitrogen compound removal and the reusability performance of the polymeric ionic liquids used for denitrification were also discussed.
2 Experimental
2.1 Reagents and materials
Vinyl imidazole (98.0%), 1,2-dichloroethane (98.0%),1,3-dichloropropane (98.0%), 1,4-dichlorobutane(98.0%), 1,5-dichloropentane (98.0%), and 1,6-dichlorohexane (98.0%) were purchased from the Aladdin Industrial Corporation (Shanghai, China). DMF(99.5%), azodiisobutyronitrile (99.5%), ethyl acetate(99.5%), dodecane (99.5%), and phosphoric acid (99.5%)were purchased from the Shanghai Titan Scientific Co.,Ltd (Shanghai, China).
2.2 Preparation of polymeric ionic liquids
An ionic liquid was synthesized with vinyl imidazole and dichloroalkane, then polymeric ionic liquids with different anions were polymerized via ionic exchange.
Preparation of ionic liquids: 0.05 mol of vinyl imidazole and 0.025 mol of dichloroalkane (1,2-dichloroethane,1,3-dichloropropane, 1,4-dichlorobutane,1,5-dichloropentane or 1,6-dichlorohexane) were poured in sequence into a 50-mL flask under a N2atmosphere.The mixture was heated at 333 K for 24 h. Then the reaction mixture was cooled down to room temperature,and the products were washed three times with ethyl acetate to remove the unreacted material. A yellow ionic liquid [Ci(Vim)2][Cl]2(i= 2, 3, 4, 5, 6) was obtained by drying at 323 K for 4 h under vacuum, and was labeled as IL-as(s= 1, 2, 3, 4, 5), as outlined in Scheme 1 (in which[C4(Vim)2][Cl]2is taken as an example).
Preparation of polymeric ionic liquids: 5 g of IL-as and AIBN (used as initiator) were added to 50 mL of DMF solution. Under a N2atmosphere, the mixture was heated at 373 K for 18 h. The as-obtained solid was filtered out and the impurity was extracted by a Soxhlet extractor using dichloromethane as the solvent,then the solid was subjected to drying at 323 K for 6 h under vacuum to generate the adsorbents PIL-as(s= 1, 2, 3, 4, 5), as outlined in Scheme 2 (in which P[C4(Vim)2][Cl]2is shown as an example).

Scheme 1 Schematic illustration of the synthesis for IL-a3

Scheme 2 Schematic illustration of the synthesis for PIL-a3
Preparation of P[Ci(Vim)2][H2PO4]2(i= 2, 3, 4, 5, 6): PIL-as(s= 1, 2, 3, 4, 5) and an equimolar amount of phosphoric acid were added to dichloromethane solution. Under a nitrogen atmosphere, the mixture was stirred at 298 K for 24 h. The product P[Ci(Vim)2][H2PO4]2(i=2, 3, 4, 5, 6) was obtained after drying at 323 K for 5 h under vacuum, which was labeled as PIL-bs(s= 1, 2, 3, 4, 5), as outlined in Scheme 3 (in which P[C4(Vim)2][H2PO4]2is shown as an example).

Scheme 3 Schematic illustration of the synthesis for PIL-b3
2.3 Characterization of polymeric ionic liquids
The polymeric ionic liquid samples were analyzed by a Nicolet 6700 type Fourier transform infrared (FT-IR)spectrometer by using anhydrous KBr as the dispersing agent with a scanning range of 4 000―400 cm-1at an accuracy of 2 cm-1. A Falion 60S type energy dispersive spectrometer (EDS) was used to study the surface elemental composition of the products. The N2adsorption-desorption isotherms of polymeric ionic liquid samples were measured by an ASAP 2010 N surface area and porosimetry system in order to estimate the specific pore size and surface area of samples under a pressure of 3 MPa. The scanning electron microscope (SEM)images were obtained with a JSM-6360LV instrument in order to observe the morphology of the products.The thermogravimetric analysis-differential scanning calorimetry (TG-DSC) was carried out by using a SDT Q600 apparatus, and the temperature was elevated from ambient temperature to 1 073 K at a temperature increase rate of 10 K/min while the nitrogen flow rate was held constant at 100 mL/min.
2.4 Preparation of raw oil fraction and denitrification
The simulated oil which contained 1 500 mg/L of nitrogen was prepared by dissolving pyridine (as a source of basic nitrogen) in dodecane.
The coal-tar diesel fraction was obtained by collecting the distillation fraction of coal tar recovered between 493 K and 633 K. The total nitrogen content was 2 717 μg/g,the basic nitrogen content was 1 542 μg/g, and the non-basic nitrogen content was 1 175 μg/g. The total nitrogen content in the sample was measured by an Antek 9 000 nitrogen/sulfur analyzer according to the ASTM D4629-17 method for testing the trace nitorgen by chemiluminescence.The basic nitrogen content was measured in accordance with the SH/T 0162―92 method for testing the basic nitrogen content in petroleum products.
Specified amounts of polymeric ionic liquids and raw oil were accurately added to a stopper flask. After a specified temperature was reached, the mixture was kept at that temperature for a predetermined period of time, and the nitrogen content of the oil was measured.
The polymeric ionic liquid was washed in batches with 100 g of petroleum ether and dried at 373 K until it reached a constant weight before next run for recycling.
3 Results and Discussion
3.1 Polymeric ionic liquids characterization
3.1.1 FT-IR analysis
The FT-IR spectra of IL-a3, PIL-a3 and PIL-b3 are displayed in Figure 1. In the FT-IR patterns of IL-a3,the peak at 3 102.9 cm-1comes from C-H antisymmetric stretching vibration of the carbon-carbon double bonds on vinyl and imidazole rings. The peak at 1 496.1 cm-1is ascribed to the stretching vibration of C=C bonds on the imidazolium cation. The peak at 1 282.6 cm-1represents the stretching vibration of C-N and C=N bonds on the imidazolium cation. The peak at 1 230.2 cm-1comes from C-H deformation vibration and C-N stretching vibration associated with tertiary N on the imidazolium cation. The peak at 1 649.1 cm-1is the stretching vibration of vinyl C=C bonds. In the FT-IR patterns of PIL-a3, the C=C absorption peak does not appear, and the strong peak at 1 159.8 cm-1is ascribed to the deformation vibration of C-H in the plane of the imidazolium cation which only occurrs in PILs. In the FT-IR patterns of IL-a3 and PIL-a3, the peaks at 3 000.9 cm-1and 2 961.3 cm-1, and those peaks at 3 130.9 cm-1and 3 089.1 cm-1correspond to the stretching vibration of C-H on alkane chains acting as cross-linking agents. These spectra can support that cross-linking takes place during polymerization of the ionic liquid to form PIL-a3. In the FT-IR patterns of PIL-b3, the emerging peaks at 1 082.5 cm-1and 987.1 cm-1are attributed to the stretching vibration of P=O and P-O bonds, indicating to the existence of H2PO4-,while the deformation vibration of C-H in the plane of the imidazolium cation is located at 1 159.6 cm-1, indicating to the formation of PIL-b3.
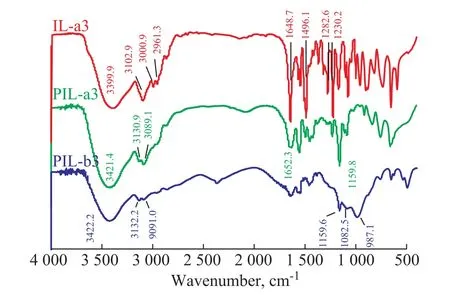
Figure 1 FT-IR patterns of IL-a3, PIL-a3 and PIL-b3
3.1.2 EDS
The ionic liquid polymers PIL-a3 and PIL-b3 were studied by EDS, with the elemental analysis results shown in Table 1.
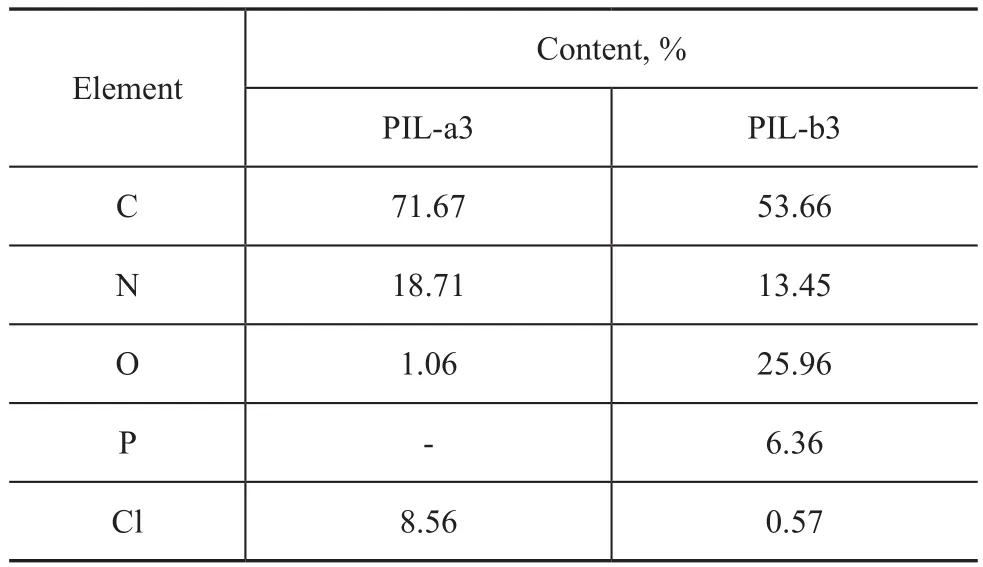
Table 1 Elemental analysis of PIL-a3 and PIL-b3
The results of elemental analysis show that the proportion of C, N and Cl in PIL-a3 was 71.67%, 18.71% and 8.56%, respectively. The carbon to nitrogen ratio of PIL-a3 is about 3.83, which is roughly equal to the carbon to nitrogen ratio of PIL-b3, viz. 3.98, and it shows that there is no obvious change in the elements on the carbon skeleton. The Cl to N ratio of PIL-b3 is 0.042,in comparison with the Cl to N ratio of 0.458 in PIL-b3,which shows the outcome of ion exchange. During the reaction process, H2PO4-groups are used to substitute for Cl-groups to increase the molecular mass, resulting in a decrease in the percentage of C and N content, but the ratio between the C and N does not change, with 90.0%of the Cl-groups being replaced.
3.1.3 Textural characterization
The specific surface area of PIL-a3 and PIL-b3 was analyzed by the BET technique, with the results presented in Table 2. The specific surface area of two samples was 15.9 m3/g and 11.78 m3/g, respectively, which could classify them as mesoporous structures.

Table 2 Textural characterization of adsorbents

Figure 2 SEM pattern of PIL-b3
Obviously, PIL-b3 is a fluffy powder. As shown in Figure 2, the surface of PIL-b3 appears to be a coarse aggregate of superfine particles. This structure suggests that the sample is suitable for use as an adsorbent with large adsorption capacity.
3.1.4 TG/DSC analyses
The thermal stability of PIL-b3 is shown in Figure 3.About 3% of weight loss occur between 313 K and 473 K, which is likely caused by loss of moisture from the product.
The initial thermal decomposition temperature of PIL-b3 is 509 K, and the main decomposition temperature is above 509 K. Judging from the DSC curve, it can be seen that there are two obvious endothermic peaks at 593 K (Tb) and 633 K (Tc), indicating that the process of PIL-b3 decomposition is endothermic. PIL-b3 can be decomposed into small alkyl imidazole molecular fragments by reversing the quaternization reaction. The subsequent inverted endothermic peak appears to be originated from the decomposition of these low molecular weight fragments. Therefore, the temperature for application of PIL-b3 should be kept at below 509 K.

Figure 3 The TGA/DSC pattern of PIL-b3
3.2 Denitrification of polymeric ionic liquids
The denitrification performance of PIL-asand PIL-bsin the simulated oil was investigated under different conditions. The effect of time for regeneration of PIL-b3 in the simulated oil and the effect of polymeric ionic liquid PIL-b3 in coal-tar diesel fraction were also investigated.
3.2.1 Effect of cross-linking agents on nitrogen removal
The effects of PIL-asand PIL-bson removal of nitrogen from the simulated oil were investigated under conditions covering a temperature of 313 K, a polymeric ionic liquid to oil ratio of 0.04 (w/w), and a reaction time of 1 h.

Figure 4 The effect of cross-linking agents on nitrogen removal
Figure 4 shows the effect of cross-linking agents on nitrogen removal. It can be seen that both PIL-a3 and PIL-b3 had the highest rate of nitride removal albeit with a slight difference when 1,4-dichlorobutane is used as the cross-linking agent, and the performance of PIL-b3 is better than PIL-a3. When the number of carbon chains is small, the distance between imidazole rings is small.The steric hindrance caused by imidazole rings can hinder the π-π interaction between imidazole ring and pyridine. When the carbon number increases, the longer carbon chains can create steric hindrance between the cationic groups and the nitrogen compounds. The spacing between the imidazole rings can exhibit a best synergistic effect on the absorption of nitrogen compounds when 1,4-dichlorobutane is used as the cross-linking agent.
3.2.2 Effect of polymeric ionic liquids/oil ratio on nitrogen removal
The effects of the ratio of PIL-a3 or PIL-b3 to oil on nitrogen removal in the simulated oil were investigated under conditions covering a temperature of 313 K and a reaction time of one hour.

Figure 5 Effect of polymeric ionic liquids to oil ratio on nitrogen removal
As displayed in Figure 5, the rate of nitrogen removal was increased with an increasing IL polymer/oil ratio. This could be likely caused by an increase in the amount of IL polymer, which would be beneficial to the probability of collisions between the pyridine and the ionic liquid. The nitrogen removal rate of PIL-b3 increased from 64.2% to 93.8% and the nitrogen removal rate of PIL-a3 increased from 20.3% to 56.3%, when the polymeric ionic liquids to oil mass ratio was raised from 0.01 to 0.06. At an identical polymeric ionic liquids/oil ratio, the nitrogen removal rate of PIL-b3 was higher than that of PIL-a3.When the polymeric ionic liquids/oil ratio was between 0.01 and 0.04, the N removal rate increased rapidly, but there was little change when the polymeric ionic liquids/oil ratio became greater than 0.04. Hence, an IL polymer to oil mass ratio of 0.04 is suitable for application.
3.2.3 Effect of denitrification temperature on nitrogen removal
The effect of temperature of PIL-a3 and PIL-b3 on nitrogen removal from the simulated oil was investigated at a polymeric ionic liquids to oil ratio of 0.04 and a reaction time of 1 h.
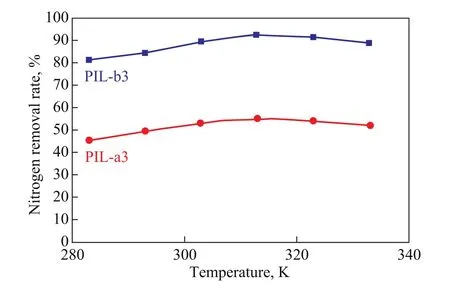
Figure 6 Effect of denitrification temperature on nitrogen removal
As shown in Figure 6, the complex denitrogenation reaction of the ionic liquid polymer was exothermic, and a low temperature was beneficial to the reaction. But at the beginning of the reaction, an elevated temperature was required, and the mass transfer rate between oil and polymeric ionic liquid could be improved. Therefore, the rate of nitrogen removal increased when the temperature rose from 293 K to 313 K. At a temperature of higher than 313 K, the nitrogen removal rate decreased, showing it was not as conducive to the exothermic denitrogenation reaction. Hence, the optimum reaction temperature was 313 K. At an identical temperature, the nitrogen removal rate of PIL-b3 was higher than that of PIL-a3.
3.2.4 Effect of denitrification time on nitrogen removal
The effect of reaction time of PIL-a3 and PIL-b3 on nitrogen removal was investigated at a temperature of 313 K and a polymeric ionic liquids/oil ratio of 0.04.

Figure 7 Effect of denitrification time on nitrogen removal
Judging from the results shown in Figure 7, it can be seen that the rate of nitrogen removal using PIL-b3 increased from 82.7% to 93.8% when the reaction time was increased from 15 min to 75 min. The rate of nitrogen removal using PIL-a3 increased from 27.89% to 61.43%,when the reaction time was increased from 15 min to 90 min. The nitrogen removal increased with a longer reaction time, but the rate of nitrogen removal became smaller. When the denitrification reaction time in the presence of PIL-b3 was more than 75 min, the nitrogen removal rate was basically unchanged, indicating that the reaction had almost reached an equilibrium, and the nitrogen removal effect tended to be stable. At the same reaction time, the nitrogen removal rate of PIL-b3 was higher than that of PIL-a3, since PIL-a3 needed more time than PIL-b3 to achieve an equilibrium. The optimum reaction time using PIL-b3 was found to be 75 min, and it should be 90 min when PIL-a3 was used.
3.2.5 Polymeric ionic liquid recycling
The above results indicate that PIL-b3 is more effective than PIL-a3 for denitrification. We performed experiments at 313 K and an IL polymer/oil ratio of 0.04 for 75 min to test the reusability of PIL-b3. After each reaction cycle,PIL-b3 was recovered by filtration and then flushed with petroleum ether. After having been dried under vacuum,the adsorbent was reused for the next run.

Figure 8 Effect of cycle times on nitrogen removal
Based on the results shown in Figure 8, the nitrogen removal rate remained at more than 90% across six runs. As the regeneration number increased, the nitrogen removal rate decreased slightly. It is possible that a small amount of nitride remained in the pores of PIL-b3 after regeneration, which could affect the efficiency for removal of nitride in the simulated oil.
3.2.6 Effect of IL polymer/oil ratio on removal of nitrogen from coal-tar diesel fraction
Due to the high nitrogen content of coal tar, the effect of different polymeric ionic liquids to oil ratios on nitrogen removal from coal-tar diesel fraction was tested using PIL-b3 at a temperature of 313 K for 75 min, with the results shown in Figure 9.

Figure 9 Effect of polymeric ionic liquids to oil ratio on nitrogen removal from coal-tar diesel fraction
When the IL polymer to oil ratio increased from 0.01(mass ratio) to 0.1, the nitrogen removal rate increased from 38.2% to 90.66%. The nitrogen removal rate did not increase significantly when the IL polymer to oil ratio was greater than 0.07. At an optimal cost, a suitable solventto-oil ratio would be 0.07, which could provide a nitrogen removal rate of 90.3%.
3.3 Mechanism of nitrogen removal from polymeric ionic liquids
Pyridine was used as the probe molecule, which was absorbed at 313 K for 30 min in PIL-a3 and PIL-b3 using anhydrous KBr as a dispersing agent (in the case of pyridine-FTIR study at a mass ratio of KBr to sample of 10:1).
It can be seen from Figure 10 that the pyridine absorption peaks appear at 1 441.1 cm-1, which indicates the existence of physical adsorption between the PIL-a3 and pyridine. Physical adsorption of nitrogen compounds may be attributed to the use of π-π interactions from the imidazole ring and multidimensional channel trapping from weak interactions.

Figure 10 Pyridine-FT-IR patterns of PIL-a3
As shown in Figure 11, the presence of pyridine peaks at 1 441.3 cm-1indicates the existence of physical adsorption between PIL-b3 and pyridine. The enhancement of absorbance near 1 550 cm-1indicates that PIL-b3 has a Brönsted acid site due to the presence of H2PO4-. Acidic sites can cause chemisorption of nitrogen compounds.Since PIL-b3 needs less time than PIL-a3 to achieve an equilibrium, we have found that chemisorption requires less time than physisorption to reach the equilibrium,which indicates that chemisorption can achieve the equilibrium more quickly. Chemisorption is generated by the acid-base complexation to provide H+ions and nitrogen compounds with a lone pair of electrons. When these effective complexation sites are fully occupied,chemisorption reaches a saturation state. Then the physisorption would take place. This type of adsorption comes from the π-π complexation between imidazolium cations and nitrogen compounds as well as weak interactions between the surfaces of polymeric ionic liquid and nitride. The three-dimensional pore structure and microstructure can play a role in the retention and adsorption of nitrogen compounds, so that the pore structure can contribute to physisorption. The adsorption mechanism of nitride is shown in Figure 12.

Figure 11 Pyridine-FT-IR patterns of PIL-b3

Figure 12 Adsorption mechanisation of two-dimensional ionic liquid polymer
4 Conclusions
An ionic liquid ([C4(Vim)2][Cl]2) containing carboncarbon double bonds was obtained by a quaternary ammonium reaction using vinyl chloride and dichloroalkane. Subsequently, a highly cross-linked polymeric ionic liquid (P[C4(Vim)2][Cl]2) was prepared by polymerization using 1,4-dichlorobutane as a cross-linking agent. Then the acidic ionic liquid polymer (P[C4(Vim)2][H2PO4]2) was obtained via ion exchange. PIL-b3 has the advantage of good thermal stability coupled with a thermal decomposition temperature of above 509 K to be a mesoporous material. PIL-a3 and PIL-b3 have been characterized and compared, leading to the conclusion that PIL-b3 had good adsorption performance, which was characterized by both chemisorption and physisorption tests, while its good regeneration performance in the simulated oil was confirmed. Under conditions covering a polymeric ionic liquid to oil ratio of 0.04, a reaction time of 1 h, and a temperature of 313 K, the nitrogen removal rate reached 93.8% from the simulated oil. In addition,the used PIL-b3 could be easily recovered and reused without any noticeable loss of activity for at least six consecutive runs. And at a polymeric ionic liquid to oil ratio of 0.07, a reaction time of 1 h and a temperature of 313 K, the nitrogen removal rate reached 90.3% from the coal-tar diesel fraction.
- 中国炼油与石油化工的其它文章
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Research on Catalytic Cracking Performance Improvement of Waste FCC Catalyst by Magnesium Modification
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield

