Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
Zhao Hong; Ye Xinxin; Yan Rui; Liu Xiaohui; Guo Yong; Wang Yanqin
(1. SINOPEC Research Institute of Petroleum Processing, Beijing 100083;2. Shanghai Key Laboratory of Functional Materials Chemistry, Research Institute of Industrial Catalysis,East China University of Science and Technology, Shanghai 200237)
Abstract: Efficient and selective production of 5-hydroxymethylfurfural (HMF) from glucose was achieved in the presence of zirconium phosphate (ZrPO) catalyst in a biphasic system. With the use of this catalyst, a high HMF yield of 56.8% was obtained from glucose in a water-tetrahydrofuran (THF) biphasic system. Characterization results showed that such catalyst had weak to strong acid sites and contained both Lewis and Brönsted acid sites. The results of comparative experiments over some other solid acid catalysts demonstrated that the Lewis acid sites on the ZrPO catalyst played a crucial role in the isomerization of glucose to fructose and the Brönsted ones were active in the dehydration of generated fructose to HMF.Moreover, less levulinic acid (LA) and formic acid (FA) (0.5%) were detected in the reaction solution, indicating that this ZrPO catalyst exhibited high selectivity towards the formation of HMF. Furthermore, the ZrPO catalyst was very stable and could maintain its activity after being used for six times.
Key words: zirconium phosphate; HMF; glucose; biphasic
1 Introduction
With the rapid depletion of fossil fuel resources and increasingly tightening environmental regulations,the production of energy and chemicals from sustainable carbon sources has become a hot topic in recent decades[1-3]. Biomass, the most abundant renewable resources on earth, is a promising alternative carbon resource for the supply of intermediates and platform chemicals to the industry[4-6]. Among them,5-hydroxymethylfurfural (HMF) is regarded as a versatile and important intermediate, which can be obtained from biomass-based carbohydrates, and has been identified as a primary building block that can be further transformed into a broad range of fine chemicals, polymeric materials,and biofuels[7-9].

Scheme 1 Scheme of HMF production from glucose by a combined isomerization/dehydration reaction
C6sugars, such as glucose and fructose, are often used as feedstocks to produce HMF. In the past few years,numerous research groups chose fructose as the preferred substrate and excellent HMF yields could be readily achieved using many proper methods and catalysts[10-12].However, it should be pointed out that fructose is seldom available and the price is high, which limits the practical production of HMF in large scale. Compared to fructose,glucose is the most abundant and cheapest hexose, which is a better candidate as a potential resource for producing HMF. However, direct production of HMF from glucose is unsuccessful because the isomerization of glucose into fructose seems to be more difficult by the conventional acid catalysts[4,13-15], resulting in low selectivity to HMF due to the formation of by-products. Generally speaking,it is considered that the conversion of glucose to HMF proceeds via two steps: at first the isomerization of glucose to fructose in the presence of enzyme, Lewis acid or base catalysts[16-17], followed by dehydration of the generated fructose to HMF (as shown in Scheme 1).Up to now, various catalysts have been developed for the production of HMF from monosaccharide, including the Lewis acid catalysts, mineral acids, organic acids(such as oxalic acid, citric acid, maleic acid, and formic acid), transition metal ions, zeolites and strong acid cation-exchanged resins. For example, Zhao[2]reported an unprecedented HMF yield of 67% by using chromium (II) chloride CrCl2in ionic liquid (1-ethyl-3-methylimidazoliumchloride,[EMIM]Cl) at 100 °C for 3 h. Yong[18]reported the efficient production of HMF from glucose in another ionic liquid, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) by using NHC/CrCl2(NHC: N-heterocyclic carbene) as the catalyst, and a high HMF yield of 81% was achieved at 100 °C for 6 h. Although good HMF yields were obtained in ionic liquid solvents, these Cr-based catalysts were toxic[19],resulting in uncertainty in the real industrial application.The reaction was also performed in the presence of homogeneous acid, such as H2SO4or HCl to give a moderate HMF yield. For example, Huang[20]reported a HMF yield of 63% from glucose by a two-step process consisting of the isomerization of glucose to fructose by enzyme and the dehydration of fructose to HMF by using HCl as the catalyst. Davis[21]also got HMF from glucose by the combination of Sn-Beta zeolite with hydrochloric acid in a low pH value environment. Unfortunately, these processes have some disadvantages such as separation and recycling in addition to the equipment corrosion. By contrast, the heterogeneous catalysts are more efficient for industrial applications owing to the easy recovery from reaction medium. Takagaki[22]reported a HMF yield of 42% at a glucose conversion of 73% through a two-step process by combining Amberlyst-15 and a base catalyst,Mg-Al hydrotalcite in DMF. Jiménez-Morales[23]reported a HMF yield of 32.8% at a 56.3% conversion of glucose by using tantalum phosphate as a catalyst in a biphasic water/methyl isobutyl ketone medium. Yang[24]used hydrated niobium pentoxide under mild conditions as an efficient catalyst for converting glucose to HMF with a yield of 49%. Wang[12]prepared a cheap Sn-Mont catalyst via the ion-exchange method. This catalyst exhibited excellent activity in dehydration of glucose to HMF(53.5%) in a mono-phase medium of tetrahydrofuran(THF)/dimethysulfoxide (DMSO) conducted at 160 °C for 3 h. Zhang[25]reported that mesoporous niobium phosphate had both Brönsted and Lewis acids and exhibited high activity in the dehydration of glucose to HMF. The maximum HMF yield reached 35% at 130 °C within a reaction time of 0.5 h in pure water. All these results have proved that solid catalysts with an appropriate ratio of Lewis to Brönsted acid sites could achieve high efficiency of glucose conversion, since both of these two acid sites played important roles in the formation of HMF.
Additionally, solvents have an important effect on the activity and selectivity for the formation of HMF from the dehydration of glucose. The dehydration of glucose has been conducted in traditional organic solvents, ionic liquids and water. But, the application of high boiling point solvents resulted in a significant problem due to the difficulty in separation and purification of HMF.A poor yield of HMF was obtained from water, since water was not favorable for the dehydration reaction and could promote the rehydration of HMF to byproducts. The disadvantages of using ionic liquids implied that they were expensive and prone to deactivation by small amounts of water formed during dehydration reactions. Alternatively, the biphasic system is a good choice to protect the acid catalysts,minimize side reactions, and enhance the selectivity of HMF,since the dehydration of monosaccharide could occur in the aqueous layer and the generated HMF could continuously extracted into the upper organic layer.
Zirconium phosphate has unique properties as a solid acid catalyst because it has both Brönsted and Lewis acid sites and high acid amounts. The objective of this study is to study the dehydration of glucose to HMF in a THF/H2O biphasic system using a novel zirconium phosphate solid acid catalyst. The influence of experimental parameters, such as reaction temperature, reaction time, and catalyst loading was systematically evaluated to optimize the HMF yields.Besides, the pathway of glucose conversion to HMF over zirconium phosphate catalyst was clearly illustrated based on the results of characterization and comparative experiments.
2 Experimental
2.1 Materials
HMF was purchased from the Alfa Aesar Chemical Reagent Company. ZrOCl2·8H2O was purchased from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai,China.) Glucose, (NH4)2HPO4, NaCl, THF, methyl isobutyl ketone (MIBK), 1,4-dioxane, 2-butanol and acetone were chemically pure solvents purchased from the Shanghai Chemicals Company. All reagents were used without further purification.
2.2 Catalyst preparation
6.445 g of ZrOCl2·8H2O were completely dissolved in 20 mL of water under vigorous stirring at room temperature to form the solution A (1 mol/L of Zr(IV)).Then 40 mL of 1 mol/L (NH4)2HPO4solution was added dropwise to the solution A. The resulting mixed solution was subject to ageing at room temperature for 5 h under stirring. The solid precipitate was collected and washed several times with deionized water until the washing water showed a pH value of 7, and was then dried at 100 ºC overnight, followed by being ground to powder and calcined at 400 °C for 4 h at a temperature increase rate of 2 ºC/min in air prior to reaction.
2.3 Characterization of the catalyst
X-ray powder diffraction (XRD) patterns were recorded on a Bruker diffractometer with CuKα radiation (λ=0.154 06 nm). Nitrogen adsorption-desorption isotherms were measured at -96 °C on a NOVA 4200e sorption analyzer (Quantachrome Co., Ltd.). Before measurements,the samples were outgassed at 180 °C for 12 h under vacuum to remove moisture and volatile impurities. The BET method was used to calculate the specific surface area. The energy dispersive X-ray analysis (EDX) was employed for the quantitative elemental analysis and measured on a Falion EDX spectrophotometer.
The pyridine adsorption infrared (IR) spectra were recorded on a Nicolet NEXUS 670 FT-IR spectrometer,with 32 scans at an effective resolution of 4 cm−1. 50 mg of catalyst were pressed into a self-supporting disk and placed in an IR cell attached to a closed glass-circulation system. The catalyst disk was pretreated by heating at 400 °C under vacuum in order to remove the physicosorption impurities. The IR spectrum background was recorded at room temperature when the cell was cooled down. Pyridine vapor was then introduced into the cell at room temperature until equilibrium was reached.
The temperature-programmed desorption of ammonia(NH3-TPD) was performed by using an apparatus PX200(Tianjin Golden Eagle Technology Company, Ltd.)equipped with a thermal conductivity detector (TCD).The sample (50 mg) was pretreated at 600 ºC for 2 h and then was cooled down to 50 ºC under a N2flow. Then,pure NH3was injected until the adsorption saturation was reached, followed by a flow of N2for 1 h at 90 ºC.Finally the temperature was raised from 90 ºC to 600 ºC at a heating rate of 10 ºC/min and the amount of desorbed ammonia was detected by using a thermal conductivity detector (TCD) at 110 ºC.
2.4 Dehydration of glucose into HMF
All the dehydration reaction experiments were conducted in a Teflon-lined stainless steel autoclave (50 mL)equipped with a temperature-controlled heating-jacket and a magnetic stirrer. A solution of glucose in water/THF (at a volume ratio of 1/5 with a total volume of 6 mL) and a given amount of the catalyst were loaded into the reactor. N2gas was used for purging air outside the reactor and keeping a certain pressure to prevent boiling. When the temperature of reactor was raised to the desired value, zero time was recorded. Then, the reactor was maintained at this temperature for a given period of time. After completion of reaction, the reactor was cooled down to room temperature by loading cooling water. The reaction medium itself was separated into two layers, viz.the upper organic layer and the lower aqueous layer, and both phases were collected for analysis by HPLC.
The analysis of the upper organic phase was carried out by means of an HPLC apparatus (Agilent 1206) equipped with an ultraviolet detector (Agilent G 1314B) at 254 nm by using an XDB-C18 column (Eclipse USA, 5 µm,4.6×250 mm) operated at 45 °C. The eluent was a mixture of methanol and water (at a volume ratio of 20:80) at a flow rate of 0.6 mL/min. The analysis of the lower aqueous phase was conducted on an HPLC apparatus(Agilent 1200) equipped with a refractive index detector(Agilent G1362A) by using a Biorad Aminex HPX-87H column operated at 55 °C. The eluent was 0.004 M H2SO4with a flow rate of 0.45 mL/min. But for the main product,the analysis of HMF both in organic phase and aqueous phase was conducted on Agilent 1206 for its efficiency in reducing the analysis time. An auto-sampler (Agilent G1329A) was used to enhance the reproducibility. All the main products were quantified based on the external standard and their structures were confirmed by GC-MS.Conversion of glucose (C, mol%) and yield (Y, mol%) of products were defined as follows:
Conversion = moles of glucose consumed in reaction/moles of starting glucose
Product yield = moles of product formed both in organic phase and aqueous phase/moles of starting glucose
3 Results and Discussion
3.1 Characterization of catalyst
The XRD pattern in Figure 1 shows that the structure of catalyst was amorphous. Even after recycle experiments,the amorphous structure of the catalyst was still retained.The content of Zr, O and P based on EDX analysis was 12.2%, 63.6% and 24.2%, respectively. The ratio of P/Zr was about 2, which complied with the initial design ratio very well. The SEM image (Figure 2) shows that the prepared sample did not display good shapes. The surface area is 110.4 m2/g calculated by the BET method,while the average pore size is 3 nm calculated by the BJH method.The acidic strength of ZrPO sample was investigated by the temperature-programmed desorption of ammonia,with the result shown in Figure 3. The sample had a broad peak in the range of 100―600 °C. The low temperature desorption peak indicated weak adsorption acidic sites on ZrPO catalyst, and the peak at high temperature was attributed to the moderate and strong acid sites.

Figure 1 XRD profile of the fresh and recycled ZrPO catalyst
Figure 2 SEM image of ZrPO catalyst

Figure 3 NH3-TPD profile of the ZrPO catalyst
The types of the acid sites on ZrPO catalyst were determined by the Py-FTIR spectroscopic study of the catalyst, with the spectra in the range of 1 430 cm-1-1 560 cm-1shown in Figure 4. There were three bands in this region. The one at 1 542 cm-1was the characteristic band of Brönsted acid sites, which could occur due to the presence of Zr-OH and P-OH groups on the surface.Another peak near 1 490 cm-1could be attributed to the adsorption of pyridine on both Brönsted and Lewis sites at the same time. The peak at about 1 450 cm-1was assigned to the adsorption of pyridine on the Lewis acid sites, which could be caused by the unsaturated-coordinate Zr. The weak sites were defined as ones from which pyridine was removed by evacuation at 200 °C; the medium acidity strength corresponded to evacuation between 200 °C and 400 °C and the strong sites still adsorbed pyridine molecules after evacuation at 400 °C. Quantification of weak, medium and strong acid sites was obtained from the spectra of adsorbed pyridine using the expressions referred to in the literature[26], with the data summarized in Table 1.
3.2 Effects of reaction time and temperature on the yield of HMF
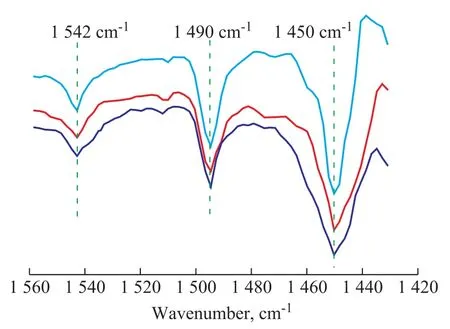
Figure 4 Pyridine-adsorbed FT-IR spectra of ZrPO catalyst
The influence of reaction temperature and time on the glucose dehydration to HMF catalyzed by ZrPO was studied, with the results presented in Figure 5 and Figure 6,respectively. Figure 5 shows that the reaction temperature had a great influence on the glucose conversion and HMF yield. At low temperatures (100 °C and 120 °C), the yield of HMF was very low and the conversion of glucose was below 40%. When the reaction was carried out at 140 °C,the glucose conversion and HMF yield reached 68.1% and 30.3%, respectively. Upon further increasing the reaction temperature to 160 °C, the activity of the ZrPO catalyst increased and a highest glucose conversion of 99.0% and a HMF yield of 56.8% were obtained within a reaction time of 3 hours. This result showed that this ZrPO catalyst containing both the Brönsted acid sites and Lewis acidsites could effectively promote the conversion of glucose via dehydration to HMF. But we found that when HMF yield reached its peak value, further increasing the reaction temperature or prolonging the reaction time could decrease the yield of HMF. This phenomenon could be attributed to the fact that at higher temperatures, the intermolecular selfpolymerization of HMF or cross-polymerization between glucose and HMF were more severe to bring about numerous polymers and humins[27]. LA and FA in the reaction system were not detected, which proved that this reaction system was very stable for HMF. Thus, a temperature of 160 °C and a reaction time of 3 h were selected as the optimum reaction conditions for the dehydration of glucose.

Table 1 Surface area and amount of Brönsted and Lewis acid sites on ZrPO after evacuation at 100 °C, 200 °C and 400 °C quantified by Py-FTIR spectra

Figure 5 Influence of the reaction temperature on glucose conversion and HMF yield

Figure 6 Influence of the reaction time on glucoseconversion and HMF yield
3.3 Effects of solvents
The reaction solvent is also an important factor for the conversion of glucose into HMF. In this work, the influence of various solvents on the yield of HMF was studied, with the results summarized in Figure 7. We can see that this catalyst exhibited low activity in pure water. The HMF yield and glucose conversion were only 5.6% and 84% at 160 °C in 3 h, respectively. This occurred because water was not favorable to dehydration of glucose and could promote the side-reactions. But when MIBK/H2O, 1,4-dioxane/H2O and THF/H2O were used as reaction medium at a volume ratio of 5:1 (organic solvent: water), the HMF yield reached 46.5%, 52.0%,and 56.8%, respectively. It was obvious that the yield of HMF could be improved by using a biphasic system,consisting of an aqueous layer saturated with sodium chloride and an organic layer. Because of the low solubility of HMF in aqueous solution, the generated HMF was gradually transferred into the upper organic phase to avoid the occurrence of further condensation reactions[4]or acetalization with glucose. Therefore,the organic phase was introduced to promote the dehydration reaction by shifting the equilibrium towards the formation of HMF by extracting the HMF formed in the aqueous medium to organic phase immediately. In this way, the side-reactions of HMF with intermediates to form solid humins and the rehydration of HMF to form levulinic and formic acid could be minimized.The addition of NaCl to the medium improved the partitioning of HMF into the organic phase by means of the “salting-out effect”[28]. Besides, the highest yield of HMF was obtained in the THF-H2O biphasic system as compared with other reaction systems, which was realized due to the favorable formation and stabilization of fructofuranose intermediates from glucose in THF solvent. So, a THF:H2O mixture of 5:1 (volume ratio)was employed as the proper reaction medium.

Figure 7 Glucose conversion and HMF yield in different solvents over ZrPO catalyst
3.4 Effects of catalyst loading
The effects of different catalyst loadings on glucose conversion and HMF yield were investigated, with the results summarized in Table 2. It can be seen from Table 2 that the said ZrPO exhibited excellent catalytic performance in the conversion of glucose into HMF. Even a small catalyst loading was adopted, a glucose conversion of 92.3% and a HMF yield of 52.1% could be achieved over 0.075 g of ZrPO catalyst. Upon further decreasing catalyst loading to 0.025 g, the glucose conversion and HMF yield could be still maintained at 83.4% and 35.2%,respectively, indicating that the total amount of acid sites and the appropriate ratio of Lewis to Brönsted acid sites on ZrPO catalyst were much favorable to the formation of HMF from glucose. Especially, the HMF yield increased to a maximum value over a catalyst loading of 100 mg.Therefore, in a bid to obtain a high yield of HMF, the mass of catalyst was chosen as 100 mg hereinafter.

Table 2 Glucose conversion and HMF yield over different amounts of ZrPO catalyst
3.5 Effects of the glucose concentration
A high substrate concentration is usually preferred to improve the efficiency of the process and make the process much more constructive and economical. So different initial concentrations of glucose were studied,with the results shown in Table 3. It can be seen that the glucose conversion and HMF yield were partly affected by the initial concentration of glucose. When the initial glucose concentration was 10% and 13%, the yield of HMF was 56.8% and 52.1%, respectively. Moreover,when the initial concentration of glucose was increased to 26%, the yield of HMF was still high (43.6%), indicating to the efficiency of the catalyst. The loss of HMF yield at high glucose concentration was most likely caused by self-polymerization of glucose and cross-polymerization between glucose and HMF[29]. Upon taking the production cost and HMF yield into consideration, an initial glucose concentration of 10% was considered to be suitable for the production of HMF from glucose.

Table 3 Effect of the glucose concentration on HMF yield
3.6 Effects of different catalysts
We have also compared the catalytic performance of ZrPO with other solid acid catalysts, including Amberlyst-15,H-ZSM-5, Nafion and NbOPO-pH7[25], used in the dehydration of glucose to HMF under the same reaction conditions, with the results summarized in Table 4. The results show that ZrPO exhibits excellent catalytic activity for the dehydration of glucose among all these tested solid acid catalysts, and can afford a complete conversion of glucose and a HMF yield of 56.8%. The strong acidic sulfonated copolymer resins, like Amberlyst-15, have low thermal stability and are normally used below 160 °C due to their organic frameworks. The strong acidity of Nafion can produce undesired by-products such as humins. At the same time, both of the Amberlyst-15 and Nafion are not favorable to the isomerization of glucose to fructose due to their single Brönsted acid sites. Although the conversion of glucose is quite complete and the yield of HMF is high over the NbOPO catalyst (which can achieve a HMF yield of 58.7%), the cost of NbOPO is higher than that of ZrPO and more LA was detected when NbOPO is used as the catalyst. When H-ZSM5 is used as the catalyst, the HMF yield is 24.5%. Upon taking various factors into account, it is believed that ZrPO is more suitable than other catalysts for the production of HMF under this condition.
The effect of calcination temperature on dehydration of glucose to HMF was investigated. A lower yield of HMF was obtained at a higher calcination temperature(Table 4, entries 2 and 3). This could occur due to the fact that more P-OH and Zr-OH on the catalyst surface disappeared at higher calcination temperature to reduce the Brönsted acid sites which were considered to play an important role on the dehydration of fructose to HMF.Another experiment was carried out to verify the role of Brönsted acid on the dehydration of glucose to HMF.By mixing the ZrPO catalyst calcined at 600 °C with Amberlyst-15 serving as co-catalyst, a high HMF yield of 55.7% was obtained (Table 4, entry 8), indicating that Brönsted acid sites could convert fructose to HMF by dehydration effectively.

Table 4 Summary of the glucose conversion and HMF yield catalyzed by various heterogeneous catalysts
3.7 Proposed mechanism
By combining the results of this study and our previous work[12], a possible mechanism on dehydration of glucose to HMF over the ZrPO catalyst is proposed and illustrated in Figure 8. There are two kinds of acid sites on the ZrPO catalyst, among which one consists of the dominating Lewis acid sites that are formed by the unsaturatedcoordinated Zr species, and the other is composed of Brönsted acid sites which are formed by –OH groups on the catalyst surface. The presence of these two kinds of acid sites allows for the conversion of glucose to HMF to be carried out over one catalyst, while the unsaturatedcoordinated Zr species can catalyze the isomerization of glucose to fructose as the Lewis acid sites, whereas the active P-OH and Zr-OH groups can convert fructose to HMF rapidly as the Brönsted acid sites.
3.8 Reusability of catalyst

Figure 8 Proposed reaction mechanism for glucose isomerisation to fructose following dehydration to HMF on ZrPO catalyst
The heterogeneous acid catalysts have advantages of being easily recovered from the reaction medium in comparison with the liquid acids, and thus can be reused after regeneration. In the present study, the recyclability of the ZrPO catalyst for converting glucose to HMF was evaluated through six repeated reaction cycles. After reaction, the catalyst was separated from the reaction solution by centrifugation, washed with deionized water,dried in oven at 100 °C before the next run, with the results shown in Figure 7. It is found that the catalyst can be reused at least six times and the HMF yields were well retained, attesting to the good reusability of such catalyst. A slight loss of activity was mainly caused by the formation of carbon deposition on the catalyst surface,which was confirmed by the weight loss of used catalyst by the TG-DTA analysis (not shown here). The XRD and SEM analysis showed that the morphology of the used catalyst was similar to the fresh one.

Figure 9 Cycle tests of ZrPO catalyst for HMF production from glucose
4 Conclusions
In conclusion, zirconium phosphate can be functioned as a heterogeneous catalyst for the dehydration of glucose to HMF in a THF/H2O biphasic medium. An excellent HMF yield of 56.8% was obtained at 160 °C for 3 h. Moreover,the catalyst was stable in the reaction medium and can be reused at least six times with a slight loss of catalytic activity. Its excellent catalytic activity could be attributed to the presence of both types of acid sites (Lewis and Brönsted sites), which could combine the isomerization process with the dehydration step in an one-pot reaction system without the addition of any other acid catalysts.The results demonstrated that the ZrPO was effective as a bifunctional catalyst for the direct transformation of glucose to HMF.
Acknowledgements:This work was supported financially by the National Science Foundation of China (No. 21273071), the Science and Technology Commission of Shanghai Municipality(13520711400, 13JC1401902, 10dz2220500), the Fundamental Research Funds for the Central Universities of China and the SINOPEC project (No.115046).
- 中国炼油与石油化工的其它文章
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Research on Catalytic Cracking Performance Improvement of Waste FCC Catalyst by Magnesium Modification
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield

