Research on Catalytic Cracking Performance Improvement of Waste FCC Catalyst by Magnesium Modification
Yuan Chengyuan; Tan Zhengguo; Pan Zhishuang; Zhang Haitao; Gao Xionghou
(Lanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, Lanzhou 730060)
Abstract: In this study, the deactivation mechanism caused by high accessibility of strong acid sites for the waste FCC catalyst was proposed and verified for the first time. Based on the proposed deactivation mechanism, magnesium modification through magnesium chloride impregnation was employed for the regeneration of waste FCC catalyst.The regenerated waste FCC catalyst was characterized, with its heavy oil catalytic cracking performance tested. The characterization results indicated that, in comparison with the unmodified waste FCC catalyst, the acid sites strength of the regenerated waste FCC catalyst was weakened, with no prominent alterations of the total acid sites quantity and textural properties. The heavy oil catalytic cracking results suggested that the catalytic cracking performance of the regenerated waste FCC catalyst was greatly improved due to the suitable surface acidity of the sample. In contrast with the unmodified waste FCC catalyst, the gasoline yield over the regenerated waste FCC catalyst significantly increased by 3.04 percentage points, meanwhile the yield of dry gas, LPG, coke and bottoms obviously decreased by 0.36, 0.81, 1.28 and 0.87 percentage points, respectively, making the regenerated waste FCC catalyst serve as a partial substitute for the fresh FCC catalyst.Finally, the acid property change mechanism was discussed.
Key words: waste FCC catalyst; regeneration; magnesium modification; catalytic cracking; acidity
1 Introduction
Fluid catalytic cracking (FCC) is currently one of the most important technologies in the oil refinery industry owing to its great ability to process heavy oil fractions and enough flexibility to selectively tune the product distributions from diesel to gasoline or liquefied petroleum gas (LPG) and olefins[1-2]. Typically, FCC catalyst consists of the Y zeolite, the matrix material and the binder, in which the Y zeolite provides most of the catalytic cracking activity due to its abundant acid sites and supercage structure[3-4]. However,during FCC reaction, the fresh FCC catalyst is often deactivated quickly because of some factors, such as the hydrothermal reaction condition, the heavy metals contamination, the physical attrition, etc., which can lead to the decrease in gasoline yield due to the increase of the undesired products such as coke, dry gas and LPG[5-6]. Therefore, to maintain the stability of the unit,it is necessary for the refinery to replace the partially deactivated waste FCC catalyst (S-Cat) with fresh FCC catalyst timely, thus resulting in a massive amount of S-Cat. As the regulations are continuously tightening for the environmental protection, the disposal of these S-Cat has become a great issue, to which more and more attention is being paid[7-9].
Out of economic and environmental considerations, the recycle of used FCC catalyst at the refinery unit after the said catalyst is subject to regeneration treatment is considered to be an optimal way for disposal of S-Cat.Currently, the regenerationof S-Cat is mainly aimed at removal of heavy metals such as V, Ni, and Fe from S-Cat by magnetic separation or acid extraction methods[10-11].Although the catalytic cracking performance of S-Cat can be improved to some extent by these reported regeneration methods, the catalyst also possesses some critical drawbacks which would extremely restrict its practical application. For example, magnetic separation only can be used to treat S-Cat that is contaminated lightly by heavy metals, and can result in substantial material loss[12]. As for the acid extraction method, this process is usually not only tedious but also can bring about a large amount of wastewater which is seriously harmful to the environment[13]. Furthermore, the regeneration of waste FCC catalyst by means of these reported methods have not been satisfactory because of their bad cracking product selectivity[14-15]. As a result, it is highly desirable to develop a simple, effective and environmentally friendly method for S-Cat regeneration.
Motivated by the above mentioned fact, we herein report a simple, effective and environmentally-friendly method for the regeneration of S-Cat by simple magnesium modification through incipient wetness impregnation. The regenerated S-Cat made by our method exhibited the remarkably improved catalytic cracking performance owing to its appropriate acid properties derived from the magnesium modification.To the best of our knowledge, there has been no similar report up to the moment.
2 Experimental
2.1 Preparation of regenerated S-Cat
S-Cat was provided by the PetroChina Lanzhou Petrochemical Company. Firstly, the received S-Cat,ammonium chloride and deionized water were thoroughly mixed under stirring at 80 °C for 1 h to carry out ion exchange with ammonium cations. After that, the slurry was filtered, washed and dried. Then, the obtained S-Cat was modified by magnesium through the incipient wet impregnation with magnesium chloride serving as the magnesium source. After being subjected to drying and calcination, the regenerated S-Cat was prepared and labeled as R-Cat.
2.2 Characterization
Ammonia temperature-programmed desorption (NH3-TPD) was performed on a Micromeritics AUTOCHEM II 2920. Pyridine (Py) and 2,6-di-tert-butylpyridine(DTBPy) adsorption IR spectra were recorded on a Bruker TENSOR 27 instrument. The textural properties of catalyst samples were obtained using nitrogen adsorption measurements on an ASAP-2010 instrument. The chemical composition of catalyst samples was analyzed by X-ray fluorescence (XRF) on a Bruker SRS 3400 XRF spectrometer.
2.3 Cracking tests
The heavy oil catalytic cracking tests of the samples were performed at 530 °C on a Kayser R+Multi advanced cracking evaluation unit (ACE) operating with a catalyst/oil weight ratio of 5. The gaseous and liquid effluents were determined by gas chromatography (HP 6890). The paraffins (P), olefins (O), naphthenes (N),and aromatics (A) contents (PONA analysis) of cracked gasoline were analyzed by a gas chromatograph (Varian CP-3380). The amount of coke deposited on the catalyst was assessed by burning the sample in a carbon analyzer(DF 190). The properties of the heavy oil are listed in Table 1.
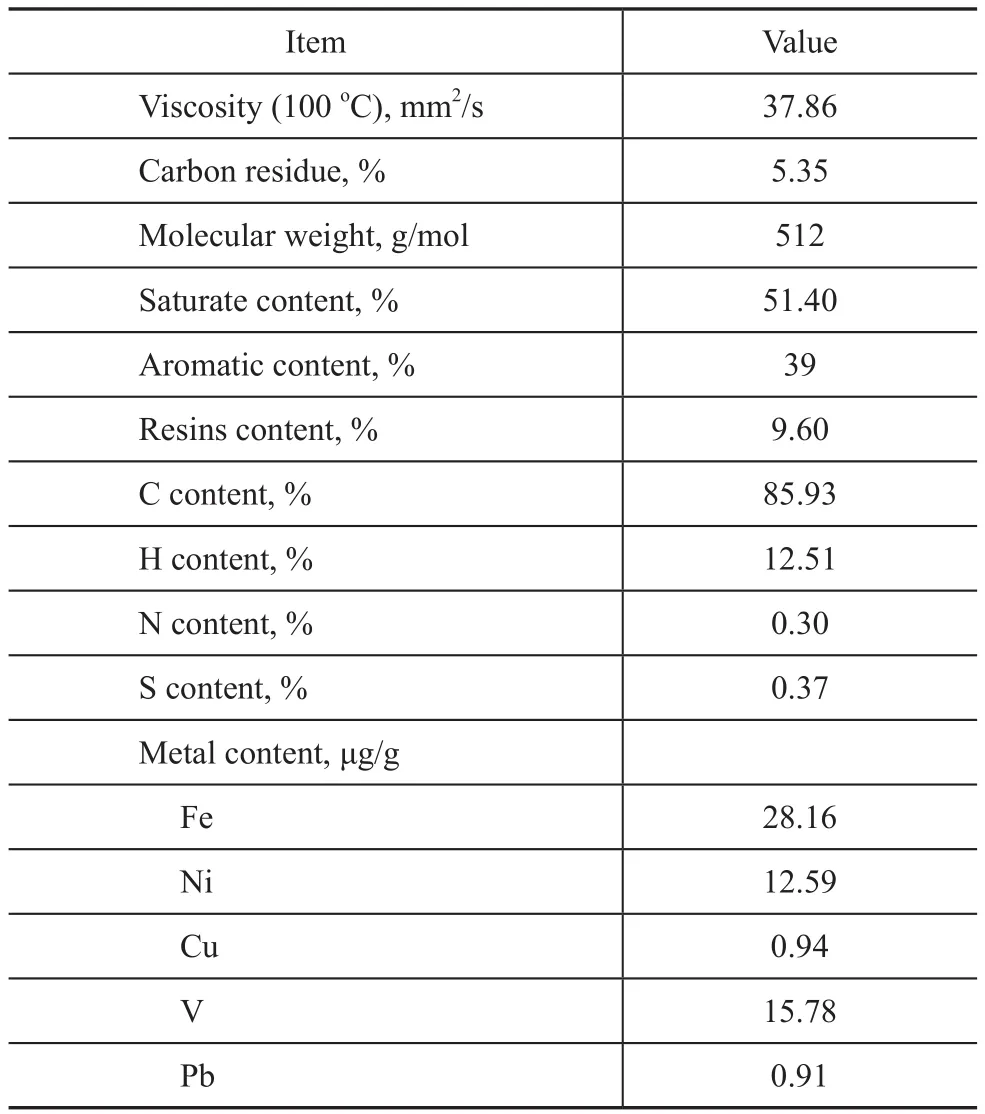
Table 1 Properties of heavy oil feedstock
3 Results and Discussion
3.1 The inference for the deactivation of S-Cat
As we know, Y zeolite is the main ingredient of FCC catalyst, which provides most of the acid sites for the FCC reaction[16-18]. In the fresh FCC catalyst, because the structure of Y zeolite is not destroyed, most of the acid sites, in particular the strong acid sites, are inside the micropores of Y zeolite, which make these strong acid sites hardly accessible to huge oil molecules[19]. As a result, during the FCC reaction, the over-cracking and coking reactions that are preferably caused by strong acid sites can be effectively restrained upon using fresh FCC catalyst, which is helpful to the increase of gasoline yield by reducing the yield of undesired products such as dry gas, LPG, and coke. However, the structure of Y zeolite is usually destroyed seriously in S-Cat, which can make the strong acid sites become more readily accessible to huge oil molecules due to the structural destruction of Y zeolite. Therefore, compared with the fresh FCC catalyst, the coking and over-cracking reactions over S-Cat could happen easily, which would lead to the so-called deactivation of the S-Cat.
Based on the above discussion, it is reasonable for us to make an inference that high accessibility of the strong acid sites may be one of reasons responsible for the deactivation of S-Cat. To verify this inference,we used Py and DTBPy adsorption IR spectra to determine the accessibility of the strong acid sites for fresh FCC catalyst and S-Cat. Because the area of IR characteristic peak is linearly correlated with the quantity of the acid sites that can be accessed by the probe molecules during adsorption, the IR peak area for Py (APy) and DTBPy (ADTBPy) after desorption at 350 °C can be used to stand for the amount of total strong acid sites and the strong acid sites that can be accessed by huge oil molecules, respectively[20]. Then,we can take the value ofADTBPy/APyratio to estimate the accessibility of the strong acid sites for the different catalyst samples[21]. BiggerADTBPy/APyvalue means higher accessibility of the strong acid sites.
Figure 1 displays the Py and DTBPy adsorption IR spectra for the fresh FCC catalyst (provided by the PetroChina Lanzhou Petrochemical Company) and S-Cat. It can be seen that the peaks at 1 540 cm-1and 3 500 cm-1could be attributed to the strong Brönsted acid sites attached by Py and DTBPy, respectively[19]. Correspondingly, theADTBPy/APyvalues calculated from IR spectra for the fresh FCC catalyst and S-Cat are shown in Figure 2. Obviously, theADTBPy/APyvalue of S-Cat was much bigger than that of fresh FCC catalyst, indicating that S-Cat possessed much higher accessibility of the strong acid sites in contrast with the fresh FCC catalyst, which was consistent with our inference. The above inference makes it possible for us to realize the regeneration of S-Cat by weakening the strong acid sites of the S-Cat. Herein, the basic magnesium species was used to weaken the strong acid sites of the S-Cat.
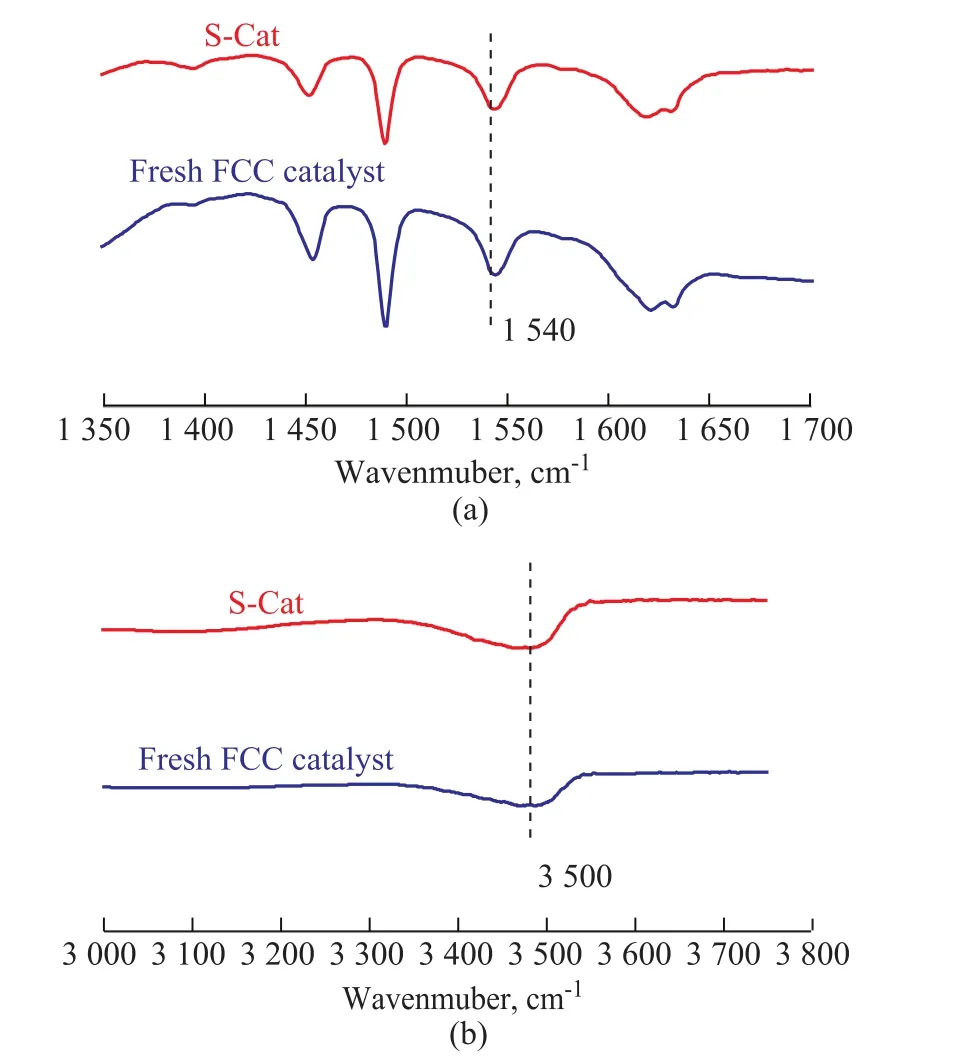
Figure 1 Py (a) and DTBPy (b) adsorption IR spectra of fresh FCC catalyst and S-Cat

Figure 2 ADTBPy / APy values of fresh FCC catalyst and S-Cat
3.2 Characterization
Figure 3 shows the NH3-TPD profiles of S-Cat and R-Cat. As shown in Figure 3, both of the two samples displayed one prominent desorption peak in the range of 120―500 °C. Compared with S-Cat, the peak area of R-Cat slightly decreased, indicating to a slight reduction of total acid sites for R-Cat. Furthermore,the position of desorption peak for the R-Cat obviously shifted to the direction of low desorption temperature in comparison with S-Cat, suggesting a decline of the acid sites strength for R-Cat. It can be concluded from the results of NH3-TPD measurements that after magnesium modification the acid sites strength of the R-Cat obviously declined without a significant reduction of total acid sites.
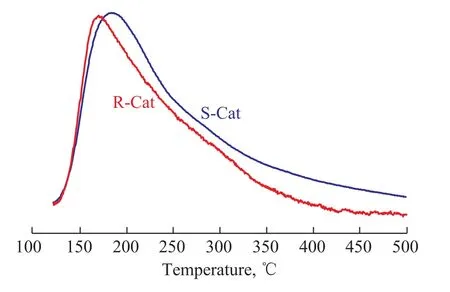
Figure 3 NH3-TPD profiles of S-Cat and R-Cat

Figure 4 Py adsorption IR spectra of S-Cat and R-Cat after desorption at 200 °C (a) and 350 °C (b)
Figure 4 displays the Py adsorption IR spectra of S-Cat and R-Cat. The peaks at around 1 450 cm-1and 1 540 cm-1could be ascribed to Py, which was bonded to the Lewis and Brönsted acid sites, respectively[19].The quantities of total acid sites and strong acid sites determined by the Py adsorption IR spectra after desorption at 200 °C and 350 °C, respectively, are shown in Table 2. As shown in Table 2, in comparison with S-Cat, there was no obvious decrease in total acid sites for R-Cat. However, the strong acid sites of R-Cat remarkably decreased in contrast to S-Cat because of the magnesium modification. The above results are in good agreement with the NH3-TPD analysis.

Table 2 Acid amount of different samples
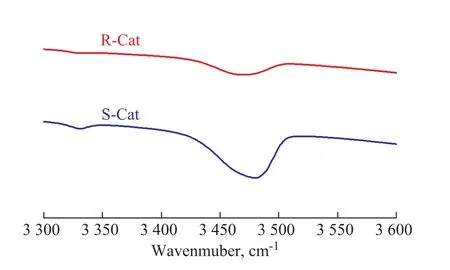
Figure 5 DTBPy adsorption IR spectra of S-Cat and R-Cat after desorption at 350 °C
Figure 5 exhibits the DTBPy adsorption IR spectra of S-Cat and R-Cat after desorption at 350oC. Compared with S-Cat, the IR peak area of DTBPy at 3 500 cm-1for R-Cat obviously decreased, indicating that the strong Brönsted acid sites that could be linked with huge oil molecules for R-Cat distinctly reduced in contrast with S-Cat.
The textural and compositional properties of S-Cat and R-Cat are exhibited in Table 3. Compared to S-Cat,the textural properties such as specific surface area,pore volume, bulk density, and attrition index of R-Cat were almost not affected by our regeneration method.Compared with S-Cat, the contents of heavy metals such as Ni, V and Fe showed no obvious change in R-Cat,indicating that our regeneration method did not lead to removal of heavy metals. Moreover, the Na2O content of R-Cat distinctly declined in comparison to S-Cat thanks to ion exchange with ammonium cations in the course of regeneration process.

Table 3 Textural and compositional properties of different samples
3.3 Catalytic cracking reactions
The heavy oil catalytic cracking performance of different catalysts is given in Table 4. It can be clearly seen that the catalytic cracking performance of R-Cat was obviously improved. Compared with S-Cat, the gasoline yield over R-Cat significantly increased by 3.04 percentage points; meanwhile, the yield of undesired products, such as dry gas, LPG, coke, and bottoms,obviously decreased by 0.36, 0.81, 1.28, and 0.87 percentage points, respectively. The above performance improvement of R-Cat could be attributed to the appropriate surface acidity which was greatly helpful to restricting the coking and over-cracking reactions during FCC reaction[22]. Furthermore, for comparison, the regeneration of waste FCC catalyst was also conducted using the traditional acid extraction method. As shown in Table 4, in contrast with R-Cat prepared by our regeneration method, although the conversion over the conventionally regenerated waste FCC catalyst used in the comparison tests increased, the gasoline yield obviously decreased by 5.1 percentage points with a distinct increase of undesired products, such as dry gas,LPG, and coke, indicating to the superior effect of our regeneration method.

Table 4 The heavy oil cracking performance of different catalysts
The constituents and octane number of the cracked gasoline were also investigated. As shown in Table 5, in contrast to S-Cat, the aromatic yield in gasoline obtained over R-Cat decreased by 2.82 percentage points, and the yield of i-paraffins and olefins increased by 2.0 and 0.64 percentage points, respectively, which finally resulted in an increase of RON by 0.6 units. The above results suggested that, upon using R-Cat, the aromatization and secondary cracking reactions of olefins in gasoline obviously decreased, which could be attributed to the lower acid sites strength of R-Cat[23]. Furthermore,with the increase of olefins in gasoline, more saturated i-paraffins were obtained via hydrogen transfer of olefins[24], which could lead to an increase of i-paraffins formed on R-Cat.

Table 5 Constituents and octane number of cracked gasoline
The cracking products selectivity on S-Cat and R-Cat at different conversion rates is shown in Figure 6. As shown in Figure 6, compared with S-Cat, R-Cat exhibited a much better selectivity of cracking products at different conversion rates with higher yield of gasoline and lower yields of dry gas, LPG, and coke.

Figure 6 Selectivity of cracking products for S-Cat and R-Cat at different conversions
If the R-Cat prepared by our regeneration method could be used in substitution for a part of fresh FCC catalyst,this idea would become our main concerned issue. In order to accomplish this target, two blended catalysts were made, with their cracking performance tested.As shown in Table 6, the catalyst blended with 85%of equilibrium catalyst and 15% of R-Cat exhibited a cracking performance that was comparable to that of the catalyst blend composed of 85% of equilibrium catalyst and 15% of fresh FCC catalyst, with no obvious decrease in gasoline yield and increase in undesired products yield,such as dry gas, LPG, coke, and bottoms. The above results suggested that R-Cat could substitute for the fresh FCC catalyst with no evident decline of catalytic cracking performance.

Table 6 Heavy oil cracking performance over different blended catalysts
The probable mechanism for acid properties change in R-Cat is shown in scheme 1. It can be seen from scheme 1 that in the process of our waste catalyst regeneration,the basic magnesium species could preferentially combine with the strong acid sites of S-Cat. As a result, the strong acid sites of R-Cat were weakened and changed into moderated acid sites. Therefore, after our regeneration,the acid sites strength of R-Cat could decline without obvious decrease in total acid sites.
4 Conclusions
This study shows that magnesium impregnation is an effective method for the regeneration of waste FCC catalyst. After magnesium modification, the acid sites strength of the regenerated waste FCC catalyst is moderately weakened with no obvious loss of total acid sites, which is believed to be very beneficial to the improvement of catalytic cracking performance of the regenerated waste FCC catalyst through suppressing the coking and over-cracking reactions during FCC reaction.Owing to the excellent catalytic cracking performance, the regenerated waste FCC catalyst prepared by magnesium impregnation can be used in substitution for a part of the fresh FCC catalyst. Furthermore, in contrast to the present regeneration methods, the method of magnesium modification also possesses its own unique features such as simple procedure, low cost and environmental friendliness, which can promise a good application prospect in the future.

Scheme 1 The acid properties change mechanism of R-Cat
Acknowledgments:This work was supported by the Exploratory Research Program of Petrochemical Research Institute (16-yk-01-03), PetroChina.
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

