Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
Wu Jianmin; Sun Qiwen; Zhang Zongsen
(Yankuang Energy R&D Co., Ltd., State Key Laboratory of Coal Liquefaction and Coal Chemical Technology,Shanghai 201203)
Abstract: The equilibrium solubilities, volumetric gas-liquid mass transfer coefficients kLa of H2 and CO were measured as functions of temperature (298―513 K), pressure (1―3 MPa), superficial gas velocity (0.5―3 cm/s) and solid volume fraction (5%―25%) in liquid paraffin/iron-based catalyst slurry bubble column reactor. The volumetric mass transfer coefficients kLa were obtained by measuring the dissolution rate of H2 and CO. The influences of the operation conditions,such as pressure, temperature, superficial gas velocity and catalyst concentration on kLa, were investigated. Two empirical correlations were proposed to predict kLa values of H2 and CO in liquid paraffin/solid particles slurry bubble column reactor.The results showed that the equilibrium solubilities of H2 and CO increased with an increasing temperature and pressure,and the solubility of CO was greater than that for H2. It was found that the equilibrium solubility can be expressed by Henry’s law. The volumetric mass transfer coefficients of H2 and CO were of the same order of magnitude, and increased with the increase of pressure, temperature and superficial gas velocity. The presence of solid particles decreased kLa values of both H2 and CO.
Key words: slurry bubble column reactor; solubility; volumetric mass transfer coefficient; iron-based catalyst
1 Introduction
Slurry bubble column reactors are widely employed in petrochemical, chemical, and biochemical processes due to their easy installation, easy operation, and high heat and mass transfer rates caused by strong gasliquid interactions[1]. These reactors are operated under high pressure in many industrial applications, such as the heavy oil upgrading and the Fischer-Tropsch (F–T)synthesis. Among the alternative energy processes, the Fischer-Tropsch synthesis for the conversion of syngas(a mixture of H2and CO produced from natural gas,coal, and/or biomass) to clean liquid fuels and chemicals has attracted significant interest from industry. In recent years, slurry bubble column reactors have become the reactors of choice for F–T synthesis because of much higher productivity in slurry bubble column reactors as compared to the fixed bed F–T reactors[2-4].
Mass transfer rates and coefficients are among the important parameters required for proper design,operation and understanding the performance of slurry bubble column reactors for F–T synthesis. It is evident that understanding the mass transfer rates among the gas-liquid-solid phases not only can study the F–T synthesis dynamics and other rate processes, but also control the product selectivity by using the mass transfer rates. So the need to investigate the mass transfer and solubility of H2and CO at the F–T synthesis conditions is necessary, which can provide more basis data for the design and development of F–T slurry bubble column reactor. However, the mass transfer characteristics in slurry bubble column reactors for F–T synthesis have not been fully studied or understood, only few relevant gasliquid mass transfer data or equations for F–T synthesis system at industrial conditions are available so far. In the past decades, a number of studies have been performed on the mass transfer coefficient in slurry bubble column reactors[5-8]. However, little research has been performed on mass transfer coefficients and solubility of reactants under industrial conditions such as F–T synthesis, and most of these studies have one or more of the following drawbacks: (1) using small diameter columns or agitated and surface-aerated vessels; (2) operating under the conditions of normal temperature and atmospheric pressure; (3) mostly using air-water-glass beads systems;(4) employing low superficial gas velocities; and (5) the equations for the volumetric liquid-side mass transfer coefficientkLaare based either on two phase equations[9]or on three phase relationships for fluidized-bed reactors[10]. Therefore, the current available experimental results and correlation equations in open literature is not sufficient to confidently satisfy the needs for quantitatively describing the mass transfer process in the real systems, and they also cannot support the commercial three phase slurry bubble column reactor design, scale-up and operation.
Thus, in order to understand the behavior of industrial slurry bubble column reactors, the mass transfer characteristics should be obtained in a wide range of operating variables typical to industrial applications.Accordingly, the primary focus of this work is to advance the mass transfer in slurry bubble column reactors under F–T synthesis conditions, in which liquid paraffin is used to mimic the physical properties of F–T wax in the presence of the iron-based catalyst for low temperature F–T synthesis. The mass transfer coefficients and solubilities of H2and CO of the three phase slurry systems have been obtained under industrial conditions based on the measurements of the mass transfer velocity among the gas-liquid-solid phases experimentally. Thereafter, the effects of pressure, temperature, superficial gas velocity,and catalyst content on mass transfer coefficients of H2and CO were investigated and were empirical, while statistical correlations of the experimental data were developed, which could be used to develop the highly efficient three phase slurry reactors for F–T synthesis or other industrial processes.
2 Experimental
2.1 Experimental setup
The experimental setup is shown in Figure 1. The experiments are carried out in a stainless steel slurry bubble column reactor, 60 mm in inner diameter and 800 mm in height. The gas distributor is a perforated plate with 19 openings, 1 mm in diameter, arranged in triangular pitch to ensure an equal initial distribution of the gas phase. The system pressure is regulated with a back pressure regulator installed at the outlet of the column. The temperature of the column is heated by the preheater and controlled by the temperature control system. Both columns can be operated up to 10 MPa and 300 °C.

Figure 1 Schematic diagram of experimental setup
The gas flow rate can be adjusted with mass flow controller. A condenser and gas-liquid separator attached to the top of column are responsible for disposing of evaporating paraffin liquid safely. The tail gas compositions are analyzed by a gas chromatograph.
H2and CO were used as the gas phase in all experimental runs with liquid paraffin serving as the liquid phase. The low temperature iron-based catalyst for F–T synthesis used in the commercial F–T synthesis reactor was employed as the solid phase. Properties of CO, H2, N2, liquid paraffin,and the iron-based catalyst particles utilized thereby are given in Tables 1 and 2. The experiments were carried out under the conditions covering a temperature ranging from 298 K to 513 K, a pressure ranging from 1 MPa to 3 MPa,and a superficial gas velocity ranging from 0.5 cm/s to 2.5 cm/s. The slurry volume fraction was varied in the range of 0%, 0.05%, 0.1%, 0.15%, 0.20%, and 0.25%, respectively. The slurry concentration is defined throughout this work as the volume fraction of solids in the gas-free slurry. The pore volume of the catalyst particles, which was filled with liquid during experiments,was counted as being part of the solid phase. At the start of each experimental investigation, the static slurry height was set at 0.55 m. During experiments, the liquid phase was regularly replenished due to the loss of some paraffin volume because of evaporation.

Table 1 Properties of liquid paraffin at 298 K

Table 2 Properties of iron-based catalyst
2.2 Calculation of the volumetric mass transfer coefficient kLa
The volumetric liquid-side mass transfer coefficientkLaof H2and CO are estimated by measuring the rate of gas absorption or dissolution with the physical absorption method[7]. To obtain the concentration of absorbed gases in the slurry, the exit gas phase is collected for measuring its composition by gas chromatography during the gas absorption process.
After the temperature of the slurry bubble column is heated to the desired value, an inert gas, such as N2, is introduced into the reactor system to purge out H2or CO from the slurry. Then gas A, which can be H2or CO, is fed into the reactor at a certain volumetric flow rateNA0at the time oft0= 0. Then the reactor is pressurized with the gas which is to be absorbed. At the time oft=ti(i =1, 2, ...,n) with equal intervals, the exit gas sample is collected after the pressure is reduced and the mole fractionyA(ti)of the samples is measured by gas chromatography. The flow rate of the exit gasNO(ti) is also recorded until the gas reaches its saturation in the slurry at the time oft=tn.Then, the instantaneous concentration of gas A in the slurrycA(ti) can be obtained by integral,

whereNA0is the flow rate of inlet gas A,VLis the volume of the slurry, and ΔnA(ti) is the decrease of mole number of gas A fromt= 0 tot=ti. The integral term in Equation (2)can be calculated approximately by the trapezoid equation

According to the mass transfer principle, the overall mass transfer rate can be determined by
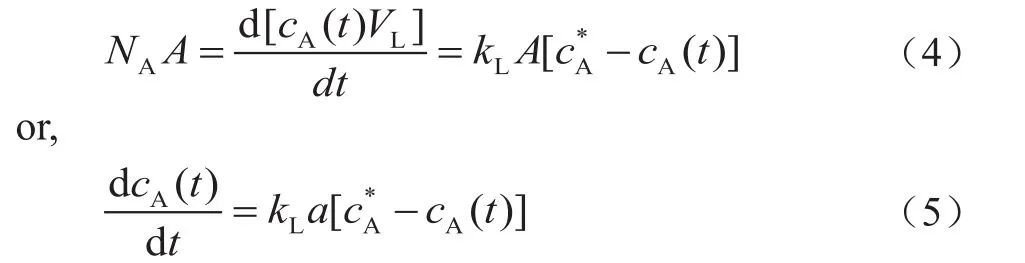
wherea=A/VLis the interfacial area of mass transfer andcA*=cA(tn) is the equilibrium concentration of absorbed gas A in the slurry phase. The integral of Equation (5)yields
Thereafter,kLacan be calculated by determining the slope of the curve of -ln[1-cA(t)] versus the time coordinate.
3 Results and Discussion
3.1 Equilibrium solubility of H2 and CO
In the F-T synthesis process, the reaction gas H2and CO must dissolve in the wax first, and then can contact with the solid catalyst and take part in the reaction. Therefore,the equilibrium solubility values of H2and CO have a great influence on gas-liquid mass transfer performance of the reaction system, which also are the critical data for gas-liquid mass transfer study.
The solubility of H2and CO in the slurry bubble column reactor was calculated using the material balance and equilibrium thermodynamic conditions after the absorption had been completed. The experimentally determined values of the equilibrium solubility of H2and CO in the slurry are illustrated in Figure 2. The reproducibility of the data was satisfactory. It should be mentioned that the presence of iron-based catalyst did not have any effect on the solubility of the gases into the liquids. It can be seen from Figure 2 that with an increasing temperature and pressure, the increase in the equilibrium solubility of CO and H2becomes remarkable.And within the same temperature and pressure range used, the equilibrium solubility of CO was greater than that for H2, whereas the solubility of H2was much more sensitive to temperature than the solubility of CO. Thus,the ratio of H2/CO solubility values increased with the temperature. In addition, the equilibrium solubility values were found to vary linearly with the gas partial pressure. In general, this phenomenon was considered to be in compliance with the Henry’s law (cA*=P/He).The Henry’s law constants (He) can be obtained from the experimental data. Yang[7], Albal[12], and Li[13]previously reported equilibrium solubility values for CO and H2in different liquids and showed similar trends as those observed in this work. Some differences may be attributed to the difference in paraffin composition.
3.2 Influence of operating conditions on the volumetric mass transfer coefficient kLa
3.2.1 Influence of pressure on kLa
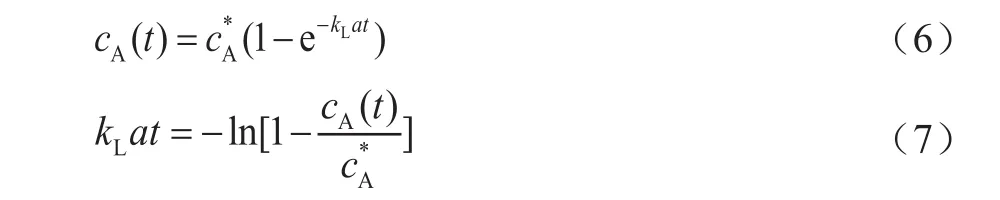
The effect of pressure onkLawas found to be dependent on the gas/liquid system, the pressure range, and the temperature range used[13]. Figure 3 shows the effect of pressure on the volumetric mass transfer coefficientskLaof H2and CO. It can be observed thatkLavalues of both H2and CO increase with an increasing pressure. The main reason of such an increase ofkLavalues with pressure can be explained that the increase of pressure increased the gas solubility in the slurry, which could result in decrease of both the surface tension and the slurry viscosity[14].

Figure 2 The equilibrium solubility of H2 and CO in the slurry
The decrease of liquid surface tension would be conducive to the formation of small gas bubbles, which would increase the gas-liquid interfacial areaa. On the other hand, an increasing pressure could cause the increase in gas bubble density and instability, which would make large bubbles split into small bubbles.Meanwhile, the decrease of surface tension would result in a reduction of bubble rising velocity and consequently a long contact time.
According to the report in the literature[12,15], the mass transfer coefficient is inversely proportional to the square root of the contact time, sokLwould decrease with a decreasing liquid surface tension. The decrease of liquid viscosity, however, would increase the mass transfer coefficient, sincekLis inversely proportional to the liquid viscosity[16]. Judging from the data presented in Figure 3, it seems that the increase ofawith an increasing pressure was stronger than the decrease ofkL,and thekLavalues were found to increase with an increasing pressure subsequently.
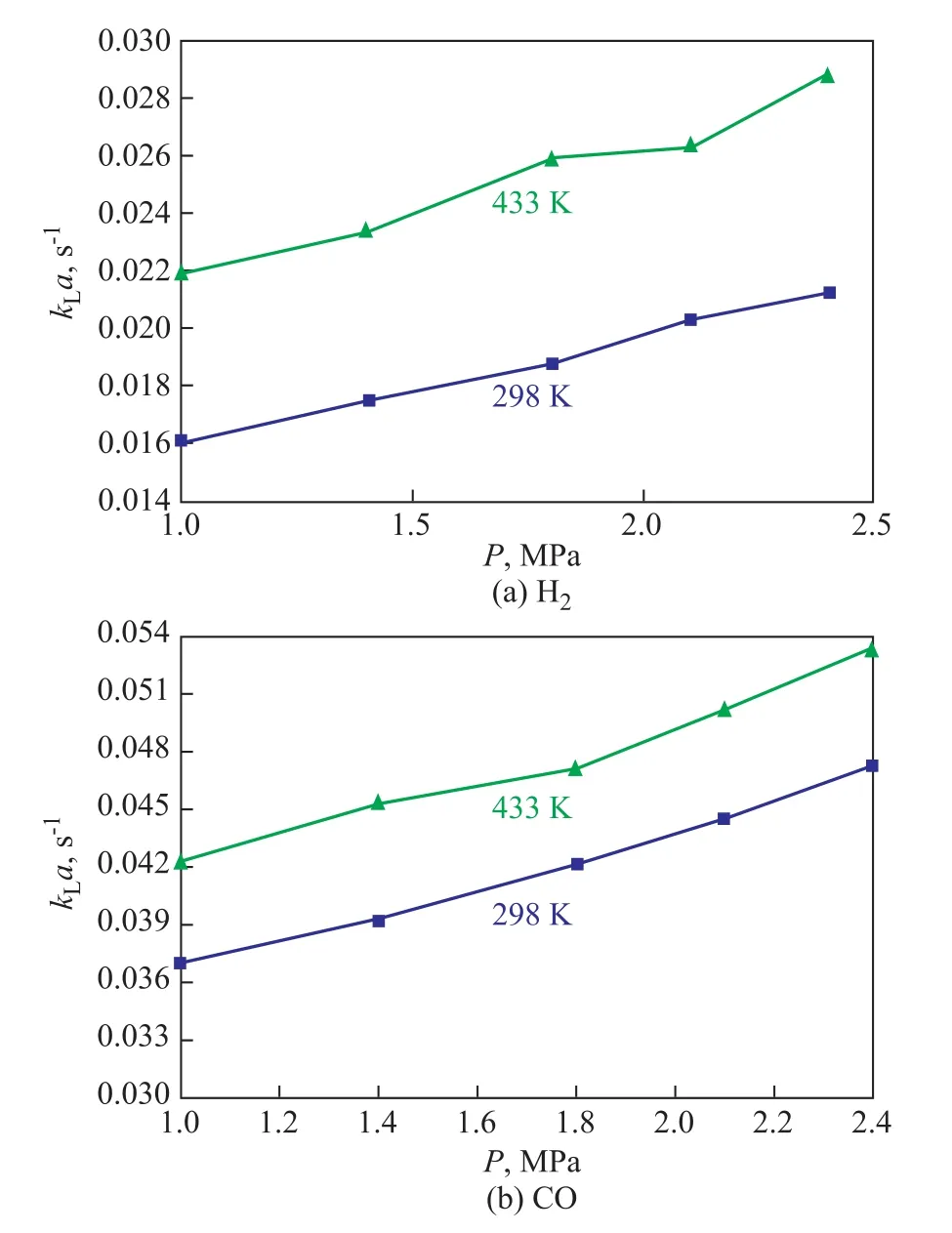
Figure 3 Effects of pressure on kLa of H2 and CO(ug=0.015m/s, Cv=5%)
3.2.2 Influence of temperature on kLa
The temperature not only can affect the physical properties of both gas and liquid phases, but can also affect the volumetric mass transfer coefficientkLa.Especially for organic systems, the temperature change will lead to the change of the system density and viscosity, and the diffusion coefficient of gas in liquid phase can also be changed. Figure 4 shows the effect of temperature onkLaof H2and CO. It can clearly be seen thatkLavalues of H2and CO increase with an increasing temperature. The influence of temperature onkLais quite complicated. The overall effect of temperature onkLawill depend on the competition between the effects of temperature onkLanda. At first, both liquid viscosity and surface tension decreased with an increasing temperature.The effect of liquid viscosity and surface tension onkLawas already analyzed above. In addition, an increasing temperature made the gas diffusivity increase, which could further result in the increase of the liquid-side mass transfer coefficientkLbecause of the liquid-side mass transfer coefficientkLwas proportional to the square root of the gas diffusivity in the liquid. However, a higher temperature could easily speed up the coalescence of small bubbles, and then the gas-liquid interfacial areaawould decrease.
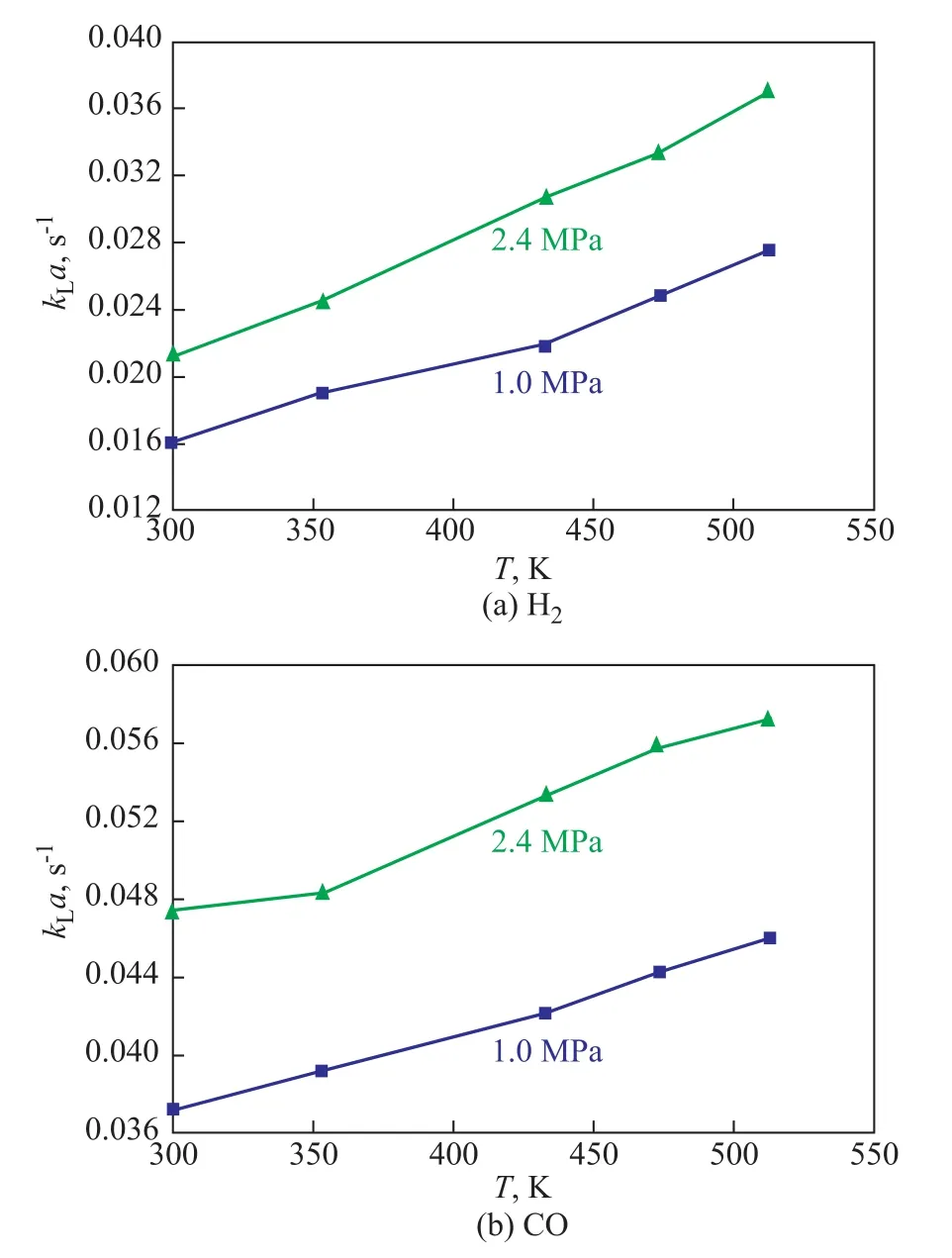
Figure 4 Effects of temperature on kLa of H2 and CO(ug=0.015m/s, Cv=5%)
3.2.3 Influence of superficial gas velocity on kLa
The effects of the superficial gas velocities onkLaof CO and H2are shown in Figure 5. The superficial gas velocity has a crucial influence on the operation of bubble and slurry bubble column reactor, since it is one of the most important operating conditions. Urseanu[17]reported that the uniform bubble flow area did not exist in the liquid with larger viscosity. The coalescence of medium-sized bubbles became faster in the lower superficial gas velocity and consequently the gas holdup decreased. On the other hand,higher superficial gas velocity improved the liquid flow over the surface of rising bubbles, which made the liquid film for mass transfer in the system become thinner, leading to an increased liquid-side mass transfer coefficientkL. As shown in Figure 5, the values ofkLaof CO and H2increased with an increasing superficial gas velocity. The main reason was that increasing the superficial gas velocity made the gas holdup increase in the slurry bubble column reactor, which could provide a larger gas-liquid interfacial area.

Figure 5 Effects of superficial gas velocity on kLa of H2 and CO (T=298K, Cv=5%)
3.2.4 Influence of solid concentration on kLa
The effects of solid concentration onkLaof CO and H2are shown in Figure 6. When the solid loading rose from 5 vol.%to 25 vol.%,kLavalues of CO and H2noticeably decreased.In earlier studies, Karandikar, et al.[18], Yang, et al.[7], and Kielbus-Rapala, et al.[19]also reported the decrease in volumetric mass transfer coefficientskLawith an increasing solid concentration. However, Gollakota, et al.[20]reported the increase in volumetric mass transfer coefficient with an increasing slurry concentration. It was noteworthy that a lot of researchers[21-22]showed that the effect of solid particles on volumetric mass transfer coefficientkLawas dependent upon the solid loading and particle characteristics.
This contradictory phenomenon may be explained by considering that solid particles may[7]: (1) enhance the surface renewal frequency and interfacial mobility at low solid concentrations, which will increase bothkLanda; (2) reduce the turbulence level and decrease the interface mobility at high solid concentrations,which will decreasekL; (3) increase the viscosity of the slurry, which will reducekL; (4) enhance the gas bubble coalescence frequency, which will decreaseaat high solid concentrations; and (5) decrease the gas bubble size,which will increasea.

Figure 6 Effects of solid concentration on kLa of H2 and CO (T=298K, P=1.0 MPa)
In a word, the overall dependency of volumetric mass transfer coefficientkLaon solid concentration will have a comprehensive consideration of the resultant effect of the solid particles on bothkLanda. Thus,kLacan be expected to increase, decrease or be independent of the solid presence. In the present work, it can be thought that the catalyst particles decreased the turbulence level and interface mobility, increased the solution viscosity and enhanced the bubble coalescence frequency, which resulted in a decrease ofkLawith an increasing solid concentration.
3.3 Correlations of kLa
Dimensional analysis was employed to obtain a correlation forkLavalues in terms of different variables such asT,P,ug,ρSL,μSL,Cv,andDA. The analysis of the experimental data led to the following dimensionless correlation for H2and CO in the liquid paraffin/catalyst particles system:

which is valid in the ranges of 1.88×106<Eu<1.14×108,16<Re< 440, and 191<Sc< 1.45×105.
Theses two empirical correlations can fit the experimentalkLavalues of H2and CO well as shown in Figure 7. The average relative error was 9.69% for H2and 5.92% for CO, respectively.
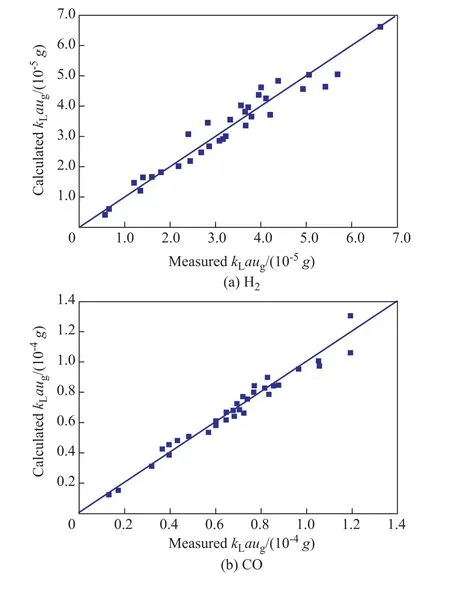
Figure 7 Comparisons of measured and calculated kLa values of H2 and CO
4 Conclusions
The equilibrium solubility, volumetric mass transfer coefficientskLaof H2and CO in liquid paraffin/iron-based catalyst slurry system were measured in a slurry bubble column reactor at elevated temperature and pressure.
The equilibrium solubility of H2and CO would become remarkable with an increasing temperature and pressure.And within the same temperature and pressure range used,the equilibrium solubility of CO was greater than that of H2, whereas the solubility of H2was much more sensitive to temperature than the solubility of CO. The volumetric mass transfer coefficientskLaof both H2and CO increased with an increasing pressure and temperature.Higher superficial gas velocity also increasedkLavalues of both H2and CO. The presence of solid particles decreasedkLavalues of both H2and CO. Two empirical correlations were proposed to predict thekLavalues of both H2and CO in liquid paraffin/catalyst slurry, which could provide a theoretical basis for the Fischer-Tropsch synthesis reactor design, magnification and optimization.
Nomenclature
a——gas-liquid interfacial area of mass transfer, m-1
A——gas-liquid mass transfer area, m2
cA——gas concentration in the slurry, kmol/m3
——saturated gas concentration in the slurry, kmol/m3
Cv——solid concentration in volume fraction, %
DA——Diffusivity, m2/s
Eu——Euler number (Eu=P/(ρSLug2))
He——Henry’s law constants, MPa·L/mol
kL——gas-liquid mass transfer coefficient, m/s
kLa——gas-liquid volumetric mass transfer coefficient, s-1
M——molar mass, g/mol
NA——rate of mass transfer per unit area, mol/(m2·s)
NA0——flowrate of inlet gas A, mol/s
NO——flowrate of outlet gas A, mol/s
P——pressure, MPa
Re——Reynolds number (Re=dρSLug/μSL)
Sc——Schmidt number (Sc=μSL/(ρSLDA))
T——temperature, K
Tc——critical temperature, K
t——time, s
ug——superficial gas velocity, m/s
VL——volume of liquid phase, m3
yA——mole fraction of the sample
ρSL——slurry density, kg/m3
μSL——slurry viscosity, Pa·s
σ——surface tension, N/m
σSL——slurry surface tension, N/m
Acknowledgement:This research was financial supported by the National High Technology Research and Development Program of China (863 Program 2011AA05A204).
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Influence of Negative Pressure Gradient on Pressure Distribution and Gas-Solid Flow Pattern of Solid Feed Systems
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

