Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
Li Xiaojuan; Song Hua,; Chang Youxin
(1. College of Chemistry and Chemical Engineering, Northeast Petroleum University, Daqing 163318;2. Provincial Key Laboratory of Oil and Gas Chemical Technology,Northeast Petroleum University, Daqing 163318)
Abstract: Deep adsorptive desulfurization of low sulfur content gasoline via zeolite is a promising process. Herein, the CuIYIIIY zeolite was prepared and the effect of adsorption conditions and aromatics on the performance of adsorptive desulfurization was studied. And the isothermal equilibrium and kinetics were also investigated. The results showed that upon using 10 mL of model oil and 0.2 g of the CuIYIIIY adsorbent during the adsorption reaction, which was carried out at 323 K for 60 min, the benzothiophene (BT) desulfurization rate reached 98.4%. The impact of aromatics on the adsorptive desulfurization over the CuIYIIIY zeolite decreased in the following order: ortho-xylene > meta-xylene > para-xylene. The equilibrium isotherm shows that the adsorption of benzothiophene over the as-prepared CuIYIIIY zeolite can be represented by the Langmuir model. And the kinetics can be more perfectly represented by the pseudo-second-order model than that of the pseudo-first-order one. The thermodynamic parameters (ΔG, ΔH) were both negative, which suggested that the adsorption process is monostratal, mass transfer controlled, spontaneous, and exothermic.
Key words: adsorption; desulfurization; isothermal equilibrium; kinetics
1 Introduction
In the past decades, deep desulfurization of fuels has been attracting more and more attention as the environment regulation on the limit of sulfur content in fuels becomes increasingly stringent[1-2]. Conventional hydrodesulfurization (HDS) process is in wide use in refineries at present.However, thiophene (TP) and benzothiophene (BT) are stable and comparatively difficult to be removed just via HDS even under severe conditions[3]. Therefore, to meet the new emission standard stipulated by The U.S. Environmental Protection Agency (EPA)[4-5], other desulfurization processes, including adsorption[6], extraction[7], oxidation[8]and bio-processes[9]have been developed rapidly in recent years. Adsorptive desulfurization has received much attention for its particular advantages, such as being able to achieve a hydrogen-free deep desulfurization under ambient conditions at a lower investment without changing the oil performance.
With regard to adsorptive desulfurization, the selection of an efficient adsorbent is vital[10]. Diversified adsorbents have been investigated and the modified Y-type zeolite is probably the best choice for the ultra-deep desulfurization process[11-12]. Yang, et al.[13]reported that among all the Cu(I)-Y (vapor phase),Ni(II)-Y (solid-state), Ni(II)-X, Zn(II)-X, and Zn(II)-Y adsorbents, the Cu(I)-Y zeolite exhibits the highest capacity. Nevertheless, the sulfur capacity decreased rapidly while the aromatic compounds simultaneously are present in the fuel. The adsorption heat of benzene onto the CuIY zeolite is 83.680—92.048 kJ/mol, which is very close to that of TP. This may be the reason why aromatics strongly compete against sulfur compounds by π-complexation[14]. Velu, et al.[15]indicated that the ion-exchanged Y zeolites with rare earth element, such as La, Ce and Y, showed higher adsorption selectivity to sulfur compounds in aromatics-containing oil because of the direct sulfur adsorbent (S-M) interaction, while the sulfur capacity of them has not reached a desired level.Wang, et al.[16]explored the adsorption performance of dibenzothiophene (DBT) over the NiIICeIVY zeolites and found that Ce as a cation in NiIICeIVY zeolite plays a promoting role in selective adsorption of DBT from model gasoline with toluene. Liu, et al.[17]have studied the hydrothermal stability of the YIIIY and CeIVY zeolites and found that the YIIIY zeolite had a better desulfurization performance than that of the CeIVY zeolite for its strong polarization effect from Y(OH)2+ions with small radius and Al-O bonds presenting as the molecular sieve frame. In summary, one might speculate that the Cu-Y bimetal ion-exchanged CuIYIIIY may be the most appropriate adsorbent for adsorptive desulfurization, especially for the aromatics containing oil.
To develop a suitable adsorbent, the studies focusing on the theory such as engineering aspects including kinetics,isotherm and thermodynamics are as important as those concerned experiments. To our knowledge, a few articles have focused on the study of adsorption of sulfur compounds onto the CuIYIIIY zeolite from the viewpoint of competitive adsorption and engineering aspects. In this paper, the Cu-Y bimetal ion-exchanged CuIYIIIY zeolite was synthesized, and the effects of the adsorption conditions and aromatics on the performance of adsorptive desulfurization over the as-prepared zeolite were tested.Furthermore, this work had focalized on the kinetics, isotherm and thermodynamics study on the adsorption process of BT over the CuIYIIIY zeolite.
2 Experimental
2.1 Materials
Thiophene (TP, 99%) and benzothiophene (BT, 98%) were purchased from the J&K Chemical Ltd., China. Powdery NaY zeolite (with a Si/Al ratio of 5 and a pore size of 1.154 nm) was purchased from the Nankai University Catalyst Co., Ltd. Cu(NO3)2·3H2O, Y(NO3)3·6H2O, ortho-xylene(OX), meta-xylene (MX) and para-xylene (PX), andn-octane were obtained as commercial analytical grade reagents without further purification.
2.2 Model gasoline
A model gasoline was prepared by blending BT into n-octane solvent to reach a total sulfur concentration of 200 mg/L, marked as M-0. To investigate the effects of the molecular dimension, steric hindrance and π-complexation effect of aromatics on selective adsorption of TP and BT from gasoline onto CuIY, YIIIY, and CuIYIIIY zeolites, M-1 was prepared by adding TP and BT into n-octane solvent to reach a total sulfur concentration of 200 mg/L. Several bottles of model gasoline were obtained by adding a specified amount of OX, MX and PX, respectively, into M-1. Each concentration of individual xylene in the obtained afore-mentioned model gasoline was 500 mg/L and was denoted as M-2, M-3, and M-4, accordingly.
2.3 Synthesis of adsorbent
The ion-exchanged Y zeolites required in this work were prepared employing the liquid-phase ion-exchange method, and the details about the preparation methods were similar to those referred to in our previous paper[18]. The CuIYIIIY zeolite was prepared by ion exchange at room temperature with NaY (with a Si/Al ratio of 5) molecular sieve as the parent with 0.1 mol/L of Y(NO3)3solution.Subsequently, the zeolite was filtered, washed thoroughly with distilled water, and dried out at 383 K for 12 h after ion exchange. Consequently, the intermediate product YIIIY zeolite was obtained after being calcined at 823 K for 4 h in the air atmosphere. The CuIYIIIY zeolite was obtained by ion exchange using YIIIY zeolite as the parent with 0.1 mol/L of Cu(NO3)2, followed by filtering, washing, drying, calcination and reduction at 463 K for 3 h in H2,resulting in a product labeled as CuIYIIIY eventually.
2.4 Static adsorption procedure
In each adsorption process, 20 mL of model gasoline was transferred into a three-neck flask containing 0.2 g of adsorbent. The mixture was subjected to stirring at a specified temperature with nitrogen stream blanketing.After adsorption, the supernatant liquid was separated by a high-speed centrifuge and analyzed for the residual sulfur concentration with a flame photometric detector-gas chromatograph (Shimadzu FPD-GC-14C) equipped with a capillary column (PH-1, 60 m × 0.25 mm). A batch of experiments was carried out under N2flow to avoid con-tact of CuIwith oxygen in the air.
3 Results and Discussion
3.1 Influence of temperature
The influence of adsorption temperature (T) was investigated, using 20 mL of model gasoline M-0 with a CuIYIIIY dosage (m) of 0.2 g at an adsorption time (t) of 60 mins (Figure 1). The effects of the adsorption temperature on BT removal over the CuIYIIIY zeolite are best illustrated by the desulfurization curve shown in Figure 1.The desulfurization degree increases firstly and then tends to taper off with an increasing adsorption temperature.Notably, there is a maximum value at 323 K on the curve.The reason may be that the adsorption rate increases faster and greater than the desorption rate with an increasing temperature initially, resulting in the gradual increase in the BT removal rate. With a further rise of adsorptio n temperature, the desorption rate increases more sharply,and therefore when the adsorption temperature is greater than 323 K, the desulfurization rate begins to decrease with an increasing adsorption temperature. This shows that the maximum BT desulfurization rate over the CuIYIIIY zeolite was detected to be 98.4% at 323 K.

Figure 1 Influence of adsorption temperature on BT removal from M-0 over CuIYIIIY zeolite (t=60 min, m=0.2 g)
3.2 Influence of adsorption time
The influence of adsorption time was investigated, using 20 mL of model gasoline M-0 with a CuIYIIIY dosage of 0.2 g at a reaction temperature of 323 K (Figure 2). As shown in Fig 2, there is an obviously increasing desulfurization tendency at the beginning and then the tendency begins to level off when the contact time is longer than 60 minutes. The desulfurization rate turns to be steady after around 120 min. The BT can be removed up to 98.4% att= 60 min, which means that the most suitable adsorption time is equal to 60 min.

Figure 2 Influence of adsorption time on the BT removal from M-0 over CuIYIIIY zeolite (m=0.2 g, and T=323 K)
3.3 Influence of adsorbent dosage
The influence of the adsorbent dosage on removal of BT is shown in Figure 3 by using 20 mL of model gasoline M-0 tested at a reaction temperature of 323 K for 60 minutes. The BT removal increased with the adsorbent dosage. When the adsorbent dosagemwas more than 0.2 g,the desulfurization rate increased not substantially. Since the sulfide in the simulated oil and adsorbent had reached the equilibrium, the adsorption of sulfide on the adsorbent has reached its saturation. Therefore, the best desulfurization efficiency obtained over 0.2 g of CuIYIIIY zeolite had confirmed that the desulfurization rate was 98.4%.

Figure 3 Influence of adsorbent dosage on BT removal from M-0 over CuIYIIIY zeolite (at t=60 min and T=323 K)
3.4 Competitive adsorption
Usually, the presence of aromatics has a negative impact on the ability to remove sulfur by the adsorbent[19]. The metal-exchanged zeolites are proved to have desulfurization performance on the basis of π-complexation between the sulfur compounds and the metal center[20-22]. During the process of adsorption over the molecular sieve, aliphatic compounds have little impact on the adsorption of heterocyclic sulfides, while aromatic compounds, with similar molecular structure to TP and its derivatives, may compete against sulfur compounds for adsorption sites via their own π-electron cloud. To find out the influence of the presence of aromatic compounds in the model gasoline, the selective adsorption of TP and BT over the CuIY, YIIIY, and CuIYIIIY adsorbents was studied, with the results illustrated in Figure 4 and Figure 5, respectively.As shown in the above-mentioned Figures, in the course of desulfurization of the model gasoline M-1 containing only TP and BT, the CuIY zeolite possessed the highest capacity for adsorption of TP and BT, whereas the YIIIY zeolite showed the lowest desulfurization rate. As regards the CuIYIIIY zeolite, the capacity for adsorption of TP and BT, respectively, is both higher than that of the YIIIY zeolite, indicating that the introduction of Cu+ions onto the Y-exchanged Y zeolite could improve its saturation loading capacity.
Desulfurization of the model gasoline M-2, which contained ortho-xylene (OX), was investigated and showed a sharp decline in the adsorption capacity over all the adsorbents, implying that the presence of OX had a great effect on desulfurizing efficiency. Among the three adsorbents, there was a most noticeable decrease of adsorption capacity over the CuIY zeolite, while the effects on the CuIYIIIY and YIIIY zeolites were less obvious. The loss of sulfur adsorption ability decreased in the following order: CuIY > CuIYIIIY > YIIIY and the sulfur adsorption capacity decreases in the following order: CuIYIIIY > YIIIY> CuIY. This indicates that Y3+ions in zeolite can play an important role in selective adsorption of sulfur when OX is present in the model gasoline. The Y3+ions could reach the I’ position in β cage in order to combine with O atom,enhance the interaction between Al atom and O atom,and consequently strengthen the stability of the zeolite[23].Both the metal-post and unsaturated adsorption positions may be occupied by the sulfur atoms. The Y3+ion on the surface of the zeolite not only can adsorb TP (or BT) via π-electronic interactions, but also can combine with TP(or BT) via direct S-M interaction. The latter one showed a better efficiency on this competitive desulfurization[24].The CuIYIIIY zeolite has both large sulfur adsorption capacity and high selectivity for TP and BT, since its metal ions take part in both S-M interaction and π-electronic interactions with thiophenic compounds.
The performance of desulfurization with model gasoline samples M-3 and M-4, which contain MX and PX(meta-xylene and para-xylene), were similar to that of gasoline M-2, the existence of MX and PX in gasoline would obstruct the adsorption of sulfur compounds. The magnitude of desulfurization efficiency loss decreases in the following order: CuIY > CuIYIIIY > YIIIY, while the sulfur adsorption capacity decreases in the following order: CuIYIIIY > YIIIY> CuIY as described above. The reason for this phenomenon was mentioned above.The CuIY zeolite adsorbs the sulfur compounds mainly through π-complexation, which has poor selectivity to sulfur compounds in the presence of aromatics. And the rare earth metal Y-exchanged Y zeolites can combine with sulfur compounds not only via π-complexation, but also via direct S-M bonds between the sulfur atoms of thiophenic compounds and the Y3+ions. Therefore, the aromatic compounds have less influence on adsorption of TP and BT over the CuIYIIIY and YIIIY zeolites. It can be concluded that the CuIYIIIY zeolite not only has high sulfur removal capacity similar to the CuIY zeolite, but also has high selectivity for adsorption of thiophenic compounds which is comparable to that of the YIIIY zeolite. According to the principle of molecular size selection[25], the adsorbents prefer to adsorb from model gasoline the molecules which have a comparable diameter/pore ratio. The size of the sodalite cage is comparable to that of xylenes, 0.66 nm[26]. TP (0.48 nm)and PX (0.5 nm) can get into the sodalite cage (0.66 nm),while the BT (0.65 nm), OX (0.636 nm) and MX(0.634 nm) can enter the super-cage (1.18 nm) only[27].On account of its small molecular size, PX cannot occupy the adsorptive sites of BT but will affect the adsorption of TP. OX and MX, which have larger size, would have great effect on adsorption of BT since they are all situated in the super-cage. It would be more difficult for TP molecules to diffuse into the sodalite cage because of steric hindrance and pore blockage during the adsorption process. Therefore, OX and MX have stronger competitive adsorption ability relative to the PX, resulting in greater influence on the efficiency for removal of TP and BT by the adsorbent. To sum up, the effect on the metal ion-exchanged Y zeolites for desulfurization decreases in the following order: OX >MX > PX.

Figure 4 Influence of aromatics on adsorption of TP onto CuIY, YIIIY, and CuIYIIIY zeolites (t=60 min, m=0.2 g, T=323K, M2/3/4=M1+OX/MX/PX)

Figure 5 Influence of aromatics on adsorption of BT onto CuIY, YIIIY, and CuIYIIIY zeolites (t=60 min, m=0.2 g, T=323 K, M2/3/4=M1+OX/MX/PX)
3.5 Equilibrium isothermal adsorption
The equilibrium isothermal adsorption study was conducted by using the model gasoline M-0 (at 100, 150,200, 250, 300, 350, 450, and 500 mg/L, respectively) in 3 h at an adsorption temperature of 293 K, 303 K, 313 K,and 323 K, respectively. Eq. (1) is employed to calculate the amount of BT adsorbed per gram of CuIYIIIY zeolite at equilibrium:

whereqe(mmol/g) is the amount of BT adsorbed per gram of the CuIYIIIY zeolite at equilibrium,V(L) is the volume of model gasoline,W(g) is the weight of CuIYIIIY zeolite,c0(mmol/L) is the initial sulfur concentration in the solution,ce(mmol/L) is the residual sulfur concentration in the solution at equilibrium.
Figure 6 exhibits the relationship ofqeversusce. It can be seen from Figure 6 that at the beginningqeincreases sharply with the increase ofce, thenqeincreases gradually and is maintained at an equilibrium value whenceis greater than 2.5 mmol/L. This indicates that most of the BT can be adsorbed onto CuIYIIIY zeolite with a lower initial concentration, whilec0is excessively high, the adsorbent reaches its saturation andqereaches the maximum adsorption (qm). Originally, the increasing initial sulfur concentration could decrease the repulsive force between the sulfur molecules in the model gasoline and on the adsorbent surface[28]. However, when the sulfur adsorption capacity is saturated, the repulsive force between sulfur molecules in the model gasoline and those on the surface of CuIYIIIY zeolite would intensify, because the possibility of occupying the empty active positions on the surface of adsorbent dwindles away[29].

Figure 6 Equilibrium isothermal adsorption of BT from M-0 over the CuIYIIIY zeolite at different temperatures
The Langmuir model can be applied in studying the sorption isotherm. It is generally accepted to match experimental data and represent adsorptive desulfurization equilibrium process based on four basic hypotheses, namely:(1) The process is a monolayer adsorption on the well-defined localised adsorption sites; (2) The solid surface is uniform and every site has the same adsorption energy; (3)There is no interaction between the adsorbed molecules;and (4) The adsorption equilibrium is a dynamic equilibrium.
The Langmuir isothermal model is:

whereqm(mmol/g) is defined as the maximum adsorption capacity theoretically.KL(L/mg) is defined as the Langmuir adsorption equilibrium constant, which is related to the adsorption energy and can represent the affinity between the adsorbent and the adsorbate molecules.
Equation 2 can be represented in the linear form:

Mathematically,KLandqmcan be obtained by transforming the slope and intercept of the straight line, which can presentce/qeagainstce. And the separation coefficientR2can be calculated by the following equation:

Here, we use the separation coefficient to evaluate the sufficiency of adsorption progress. The process of adsorption will prefer going on, when 0 <R2< 1. By contrast, the process of adsorption will meet obstruction, whenR2> 1.WhenR2=1, the adsorption process curve will resemble a line. WhenR2=0, an irreversible adsorption process will take place.
A linear correlation betweence/qeandcein Eq. (2) at different temperatures is shown in Figure 7. The results and the corresponding regression coefficient (R2) at different temperatures are listed in Table 1. TheR2at different temperatures are all near 0.99, showing that the experimental data can be fitted to the Langmuir adsorptive isothermal equation very well. As shown in Figure 7,the slope of the line decreases slightly with an increasing temperature, which proves thatqmincreases slightly with the increase in temperature, indicating that the affinity between the CuIYIIIY zeolite and BT molecules is enhanced with an increasing temperature. The values ofqmobtained from Figure 7 are in good agreement, with the results shown in Figure 6. The Langmuir constant KLdecreases with an increasing temperature as evidenced by the data from Table 1, illustrating that the adsorption process is exothermic.

Figure 7 Isothermal adsorption of BT from M-0 at different temperatures over CuIYIIIY zeolite (linear fitting by Langmuir model)

Table 1 Langmuir adsorption isotherm equation parameters
3.6 Adsorption dynamics
3.6.1 Kinetic model
To better understand the adsorption process, a group of kinetic models are simulated to test the experimental data.More precisely, the pseudo-first-order and the pseudo-second-order models, and the Langmuir models, which are the frequently used kinetic models, are employed to investigate the adsorption of sulfur compounds over the CuIYIIIY zeolite. Theoretically, we should assume the following major premises: (1) The adsorption process is a kind of monolayer adsorption; (2) The adsorbent surface is uniform; (3) The molecules on the surface of the adsorbent have no interaction with each other; and (4) The adsorption equilibrium is a dynamic equilibrium.
The equation for the pseudo-first-order/pseudo-secondorder rate models is represented by Eq. (5) and Eq. (6),respectively:

whereqt(mmol/g) is the amount of adsorbed sulfur over the CuIYIIIY zeolite at timet(min),qe(mmol/g) is the equilibrium amount of the pseudo-first-order/pseudo-second-order adsorption,k1(min-1) andk2(g/(mmol·min))are the pseudo-first-order/pseudo-second-order rate constants. By integrating and applying the boundary conditions ranging fromqt= 0 (att= 0) toqt=qt(att=t), Eq. (5)and Eq. (6) can take the following forms, respectively:

Upon taking the logarithm of Eq. (7) and Eq. (8) respectively, the resulting equations will be:

To gain the value ofqeandk1, the ln(qe-qt) should be plotted versustbased on Eq. (9). We can also get a straightline graph witht/qtagainsttto obtain the value ofqeandk2. Obviously, while using the pseudo-first-order model or the pseudo-second-order model, there is no need to obtain theqefrom experimental data[30]. Both a pseudo-first-order model and a pseudo-second-order model are suitable for describing the kinetics of the adsorption of BT over the CuIYIIIY zeolite at 323 K quite well.
The representation of kinetic analysis is shown in Figure 8 schematically, and the model parameters are listed in Table 2. It can be seen from Figure 8 that the experimental data are well available for both the adsorption kinetic models.The correlation coefficientR2of the pseudo-first-order model is 0.9965, which is slightly lower than that of the pseudo-second-order model (0.9970), confirming that the pseudo-second-order model is a bit more suitable for representing the adsorption kinetics of this adsorption process.
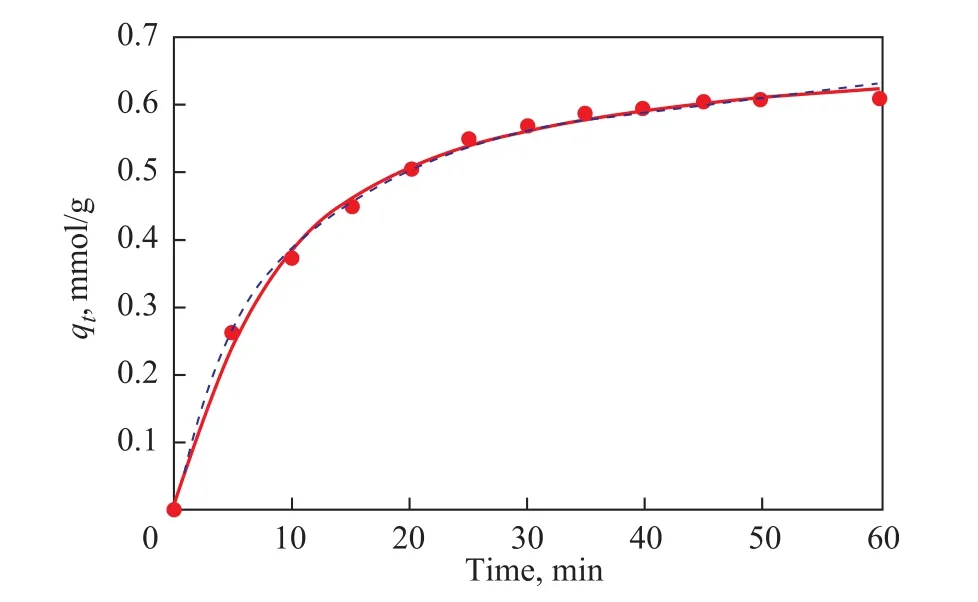
Figure 8 Nonlinear fitting to pseudo-first-order/pseudosecond-order models for adsorption kinetics of BT from M-0 over CuIYIIIY zeolite at 323 K

Table 2 Pseudo-first-order/pseudo-second-order model parameters
3.6.2 Influence of temperature on adsorption capacity and apparent activation energy
In order to thoroughly investigate the influence of temperature on adsorption capacity, the adsorption experiments run at 303 K, 313 K, and 323 K, respectively, in 5, 10, 15, 20, 25, 30, 40, 50, and 60 min were studied.Figure 9 shows thatt/qtdecreases with an increasing temperature, confirming that the adsorption capacityqtincreases gradually. To gain more insight into the reality,the experimental data were fitted in with the pseudo-second-order model, with the correlation parameters listed in Table 3. As shown by Table 3, the theoretical adsorptive amount of BT over the CuIYIIIY zeolite (qe) and the pseudo-second-order rate constant (k2) are both associated with the temperature and would rise with an increase in temperature. Overall, the adsorption of BT over the CuIYIIIY zeolite is more effective at higher temperature.

Figure 9 Influence of temperature on the adsorption kinetics over CuIYIIIY zeolite

Table 3 Pseudo-second-order kinetic parameters obtained at different temperatures
To further confirm the influence of temperature on the ad-sorptive rate, the Arrhenius equation can be employed:

wherek0(g/(mmol·min)) is a temperature-independent factor,Ea(kJ/mol) is the activation energy for adsorption,Rg(8.314 (J/(mol·K)) is the universal gas constant, andTk(K) is the temperature of adsorption. Hence, Eq. (11) can be rearranged as follows:

Typically, the activation energy can be evaluated by the slope of the plot of lnk2versus1/Tk. According to the data listed in Table 3, a line showing the result of plotting lnk2against1/Tkis illustrated in Figure 10. The evaluated activation energy is 35.9 kJ/mol. The low activation energy determined for the adsorption of BT over the CuIYIIIY zeolite suggests that the adsorption is controlled by mass transfer and not by temperature[31]. That illustrates why the adsorption could proceed successfully at atmospheric pressure and low temperature theoretically.

Figure 10 Plots of ln k2 versus 1/Tk
3.6.3 Study on effect of diffusion on adsorptive desulfurization
The adsorption behavior in an adsorption process may be represented by the intraparticle diffusion model via determining the rate controlling step of adsorption. The intraparticle diffusion model can be written as:

whereki(mmol·g-1·min-1/2) is the internal diffusion rate constant andC(mmol·g-1) is the association constant of the adsorption energy. They can be obtained from the intercept and slope of the straight lines which consist of plots ofqtagainstt1/2in Eq. (13). It is commonly known that a straight line passing through the origin that signifies the intraparticle diffusion is the key control process.(31)As shown in Figure 11, the plots ofqtagainstt1/2in Eq. (13)can be fitted by straight lines, (R2323K=0.90,R2313K=0.94,R2303K=0.97), while there is no available fitted line across the origin, illustrating that the intraparticle diffusion has little effect on the adsorption over the CuIYIIIY zeolite,which is not a rate-controlling step.
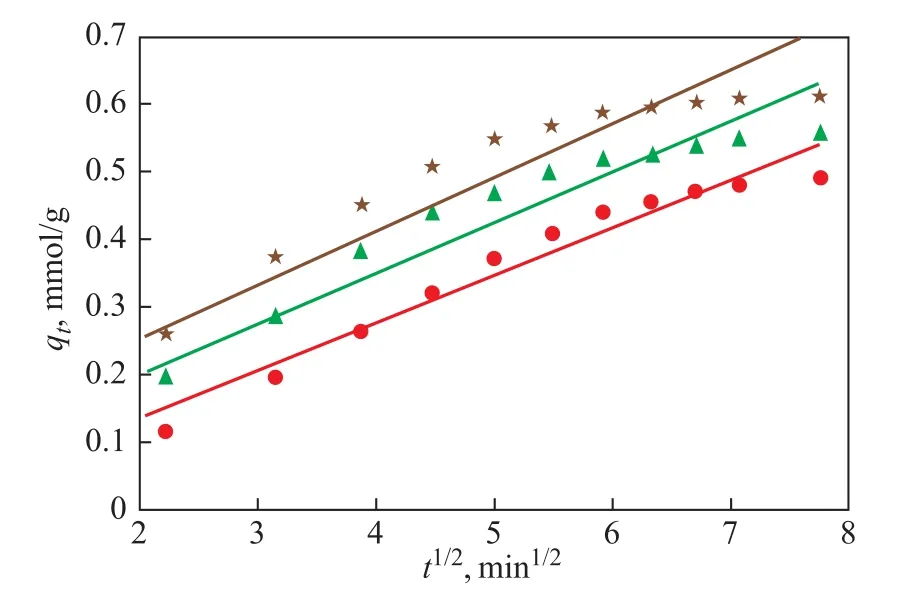
Figure 11 Plots of qt versus t1/2
3.7 Thermodynamics
The Gibbs free energy change (ΔG, kJ/mol) and the isosteric heat of adsorption (ΔH, kJ/mol) not only can directly represent the interaction between adsorbents and adsorbates or solution, but also can estimate the influence of temperature on the equilibrium adsorption coefficient.ΔGand ΔHcan be calculated by:

Upon substituting Eq. (14) into Eq. (15), the equation can be rearranged in the following form:
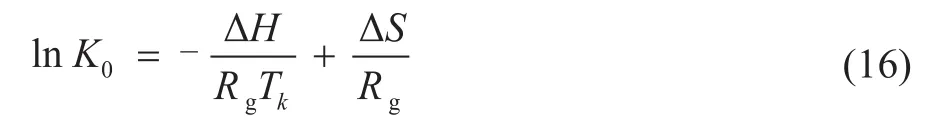
whereK0is the equilibrium coefficient and the Langmuir adsorption constantKLcan be its representation,Rgis 8.314 J/(mol·K),Tk(K) is the adsorption temperature, and ΔS(J/(mol·K)) is the entropy of adsorption.
On the basis of theKLobtained at different temperatures which are listed in Table 1, the values of ΔGcan be deduced accordingly. As shown in Figure 12, the plots of lnKLagainst 1/Twhich are based on the data in Table 1 show a good linear relationship, and then, the values of ΔHcan be deduced by the slope of the lines. The adsorption rate and desorption rate will be equal, while the ad-sorbent and adsorbate would reach an equilibrium. Meanwhile, the thermodynamic parameters are obtained and listed in Table 4. Obviously, since each ΔHis negative,the adsorption of BT on the CuIYIIIY zeolite is an exothermic process. When each ΔGis also negative, then the adsorbates are eager to be adsorbed onto the surface of the adsorbents from solution and the adsorption process is spontaneous.
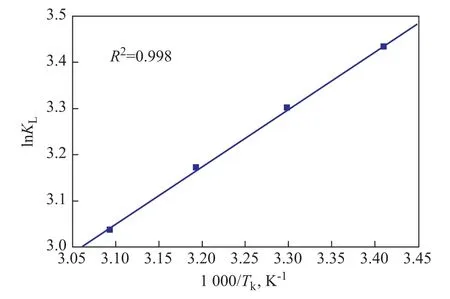
Figure 12 Plots of ln KL versus 1/Tk

Table 4 Thermodynamic parameters of adsorption
4 Conclusions
Herein, the CuIYIIIY zeolite was successfully prepared and its performance for adsorptive desulfurization was tested and analyzed. Test results have shown that the CuIYIIIY zeolite can effectively adsorb refractory sulfur compounds from the model gasoline. Upon using 10 ml of model oil and 0.2 g of CuIYIIIY adsorbent in an adsorption process carried out at 323 K for 60 min, the BT desulfurization rate reached 98.4%. The presence of aromatics has a negative impact on the ability of adsorbent to remove sulfur compounds because of their similarπ-electron cloud and their influence decreases in the following order: OX >MX > PX. In terms of equilibrium isotherm,the adsorption of BT onto the CuIYIIIY zeolite can be well fitted by the Langmuir model, which means that the adsorption process is a monolayer type. Subsequently, the maximum adsorption capacity (qm) increases with an increasing temperature, whileKLdecreases instead. Judging from the standpoint of kinetics, the adsorption process can be represented by the pseudo-second-order model.The equilibrium adsorptive capacity (qe) and the kinetic constant of pseudo-second-order reaction (k2) increase with an increasing temperature, suggesting that a superior temperature is favorable for enhancing the efficiency of adsorption. Nevertheless, since the activation energy,Ea= 35.9 kJ/mol, is a little bit low, hence the adsorption is controlled by mass transfer and not by temperature. Furthermore, the intraparticle diffusion has little effect on the adsorption over the CuIYIIIY zeolite, which is not a rate-controlling step. In the light of thermodynamics, ΔG< 0 and ΔH< 0 indicate that the adsorption of BT onto the CuIYIIIY zeolite is a spontaneous and exothermic process.
Acknowledgments:The authors acknowledge the financial support from the National Natural Science Foundation of China(21276048) and the Natural Science Foundation of Heilongjiang Province (ZD201201).
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Research on Catalytic Cracking Performance Improvement of Waste FCC Catalyst by Magnesium Modification
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield

