Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
Zhenwei Zhang ,Peng Wang ,Fei Wang ,Yaqing Li,Wei Lu ,Xingmao Jiang ,4,Xia Gui,Zhi Yun ,*
1 College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 College of Mathematics,Science and Chemical Engineering,Changzhou Institute of Technology,Changzhou 213022,China
3 Department of Chemical Engineering,Changzhou University,Changzhou 213016,China
4 Hubei Key Lab of Novel Reactor&Green Chemical Technology,Key Laboratory for Green Chemical Process of Ministry of Education,School of Chemical Engineering and Pharmacy,Wuhan Institute of Technology,China
1.Introduction
Molybdenum disulfide(MoS2)is a transition metal chalcogenide material widely used in photocatalysis[1],catalyst synthesis[2],hydrodesulfurization[3],and hydrodeoxygenation[4].Two-dimensional MoS2is widely used in electronic[5],optical[6],mechanical[7],even in hydrogen evolution reaction(HER)[8].Morphology and particle size of MoS2can obviously influence their chemical and physical properties,but possessing controllable morphology and dispersion of MoS2nanocrystal still remain big challenges.A variety of methods to controllably synthesize MoS2nanomaterials with different shapes have been reported using different precursors and reaction conditions.Early method was sulfidation of molybdenum oxides MoO3by sulfur compounds(KSCN[9],Na2S[10],sulfur powder[11])in a stainless steel autoclave under a suitable temperature to obtain MoS2with high crystallinity but their surface area was only<100 m2·g-1.Other precursors such as Mo(CO)[12],MoCl5[13]in the reaction decomposed quickly and uncontrollably.(NH4)2MoS4is a new precursor,which can decompose easily in reducing gas or solvent,and form MoS2with high crystallinity,but morphology of MoS2cannot be also controlled well.Recently,template method is probably the most effective and general route to prepare nanostructures and can be used to controllably adjust morphology of MoS2.MoS2nanospheres were synthesized by using Pluronic P-123 and other surfactants,unfortunately,surface area was lower than 140 m2·g-1and these organic compounds interact with MoS2removing difficultly.MoS2nanotubes,nanorods,and nanospheres were successfully prepared by using hard templates such as:mesoporous Al2O3[14],MoO3nanorods[15]and carbon nanospheres[16].However,size of these MoS2nanomaterials was difficult to control and the rigid templates were hard to remove completely.Skrabalak and Suslick[17]reported that mesoporous MoS2with a narrow pore size distribution and high surface area of 200 m2·g-1using SiO2nanoparticles as hard templates was synthesized successfully and the SiO2template can be easily removed by HF,but particle size and surface area of these mesoporous MoS2cannotbe efficiently controlled.In addition,other novel processes were also introduced to control the size of nanoparticles.Monodisperse MoS2nanospheres can be prepared in cyclohexane-water reverse microemulsion within a low surface area[18].Therefore,how to synthesize a high surface area MoS2nanosphere owning a controllable morphology and particle size is becoming an interesting topic and challenging problem.
Herein,we found a simple method to synthesize monodisperse MoS2nanospheres using SiO2as hard template.The size and morphology of Mo S2nanospheres can be controlled by changing the amount of SiO2and sulfur precursors.Moreover,nanosized MoS2applied in hydrodesulfurization without catalyst support can eliminate the adverse effect in HDS[19].These monodispersed MoS2nanospheres exhibited high reaction rate for hydrodesulfurization of Dibenzenethiophene mainly due to highly exposed active sites and reduced mass transfer resistance.
2.Experimental
2.1.Materials
Ammonium molybdate(VI)tetrahydrate((NH4)6Mo7O24·4H2O,AR,>99%),Tetradecane(C14H30,AR,>99%),Ammonium sulfide solution((NH4)2S,22-24 wt%water solution,sulfur content≥8.0%),Dibenzenethiophene((short as DBT,AR,>98%),Aladdin Industrial Corporation),Thiourea(CS(NH2)2,AR,>99%),Ammonia aqueous(AR,NH3≥17%)and Tetraethoxysilane(TEOS)were purchased from Shanghai Lingfeng Company.Deionized(DI)water was made from OKP equipment(Shanghai Lakecore Company).
2.2.MoS2 nanosphere preparation
According to the literature[20](NH4)2MoS4was synthesized by the(NH4)6Mo7O24·4H2O and(NH4)2S(20 wt%water solution).15 g of(NH4)6Mo7O24·4H2O was added to 200 ml of a 20 wt%solution of(NH4)2S at ambient temperature.The precipitated red crystals were thoroughly washed with small amounts of ethanol and tetrahydrofuran(THF)and then dried and stored under nitrogen.
20 nm SiO2nanoparticles were synthesized using TEOS and Ammonia aqueous by the Stöber method.5 g Concentrated ammonia(17 wt%)was dissolved in 158 ml ethanol,and 20 g of TEOS was dissolved in 60 ml ethanol,then the two solutions were mixed together and stirred under ambient conditions for 6 h,the solution was dried in a rotary evaporator under 60°C to obtain a white powder,which is silica nanospheres about 20 nm.
MoS2nanospheres were synthesized according to Fig.1:0.10 g of(NH4)2MoS4was dissolved in 10 ml deionic water to get a red homogeneous solution,8.0 g SiO2nanoparticles were added into 20 ml deionic water and stirred to form a transparent sol.Then the above two solutions were mixed togetherand stirred for 30 min.After that the solution was poured into Teflon lined autoclaves using hydrothermal treatment at a specific temperature for 72 h.Then the autoclave was cooled down to room temperature and the resultant precipitate was centrifuged at8000 r·min-1for 3 min,washed with 10%HF solution for several times and dried at 70°C for 12 h,leading to MoS2nanospheres named as M-1,when the amount of SiO2was increased to 16 g,the resulting MoS2nanospheres were named as M-2.
Using thiourea and(NH4)6Mo7O24·4H2O as the precursors:0.41 g of(NH4)6Mo7O24·4H2O and 0.52 g thiourea were dissolved in 10 ml deionic water to get a transparent homogeneous solution,the next steps were the same as(NH4)2MoS4.The as prepared MoS2nanospheres were named as:T-1 and T-2.
Using Ammonium sulfide solution and(NH4)6Mo7O24·4H2O as the precursors:0.41 g of(NH4)6Mo7O24·4H2O and 5.5 ml Ammonium sulfide solution were dissolved in 10 ml deionic water to get a transparent homogeneous solution,the next steps were the same as(NH4)2MoS4.The as prepared MoS2nanospheres were named as:S-1 and S-2.
MoS2nanospheres were activated for hydrodesulfurization in a tube furnace under H2atmosphere at 400°C for 3 h.
2.3.Hydrodesulfurization property test
The catalytic activities were tested by utilizing Dibenzenethiophene(DBT)as the probe reactant.In a typical procedure,catalyst 0.10 g of Mo S2was dispersed in 40 ml heptane solution containing Dibenzenethiophene(40 mg)in a steel autoclave.The reactor was then purged and pressurized with H2to 4 MPa and heated up to 300 °C at a 10 °C·min-1heating rate under magnetic stirring.After reaching the working temperature,the products were collected for sulfur fluorescence analysis every hour to determine conversion versus time dependence.
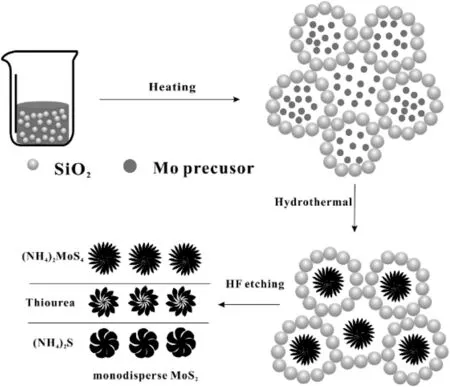
Fig.1.An approach to synthesis of monodisperse MoS2.
2.4.Characterization
The crystallographic information of the obtained MoS2nanocrystals was established by X-ray diffraction(XRD,using nickel- filtered Cu Kαradiation),scan rate 0.1(°)·s-1.The morphology of MoS2nanospheres was examined using a transmission electronic microscope(TEM,JEM-2100)and scanning electron microscopy(SEM,supra 55)and element analysis ofMoS2nanospheres determined byEnergy Dispersive Spectroscopy(EDS)(Oxford Inca Energy-dispersive X-ray spectroscopy).BET specific surface areas were determined using a Micromeritics Gemini instrument(type ASAP2010C).Concentration of sulfur was determined by a Sulfur Fluorescence Analyzer(KDS-3000).
3.Result and Discussion
In the preparing process,MoS2crystals were formed slower than SiO2crosslinking reaction,SiO2nanoparticles were crosslinking together to form a gel rapidly under hydrothermal condition,the coupled SiO2nanoparticles played a barrier role to give a kinematic viscosity condition causing lower collision probability between MoS2crystals,giving monodisperse MoS2nanospheres.The larger the amount of SiO2made the smaller the diameter of MoS2nanospheres.In Fig.2 diameters of MoS2nanospheres were tuned by the amount of SiO2,(NH4)2MoS4as precursor,MoS2nanospheres decreased from 390 nm to 90 nm.
The influences of sulfur precursors on the morphology of the product were also investigated(Fig.2).When using(NH4)2MoS4,uniform M-1 nanospheres with the size of about 90 nm were obtained(Fig.2(a)).From TEM images in Fig.2(c),we found that these nanospheres were composed of some nanosheets with thickness of about 3.4 nm.When using thiourea and Ammonium molybdate tetrahydrate as raw materials,MoS2nanospheres T-1 about 210 nm were obtained(Fig.2(d)),but thickness of as-synthesized samples changed.TEM images show size of MoS2sheets thicker than M-1 in Fig.2(f),according to literature MoS2prepared by thiourea can form a sheet like morphology[21].When using(NH4)2S and Ammonium molybdate tetrahydrate as raw materials,MoS2nanospheres S-1 about 400 nm were obtained,morphology of MoS2nanospheres was also changed to sheets.
XRD was used to determine the crystallinity and crystal size of MoS2nanospheres;Fig.3 showed the XRD patterns of as-prepared samples under different sulfur precursors.The sample M-1 obviously exhibited four typical peaks centered at 13.8°,33°,39°,and 59.8°corresponding to the(002),(101),(103)and(110)crystal planes,which could be assigned as hexagonal MoS2phase(JCPDS No.37-1492).According to the Debye-Scherrer formula,the crystal size of MoS2was about 8.6 nm.XRD peak intensities of MoS2steadily became stronger and the width of XRD diffraction peaks of MoS2became slightly narrower,indicating the formation of more and greater MoS2crystallites and an enhancement of crystallization in sample T-1.However,XRD peak intensities of S-1 were lower than M-1 and T-1 indicating lower crystallization according to TEM analysis.Therefore,these results indicated that sulfur precursors can significantly influence the crystallinity and crystal size of MoS2.

Fig.2.SEM and TEM images of as-synthesized MoS2 samples by changing amount of SiO2[SEM:(a)M-1,(b)M-2,(d)T-1,(e)T-2,(g)S-1,(h)S-2,TEM:(c)M-2,(f)T-2,(i)S-2].

Fig.3.XRD patterns of MoS2 prepared under different hydrothermal temperatures.
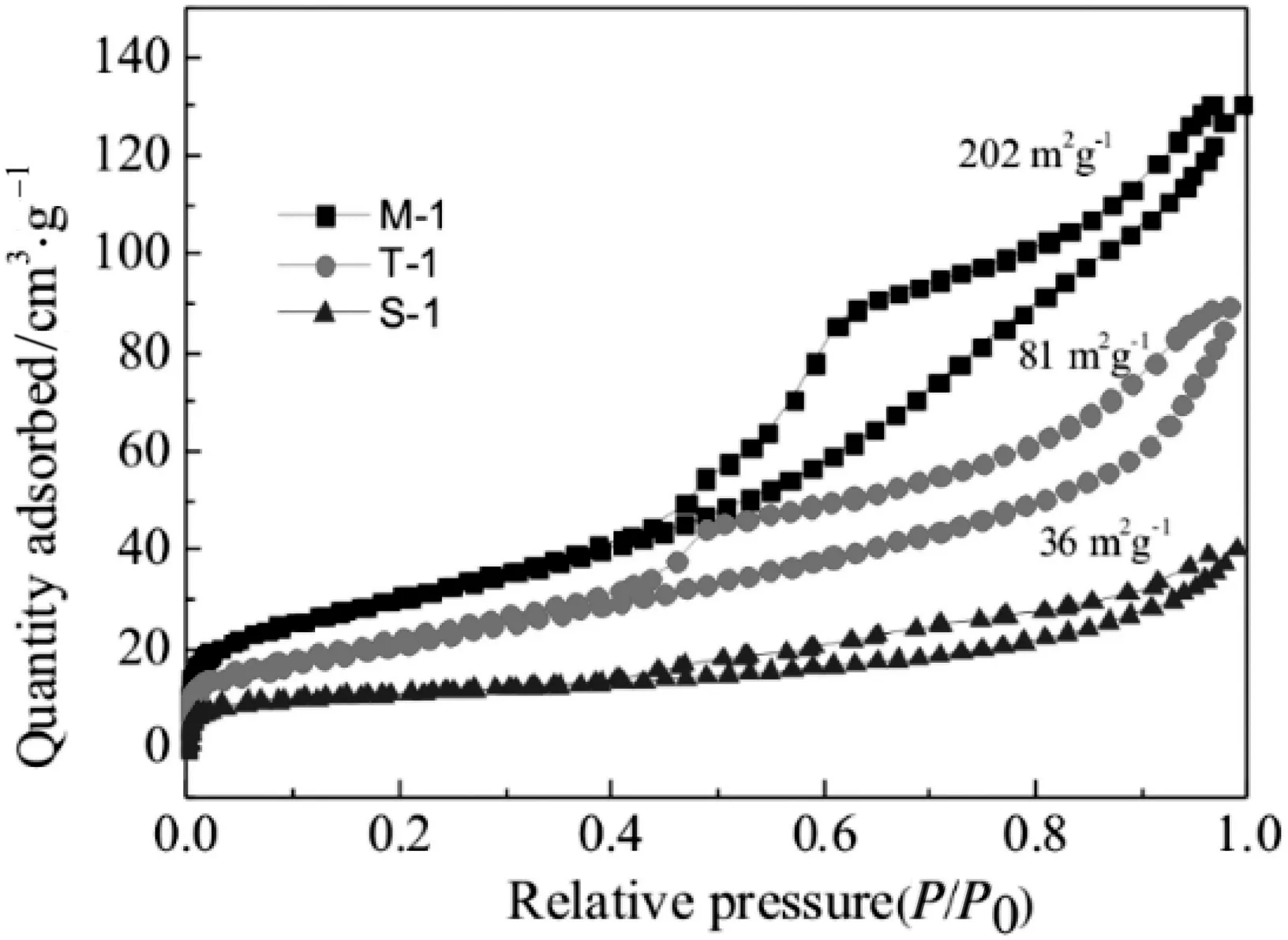
Fig.5.N2 adsorption-desorption isotherm analysis of M-1,T-1 and S-1.
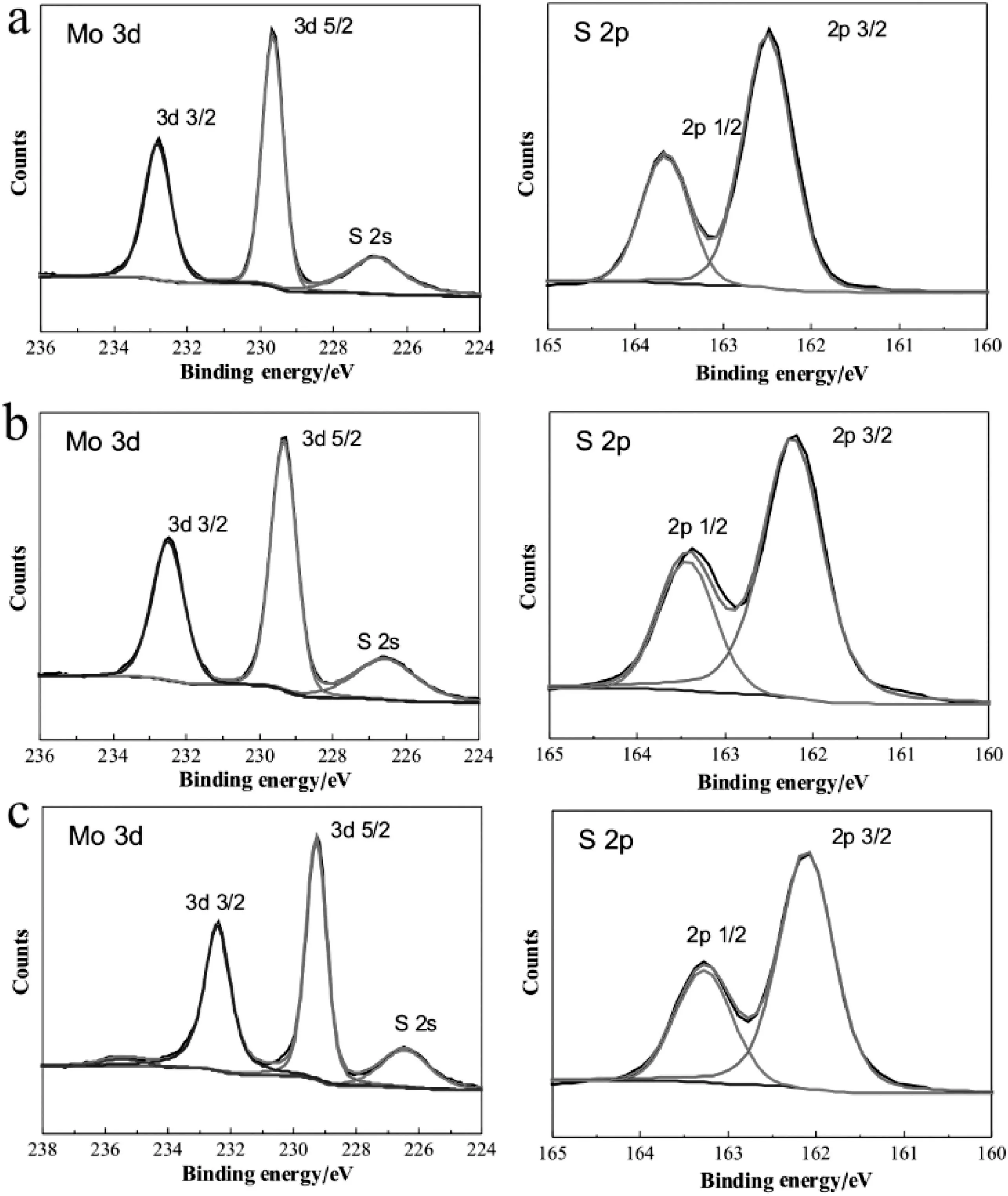
Fig.4.XPS analysis of MoS2 nanospheres[(a)M-1,(b)T-1,(c)S-1].
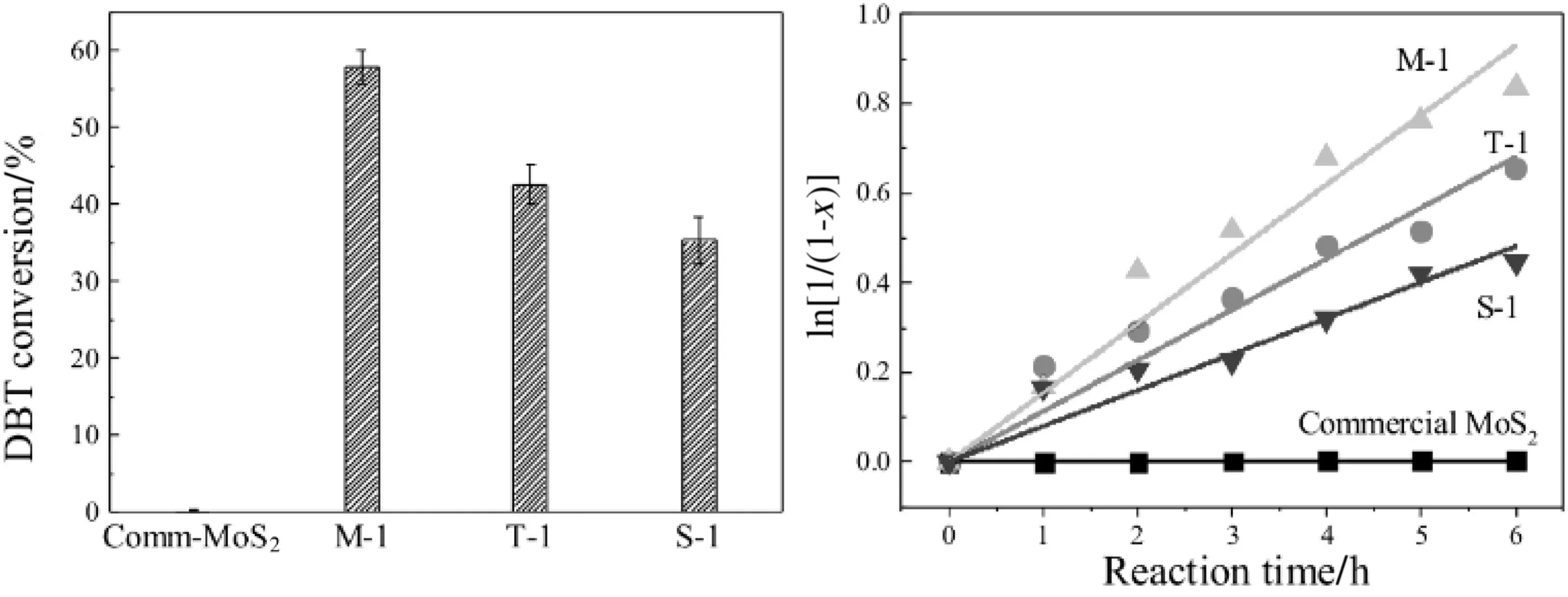
Fig.6.Hydrodesulfurization performance of MoS2 nanospheres.
To further clarify covalent value of molybdenum and sulfur in different morphology of MoS2nanospheres,X-ray photoelectron spectroscopy(XPS)analysis was performed in Fig.4.Typical XPS spectra of Mo(3d)and S(2p)core level region of as-prepared MoS2sample M-1 were shown in Fig.4a,the high intensity Mo(3d5/2)peak at 229.5 eV and the low intensity Mo(3d3/2)peak at 232.5 eV could be attributed to MoS2,and S(2p)spectra show a strong XPS peak at 162.5 eV and a weak peak at 163.7 eV,which was ascribed to S2-.XPS spectra of T-1 were shown in Fig.4b,peak values of Mo(3d)were about 229.3 eV and 232.5 eV,peaks of S(2p)spectra show at 162.6 eV and 163.8 eV similar with M-1.These results indicated that only pure MoS2was present without oxidized phase in M-1 and T-1.The peak values were nearly the same binding energies as those of reported[22].In Fig.4c,Mo(3d)spectra of sample S-1 show a little change at235.6 eV presenting an oxidized phase compared with M-1 and T-1.
Nitrogen adsorption/desorption isotherms of as-prepared MoS2samples M-1,T-1 and S-1 were shown in Fig.5.M-1 and T-1 exhibited type-IV isotherms with a regular type H1 hysteresis loop at relative pressure(P/P0)of 0.8-1.0.The obtained M-1 has a large surface area of about 202 m2·g-1.Mesopores on T-1 surface formed by removing SiO2nanospheres and made it owning a surface area about 81 m2∙g-1.The surface area decreases due to higher crystallinity and crystal size of MoS2nanosheets on spheres.And S-1 owns the lowest surface area of about 36 m2·g-1.
MoS2nanospheres were activated for hydrodesulfurization in a tube furnace under H2atmosphere at 400°C for 3 h.The catalytic activities were tested by utilizing Dibenzenethiophene(DBT)as the probe reactant.The procedure was seen in experiment details.The catalytic activity of HDS for as-synthesized MoS2nanospheres compared with commercial MoS2was presented in Fig.6.Commercial MoS2was used as catalyst for HDS,and conversion of DBT was only about 0.95%in Fig.5.All as-synthesized MoS2nanospheres exhibited better catalytic activity than commercial MoS2.With surface of MoS2nanospheres increasing catalytic activity of as-prepared MoS2nanospheres increased gradually and M-1 sample showed a much higher conversion of DBT.Combining N2adsorption-desorption isotherms,M-1 owned the largest surface areas,which in turn increased the number of active sites,leading to increase of catalytic activity[23].Another reason may be explained by the mechanism of the “Rim-edge model”in the HDS of MoS2.MoS2made by(NH4)2MoS4exhibits a positive role in the activity by increasing the number of accessible sites[24].Through MoS2nanospheres with high surface area exposed more active sites,resulting in improvement of the reaction rate.In the kinetic studies of HDS reactions of thiophenic compounds,either the Langmuir-Hinshelwood(L-H)model or the pseudo first-order model was used in the literature[25].Kinetic plots of HDS reaction of thiophene catalyzed by each as-synthesized MoS2sample with different morphology were shown in Fig.6.The HDS of each sample was treated as a pseudo- first-order reaction and the approximate linear relationship was observed for each sample.Rate constants obtained with different MoS2nanospheres were calculated from the regression lines.M-1 sample showed the fastest rate(k=0.15493 h-1)than the other sample.
4.Conclusions
Monodisperse MoS2nanospheres owning a controllable morphology and particle size were synthesized by SiO2gel as a barrier agent.Amount of SiO2had an effect on particle size of MoS2nanospheres.Different sulfur precursors greatly affect crystallinity and surface area of the MoS2nanospheres.Moreover,MoS2nanospheres M-1 prepared by(NH4)2MoS4exhibited better catalytic activity for hydrodesulfurization of DBT due to their high surface area and more active sites exposed.
[1]L.Zhi,H.Zhang,Z.Yang,W.Liu,B.Wang,Interface coassembly of mesoporous MoS2based-frameworks for enhanced near-infrared light driven photocatalysis,Chem.Commun.52(2016)6431-6434.
[2]G.Shi,L.Yu,X.Ba,X.Zhang,J.Zhou,Y.Yu,Copper nanoparticle interspersed MoS2nanoflowers with enhanced efficiency for CO2electrochemical reduction to fuel,Dalton Trans.46(2017)10,569-10,577.
[3]C.Liu,H.Liu,C.Yin,Preparation,characterization,and hydrodesulfurization properties of binary transition-metal sulfide catalysts,Fuel154(2015)88-94.
[4]W.Wang,L.Li,K.Wu,K.Zhang,J.Jie,Y.Yang,Preparation of Ni-Mo-S catalysts by hydrothermal method and their hydrodeoxygenation properties,Appl.Catal.A Gen.495(2015)8-16.
[5]D.Lee,S.Kim,Y.Kim,J.H.Cho,One-transistor-one-transistor(1T1T)optoelectronic nonvolatile MoS2memory cell with nondestructive read-out,ACS Appl.Mater.Interfaces9(2017)26,357-26,362.
[6]Y.Chen,X.Wang,P.Wang,Optoelectronic properties of few-layer MoS2FET gated by ferroelectric relaxor polymer,ACS Appl.Mater.Interfaces8(2016)32,083-32,088.
[7]Y.Liu,X.He,D.Hanlon,Electrical,mechanical,and capacity percolation leads to high-performance MoS2/nanotube composite lithium ion battery electrodes,ACS Nano10(2016)5980-5990.
[8]L.Zhang,H.B.Wu,Y.Yan,X.Wang,X.W.Lou,Hierarchical MoS2microboxes constructed by nanosheets with enhanced electrochemical properties for lithium storage and water splitting,Energy Environ.Sci.7(2014)3302-3306.
[9]Y.Tian,Y.He,Y.Zhu,Low temperature synthesis and characterization of molybdenum disulfide nanotubes and nanorods,Mater.Chem.Phys.87(2004)87-90.
[10]W.-J.Li,E.-W.Shi,J.-M.Ko,Z.-Z.Chen,H.Ogino,T.Fukuda,Hydrothermal synthesis of MoS2nanowires,J.Cryst.Growth250(2003)418-422.
[11]Y.Peng,Z.Meng,C.Zhong,Hydrothermal synthesis of MoS2and its pressure-related crystallization,J.Solid State Chem.159(2001)170-173.
[12]H.Hwang,H.Kim,J.Cho,MoS2nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials,Nano Lett.11(2011)4826-4830.
[13]L.Liu,H.Qiu,J.Wang,G.Xu,L.Jiao,Atomic MoS2monolayers synthesized from a metal-organic complex by chemical vapor deposition,Nanoscale8(2016)4486-4490.
[14]C.M.Zelenski,P.K.Dorhout,Template synthesis of near-monodisperse1microscale nanofibers and nanotubules of MoS2,J.Am.Chem.Soc.120(1998)734-742.
[15]K.Zhang,Y.Zhao,S.Zhang,MoS2nanosheet/Mo2C-embedded N-doped carbon nanotubes:synthesis and electrocatalytic hydrogen evolution performance,J.Mater.Chem.A2(2014)18,715-18,719.
[16]L.Zhang,X.W.Lou,Hierarchical MoS2shells supported on carbon spheres for highly reversible lithium storage,Chem.Eur.J.20(2014)5219-5223.
[17]S.E.Skrabalak,K.S.Suslick,Porous MoS2synthesized by ultrasonic spray pyrolysis,J.Am.Chem.Soc.127(2005)9990-9991.
[18]M.Liu,X.Li,Z.Xu,B.Li,L.Chen,N.Shan,Synthesis of chain-like MoS2nanoparticles in W/O reverse microemulsion and application in photocatalysis,Chin.Sci.Bull.57(2012)3862-3866.
[19]F.Cesano,S.Bertarione,A.Piovano,et al.,Model oxide supported MoS2HDS catalysts:Structure and surface properties,Catal.Sci.Technol.1(2011)123-136.
[20]J.W.McDonald,G.D.Friesen,L.D.Rosenhein,W.E.Newton,Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates,Inorg.Chim.Acta72(1983)205-210.
[21]S.-K.Park,S.-H.Yu,S.Woo,et al.,A facile and green strategy for the synthesis of MoS2nanospheres with excellent Li-ion storage properties,CrystEngComm14(2012)8323-8325.
[22]L.Hu,Y.Ren,H.Yang,Q.Xu,Fabrication of 3D hierarchical MoS2/polyaniline and MoS2/C architectures for lithium-ion battery applications,ACS Appl.Mater.Interfaces6(2014)14644-14652.
[23]G.Alonso,G.Berhault,A.Aguilar,Characterization and HDS activity of mesoporous MoS2catalysts prepared by in situ activation of tetraalkylammonium thiomolybdates,J.Catal.208(2002)359-369.
[24]M.Daage,R.R.Chianelli,Structure-function relations in molybdenum sulfide catalysts:the “rim-edge”model,J.Catal.149(1994)414-427.
[25]Y.Wang,Z.Sun,A.Wang,Kinetics of hydrodesulfurization of dibenzothiophene catalyzed by sulfided Co-Mo/MCM-41,Ind.Eng.Chem.Res.43(2004)2324-2329.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
- An innovative trigeneration system using biogas as renewable energy
