Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
Preeti Dauthal,Mausumi Mukhopadhyay *
1 Department of Chemical Engineering,Sardar Vallabhbhai National Institute of Technology,Surat 395007,Gujarat,India
2 Department of Chemical Engineering,Marwadi University,Gauridad,Rajkot 360003,Gujarat,India
1.Introduction
Various noble metal nanoparticles have attracted a significant attention because of their size and shape dependent specific properties[1].During recent years phytosynthesized noble metal nanoparticles and their applications have drawn interest of various researchers in the fields of biomedical science[2].Phytosynthesis is an eco-benign,convenient,safe,cost-efficient and rapid method for synthesis of biocompatible metal nanoparticles.Use of metal nanoparticles as an antioxidant is one such application which has attracted significant attention of researchers.It is well known that antioxidant plays a crucial role for proper running of various bio-systems by scavenging various harmful radicals,which cause oxidative stress to various cellular components.Free radicals are by-products of normal metabolic reactions of biological systems.In living organism like humans,free radicals are known to be responsible for the development of many pathological conditions ranging from cancer to cardiovascular disease,Alzheimer's disease,atherosclerosis,diabetes and aging[3-5].Besides this,free radicals are also responsible for oxidative deterioration of food materials.Effective scavenger of free radicals may serve as potent compounds to treat free radical mediated diseases as well as for food material preservation[6,7].Till now,various research groups are involved generally in the exploration of antioxidant potential of various bio-resources.However,recently free radical scavenging activity of nanomaterials is also reported in variousin-vivoandin-vitrosystems[8-12].
Theoretically,fabrication of large number and variety of nanostructure material is possible through various chemical and physical routes.Most of these strategies require high energy and toxic chemicals.Thus,these methods are potentially dangerous to various biological systems and hazardous to the environment[13].Therefore,biofabrication approaches are more economical,convenient and eco-benign and better substitutes for the classical procedures due to availability of various biological entities,rich biodiversity and easy availability[14].Biosynthesis methods have developed as a highlight of the intersection between biotechnology and nanotechnology.Thus,these methods have been received increasing attention in the last decade.Biofabrication of metallic nanoparticles is an eco-friendly approach without the use of toxic,harsh and expensive chemicals and safe for human therapeutic use.Nowadays,because of their non-pathogenic and nil toxic profile,use of different medicinal plants extract,is gaining importance for biofabrication of high quality nanostructures with sustainable commercial viability.Use of eco-friendly and biocompatible nanostructure materials is also important in terms of their biomedical utility.Recently,Suriyakalaaet al.have shown the strategy for biofabrication of biocompatible nanoparticles and their utility as a hepatocurative agent[15].
In the present studyDelonix regia,an ethno-medicinal plant of theCaesalpiniaceaefamily is selected for biofabrication.The bio-reducing ability ofD.regiais recently reported for the synthesis of monometallic palladium[16],gold[17]and bimetallic gold-palladium nanoparticles[18]in our previous studies.Biofabrication of silver nanoparticles is also stated earlierusing one another plant fromDelonixgenus(Delonix elata)[19].Thus,the present study is focused on the exploration of the bioreducing potential ofD.regiafor the synthesis of noble monometallic platinum nanoparticles(Pt-NPs)and bimetallic gold-silver nanoparticles(AuAg-NPs).Till now,biofabrication of nanoparticles has been focused mainly on the fabrication of noble monometallic nanoparticles of silver,gold and palladium.Albeit,platinum is also in the category of noble metal,but the biofabrication of platinum nanoparticles(Pt-NPs)has not been explored to the same extent as that of Au and Ag[20].Very few reports are available for biofabrication of Pt-NPs[13].Similarly,despite of the significant potential of alloy bimetallic nanoparticles in various domains due to bi-functional or synergistic effects,biofabrication of bimetallic nanoparticles has been explored very less,compared to monometallic nanoparticles[18].Therefore,the present study is dedicated for the fabrication of Pt-NPs and AuAg-NPs using widely available bio-resourceD.regia.Biomedical utility of both nanoparticles is further explored based on their antioxidant potential.Two model radicalsi.e.,(2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt radical(ABTS·+))and(1,1′-Diphenyl-2-picrylhydrazyl radical(DPPH·))have been utilized for assessment of the antioxidant potential of biofabricated nanoparticles.
2.Experimental
2.1.Materials
Leaves ofD.regiawere collected fromthe college campus of S.V.N.I.T.,Surat,Gujarat.Potassium persulfate,ABTS,ethanol and methanol were procured from Germany(Merck,Darmstadt).Folin-Ciocalteu's reagent,quercetin,bovine serum albumin,gallic acid and DPPH·were obtained from Germany(Sigma-Aldrich,Steinheim).Gold chloride trihydrate,silver nitrate,chloroplatinic acid hexahydrate,ascorbic acid,potassium phosphate,potassium ferricyanide,trichloroacetic acid,ferric chloride,sulfuric acid and sodium phosphate were acquired from India(Himedia,Mumbai).Ammonium molybdate was purchased from India(Merck,Mumbai).Sodium carbonate,aluminium chloride,and potassium acetate were boughtfrom India(Qualigens,Mumbai).Double distilled deionized water was collected from Elix Millipore system.
2.2.Preparation of D.regia leaf extract
To prepare the extract ofD.regialeaves,30 g ofD.regialeaves,was added to 120 ml of deionized water in round-bottom flasks of 500 ml capacity.The mixture was then heated for 10 min at60°C.The resultant extract was filtered with Whatman filter paper no.40.Thereafter,filtrate was used for further experiments.
2.3.Biofabrication of nanoparticles
Aqueous extract of fresh leaves ofD.regiawas prepared according to the procedure described in literature[16].In a typical reaction,about 400 ml of 1 × 10-3mol·L-1chloroplatinic acid hexahydrate solution was mixed with 100 ml aqueous leaf extract ofD.regia.Thermal assisted reduction ofchloroplatinic acid hexahydrate was carried out by keeping this reaction mixture in a sealed flask for 30 min(at 90°C)on a rotary shaker(500 r·min-1).
Similarly,biofabrication of AuAg-NPs was started by the addition of a well-mixed 800 mlaqueous solution of gold chloride trihydrate(400 ml;1 × 10-3mol·L-1):silver nitrate(400 ml;1 × 10-3mol·L-1)with 100 mlaqueous extract of fresh leaves ofD.regia.Spontaneous reduction of a mixture of salt solutions was carried out by keeping this reaction mixture in a sealed flask for 10 min on a rotary shaker(500 r·min-1).
Both reduced nanoparticle solutions were kept for sonication(10 min)to separate nanoparticles from the bioorganics of leaf extract followed by repetitive centrifugation at 15,000 r·min-1for 10 min for nanoparticle recovery.After centrifugation,pellets were washed with double distilled deionized water to remove the impurities followed by drying at 60°C for 2 h.Control reactions were performed with double distilled deionized water instead of leaf extract under the same reaction condition.
2.4.Calorimetric assay for total phenolic acid(TPA),total proteins(TP),total flavonoids(TF),reducing power capacity(RPC)and total antioxidant capacity(TAC)
The concentrations of TPA,TF and TP inD.regialeaf extract were determined by Folin-Ciocalteu's,aluminium chloride,and Bradford protein calorimetric methods respectively[16].The quantities of TPA,TF and TP were stated in gallic acid equivalent(GAE),quercetin equivalent(QE)and bovine serum albumin equivalent(BSAE)respectively.The quantity of these bioorganic compounds was expressed in mg of respective equivalent per gram of fresh weight(fw)of leaves.
The RPC ofD.regialeaf extract was determined by following the procedure explained by Oyaizu with slight modifications[21].In a typical reaction,0.5 ml sample ofD.regialeaf extract was mixed with 2.5 ml of potassium ferricyanide(1%)and 2.5 ml of phosphate buffer(0.2 mol·L-1;pH 6.6)followed by incubation for 20 min(50°°C).Then,2.5 ml of trichloroacetic acid solution(10%)was mixed with the above mixture.This mixture was kept for centrifugation at 3000 RPM(10 min).Then,2.5 ml of distilled water and 0.5 ml of ferric chloride solution(0.1%)were mixed with 2.5 ml of supernatant solution(obtained after centrifugation).The quantity of iron-(II)-ferricyanide complex formation in the reaction mixture was measured by the formation of Perl's Prussian blue color.The absorbance value of this reaction solution was measured at 700 nm against a blank.A typical blank solution contains an equivalent volume of double distilled water,instead of leaf extract.Quantification of RPC was done on the basis of standard curve of ascorbic acid(AA)and it was stated in mg of ascorbic acid equivalent(AAE)per gram of fw of leaves.
The TAC ofD.regialeaf extract was assayed following the procedure of Prietoet al.with few alterations[22].A 0.2 ml aliquot of aqueous extract ofD.regiawas mixed with 2.0 ml of reagent solution(1:1:1 ratio of 0.6 mol·L-1sulfuric acid,28 mmol·L-1sodium phosphate and 4 mmol·L-1ammonium molybdate)in a capped sample vial followed by incubation in a water bath at 95°C(90 min).This solution was further cooled to room temperature.The amount of green phosphate/Mo(V)complex formation in the reaction mixture was measured at 695 nm against a blank.A typical blank solution consisted 2.0 ml of reagent solution with 0.2 ml of the double distilled water.Quantification of TAC was done on the basis of standard curve of AA and it was stated in mg of AAE per gram of fw of leaves.
2.5.In-vitro antioxidant assay of biofabricated nanoparticles
The antioxidant activity of biofabricated nanoparticles was evaluated by measuring their capability to scavenge synthetic stable radical of DPPH·and ABTS·+using the method given by Serpenet al.with minor modifications[23].
For DPPH·scavenging reaction,nanoparticle samples of 2.5,5,10,15,20 and 30 mg were mixed with 4.0 mlofDPPH·solution(400 mmol·L-1in 80vol%ethanol)in different Eppendorf tubes followed by ultrasonication for 30 min to allow the surface reaction of nanoparticles with DPPH·.Then reactions were quenched by removing the nanoparticles from reaction mixtures.For this different reaction mixtures were centrifuged at 10000 r·min-1(2 min)followed by filtration using a 0.1 μmol·L-1membrane filter.The absorbance of different filtrates was then measured at λmax(521 nm)to understand the scavenging activity of biosynthesized nanoparticles for DPPH·.DPPH·is a stable radical and its scavenging reaction usually takes a longer time when compared with ABTS·+[23,24].Therefore,all investigations were performed after 30 min of reaction.
To evaluate the ABTS·+scavenging activity of nanoparticles, first ABTS salt(7.0 μmol·L-1)was mixed with potassium persulfate(2.45 μmol·L-1).For synthesis of ABTS cation radical(ABTS·+),the above reaction mixture was kept in the dark for 16 h(23 °C).Then,ABTS·+was diluted with methanol(80vol%),till absorbance of solution reached 0.700 ± 0.005 at λmax(734 nm).
For ABTS·+scavenging reaction,D.regiamediated nanoparticles(2.5,5,7.5,10,and 12.5 mg)were transferred with 4.0 ml of ABTS·+in different Eppendorf tubes followed by ultra-sonication for 6 min to assistthe surface interaction ofABTS·+with nanoparticles.Completions of reactions were observed by color change from bluish-green to colorless.Then different reaction mixtures were centrifuged at 10000 r·min-1(2 min)to recover nanoparticles.The scavenging activity of nanoparticles for ABTS·+was measured by the absorbance values of the different filtrates at λmax(734 nm).All the above tests were accomplished in triplicate and the results were then averaged.
Radical scavenging activity(RSA)of nanoparticles was expressed in percentage using the following Eq.(1),where AS:absorbance of the free radicals(DPPH·and ABTS·+)with nanoparticles and AC:absorbance of the free radicals without nanoparticles.The RSA ofD.regialeaf was also measured as positive control.
2.6.Characterization
A UV-Visible spectrophotometer,(DR 5000,HACH,USA),has been used for the measurement of optical absorbance and RSA of nanoparticles along with quantification of bioorganic compounds and antioxidant potential of biological extract used in the present study.A dynamic light scattering(DLS)instrument,(Zetasizer Nano ZS90,Malvern,UK)has been utilized to analyse the distribution of hydrodynamic diameter and ζ potential of colloidal nanoparticles.A transmission electron microscopy(TEM)instrument,(CM-200,Philips,UK)has been used for direct imaging and selected area electron diffraction(SAED)pattern analysis of nanoparticles.An X-ray diffractometer,(X'Pert Pro,PANalytical,Holland)has been used to analyse the X-ray diffraction(XRD)pattern and crystalline structure of nanoparticles.A scanning electron microscope,(JSM-6380LV,JEOL,Japan)equipped with an energy dispersive X-ray spectroscopy(EDX)detector,(INCA X-sight,Oxford Instruments,UK)has been utilized for the determination of elemental composition of nanoparticles.A thermal analysis system,(Perkin Elmer,Diamond TG/DTA,USA)has been used for the thermogravimetric analysis(TGA)of the nanoparticles.
3.Results and Discussion
3.1.Structural characteristics of nanoparticles
The gradual change in the color of platinum reduced solutions from pale yellow to light brown within reaction periods of 30 and 60 min at 90°C,suggested the fabrication of colloidal Pt-NPs.Fig.1(a)represents UV-Visible spectra of the colloidal Pt-NPs and precursor H2PtCl6·6H2O.The absorption peak for H2PtCl6·6H2O was observed at around 260 nm.On thermal treatment of the solution containing H2PtCl6·6H2O andD.regialeafextract,the absorption peak corresponding to H2PtCl6·6H2O present at 260 nm disappeared.This peak was replaced by a broad spectral continuum spanning from the visible to ultra-violet region,suggesting complete bioreduction of platinum ions(Pt4+)to zero valent form(Pt0).

Fig.1.UV-Visible absorption spectra of(a)Pt-NPs and(b)AuAg-NPs.
Similarly,fabrication of AuAg-NPs was con firmed,by comparing the absorbance pattern of AuAg-NPs with their monometallic counterparts along with their physical mixture[Fig.1(b)].Appearance of high and comparatively low intensity SPR peak(in two different spectra)present at 542 and 431 nm con firmed the biosynthesis of Au-NPs(10 min)and Ag-NPs(20 min)respectively.Faster reduction rate and higher intensity SPRpeak of Au-NPs,compared to Ag-NPs attributed to the higher reduction potential of Au3+/Au0than Ag+/Ag0[25,26].It was also noticed that reddish purple color of AuAg-NP colloid appears within 10 min of reaction time,due to acceleration of the reduction rate of Ag+in the presence of Au3+[25].A single broad high intensity SPR peak observed at 500 nm for bimetallic AuAg-NPs in co-reduced solution,proved the presence of alloy bimetallic nanoparticles,despite the physical mixture of both nanoparticles.
Generally,bimetallic nanoparticles exhibit a single SPR peak,which is normally located between SPR peaks of pure nanoparticles[27].However,the exact position,broadness and intensity of SPR peak(λmax)of bimetallic nanoparticles are generally based on the particle size and environment of nanoparticle's surface(chemical interface damping)[28,29].The increased SPR intensity of bimetallic AuAg-NPs might have been caused by an ingression of Au-NPs into the Ag-NPs after the reduction of the Au ions as higher reduction potential of Au3+/Au0as compared to Ag+/Ag0promotes faster reduction rate of Au3+as compared to the Ag+and facilitates ingression of Au-NPs into the Ag-NPs[25,26,30].As far as visual observation of blank solution is concerned,there was no color variation observed in the control reactions,suggesting the formation of nanoparticles was only due to the biological components ofD.regialeaf extract not due to any abiotic feature.
DLS pattern revealed that the size distribution of colloidal Pt-NPs was found in the range of 11-58 nm(average hydrodynamic diameter:33.99 nm)and polydispersity index(PDI)of 0.192[Fig.2(a)].However,size distribution of colloidal AuAg-NPs was observed in the range of 21-105 nm(average hydrodynamic diameter:65 nm)[Fig.2(c)].The PDI was observed to be comparatively higher for AuAg-NPs(0.233).This observation suggested that AuAg-NPs were distributed in a broad range as con firmed also from TEM analysis.
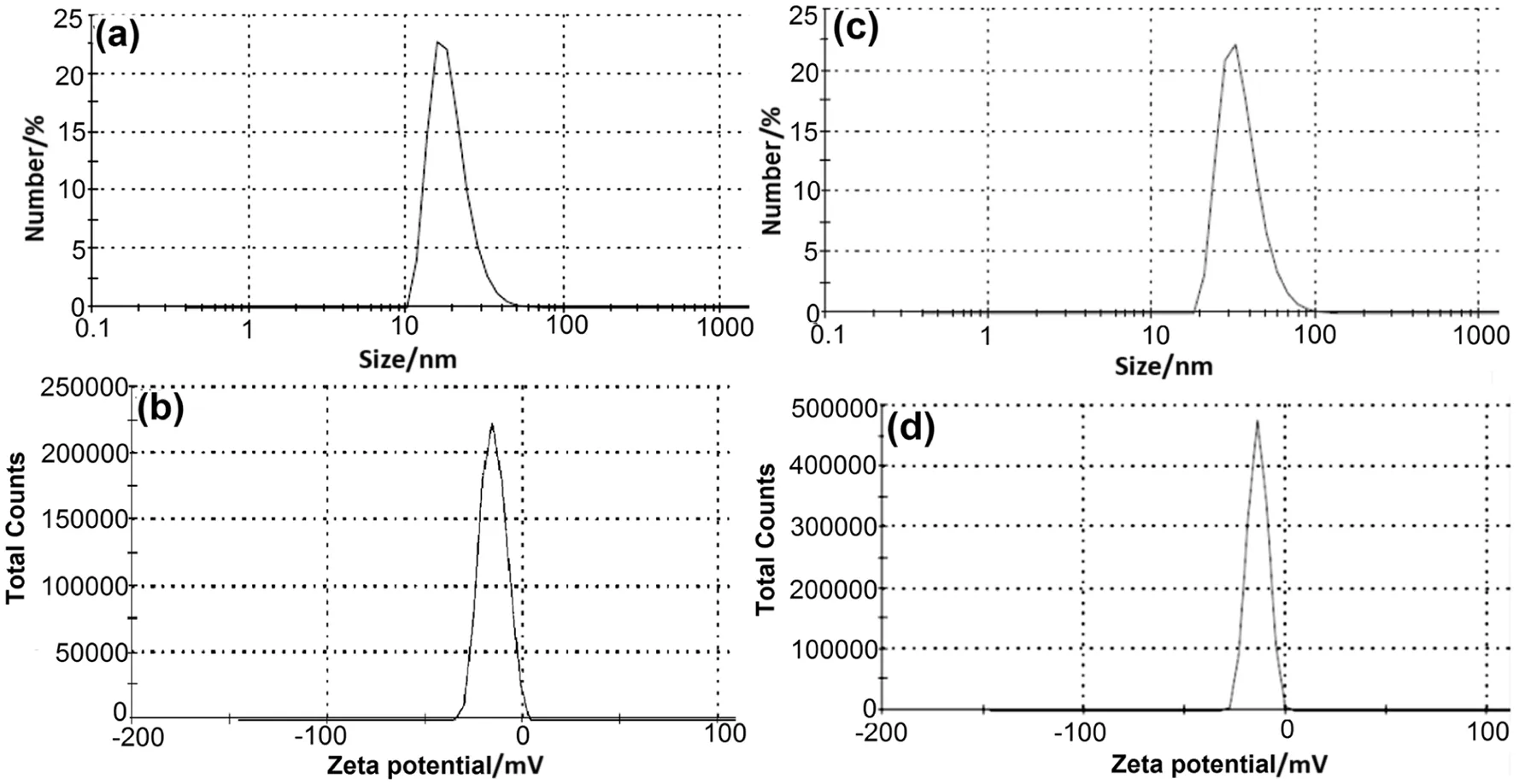
Fig.2.Hydrodynamic size distribution of(a-c)Pt-NPs and AuAg-NPs;ζ potential distribution of(b-d)Pt-NPs and AuAg-NPs.
The colloidal solutions of both nanoparticles were found negatively charged having an average ζ potential of-15.2 mV(Pt-NPs)and-13.9 mV(AuAg-NPs)[Fig.2(b)-(d)].The negative values of ζ potential could be due to the capping of nanoparticles by polyphenolic constituents ofD.regiaextract.It is well known that the magnitude of ζ potential anticipates the stability of nanoparticles in colloidal solution.Negative values of ζ potential suggested the existence of repulsive forces among biosynthesized nanoparticles which provide stability.
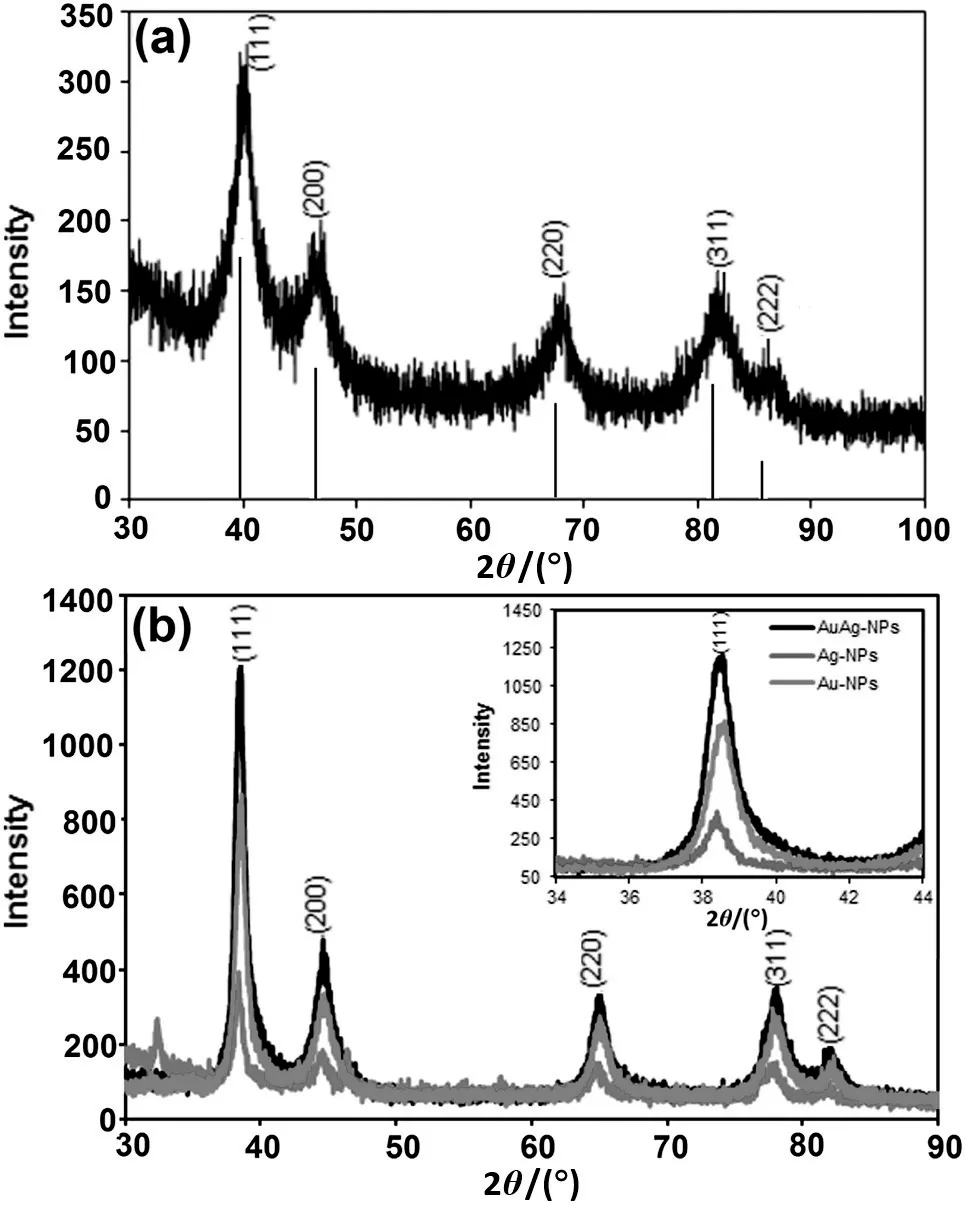
Fig.3.XRD diffractogram of(a)Pt-NPs and(b)AuAg-NPs.
Further,crystalline structures of dried nanoparticles were evaluated using XRD analysis[Fig.3(a)].XRD pattern of the biofabricated Pt-NPs indicates five distinct Bragg's peaks observed at(2θ)=39.97°(111),46.99°(200),68.07°(220),81.35°(311)and 85.31°(222)corresponding to different planes of Pt-NPs(JCPDS no.04-0802).The crystallite size of 7 nm was calculated using the full-width-at-half-maximum of highest intensity Bragg peak(111)using the Debye-Scherrer method.Lattice constant value calculated from this pattern was 3.928 nm;con firming the face-centred-cubic structure of Pt-NPs.Similarly,to confirm the crystallinity of AuAg-NPs,XRD pattern of AuAg-NPs[Fig.3(b)]was compared with pure Au-NPs and Ag-NPs.Bimetallic AuAg-NPs displayed Bragg reflections at 38.56°,44.61°,64.95°and 77.92°and 82.08°positions.The positions of Bragg reflections of AuAg-NPs were very similar to individual Au-NPs and Ag-NPs.This was due to the minor difference of their lattice constants(4.069 nm:Au-NPs and 4.079 nm:Ag-NPs)[31].
For detail comparison,highly intense(111)Bragg peak present at 38.56°of AuAg-NPs was compared with(111)reflection of pure nanoparticles(38.46°of Au-NPs and 38.39°of Ag-NPs)[inset in Fig.3(b)].It was observed that the intensity of(111)Bragg reflections was comparatively less intense in monometallic Au-NPs and Ag-NPs,as compared to the bimetallic AuAg-NPs.Significant enhancement in the intensity of(111)Bragg reflections ofAuAg-NPs,suggests the ingression of Au-NPs into the Ag-NPs after the reduction of the Au ions as higher reduction potential of Au3+/Au0as compared to Ag+/Ag0promotes faster reduction rate of Au3+as compared to the Ag+which facilitates ingression of Au-NPs into the Ag-NPs[30].The result of XRD analysis is very much coherent with the results of SPR peak enhancement in bimetallic nanoparticles.However,more thermodynamic driving force for the Ag on the surface rather than Au supports more distribution of Ag on the shell and Au on the core,despite their alloying interaction[32,33].This result provided evidence that biofabricated bimetallic nanoparticles had alloy bimetallic structure with Au-rich core and Ag-rich surface,further con firmed in TEM analysis.Crystallite size of AuAg-NPs calculated using full-width-at-half-maximum of(111)reflection,was 15 nm.
TEM imaging of nanoparticles was also performed to visualize the morphological characteristics of biosynthesized nanoparticles[Fig.4(a)-(b)].Biosynthesized Pt-NPs were distributed in the narrow range of 2-4 nm.Distorted spherical and irregular aggregated Pt-NPs were observed in TEM image.This aggregate formation was due to the high temperature and also the polyphenolic compounds ofD.regiathat coated the nanoparticles kept them close[34].Bright circular concentric rings in SAED pattern[inset in Fig.4(a)]also indicated the crystalline structure of Pt-NPs.

Fig.4.TEM micrograph of(a-b)Pt-NPs(c-d)AuAg-NPs(e)size distribution histogram of AuAg-NPs(inset represented SAED pattern of Pt-NPs and AuAg-NPs).
TEM images of AuAg-NPs also signified the presence of distorted spherical and irregular bimetallic AuAg-NPs with particle size ranging from 6 to 40 nm[Fig.4(c)-(d)].Particle size histogram of AuAg-NPs suggests that,60%of nanoparticles were present in the size range of 30-40 nm[Fig.4(e)].Despite the alloying interaction of Au-NPs and Ag-NPs for the formation of alloy bimetallic AuAg-NPs as con firmed earlier by XRD,UV-Visible analysis,few core-shell structures were also visible in TEM images[Fig.4(c)].However,no clear demarcation between core and shell,suggests that the core and shells might be AuAg alloys rather than pure Ag and Au.Cores are partially distinguished from the shells because higher atomic number of Au provides more contrast in TEM as compared to Ag[35].Higher thermodynamic driving force for Ag on the surface facilitates the formation of alloy nanoparticles with Au-rich core and Ag-rich shell[32].If the core and shells are AuAg alloys,the composition is likely graded,so that they are the most Ag-rich on the nanoparticle surface and Au-rich on the core.Grading and distribution of Au and Ag may arise from a kinetically regulated process.As expected,the contrast becomes uniform upon alloying.The Au,Ag,and AuAg phases are nearly isomorphous,which prevents the use of diffraction methods for distinguishing between distinct phases as confirmed earlier in XRD.In addition to this,the presence of four spotty diffraction rings in SAED pattern instead of eight(produced by two phase-segregated,core-shell metals)suggested the synthesis of alloy bimetallic AuAg-NPs[inset in Fig.4(d)].
Quantification of different elements associated with biofabricated nanoparticles was ascertained by quantitative EDX analysis[Fig.5(a)-(b)].The presence of a strong signal at around 2.10 keV indicates the presence of nano-sized particles of platinum and existence of oxygen and chloride signals in EDX acclaimed the surface adsorption of bioorganic compounds ofD.regialeaf extract.Elemental composition and their distribution ratio in biosynthesized Pt-NPs are shown in Table 1.
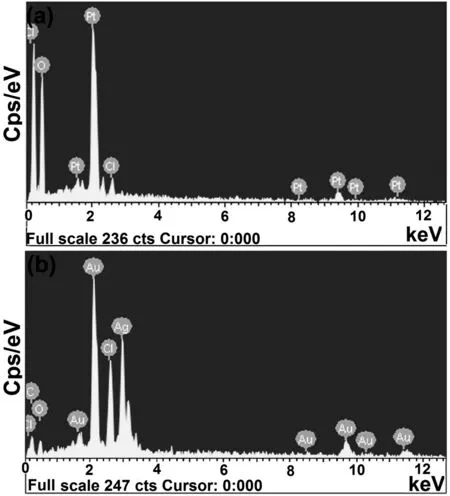
Fig.5.EDX spectra of(a)Pt-NPs and(b)AuAg-NPs.

Fig.6.TGA plots of(a)Pt-NPs and(b)AuAg-NPs.

Table 1 Mass content(%)of different elements present in biofabricated nanoparticles
EDX spectrum of biofabricated AuAg-NPs evidenced the co-existence of Au and Ag,in bimetallic nanoparticles(characteristic X-ray peaks present at 2.20 keV:pure Au and 3 keV:pure Ag).Table 1 represented the distribution ratio of different elements(%)in bimetallic nanoparticles.The proportions of Au and Ag which were measured using EDX were of 38.57 and 32.70%,respectively;almost consistent with that of the feed solution(1:1).The uniformity of Au and Ag content con firmed the homogenous distribution of both metal for fabrication of bimetallic AuAg-NPs despite the higher probability of Au in the core and Ag in the shell.
TGA plots ofD.regiamediated nanoparticles represented the initial mass loss of 2.14%for Pt-NPs[Fig.6(a)]and 8.15%for AuAg-NPs[Fig.6(b)]till 100°C.These mass losses were attributed to loss of water molecules present on the surface of nanoparticles.Thereafter,there were steady mass losses of approximately 11.33%(Pt-NPs)and 40.29%(AuAg-NPs)up to 615°C.The observed behavior is most likely as a consequence of surface desorption of bioorganic compounds of leaf extract present in the nanoparticles[36].Thus,these nanoparticles are expected to be made up of bioorganic compounds responsible for reduction of metal ions and stabilization of nanoparticles.
3.2.Probable mechanism of D.regia leaf extract mediated nanoparticle synthesis
The involvement of bioorganic compounds ofD.regialeaf extractduring biosynthesis of nanoparticles was initially con firmed by UV-Visible spectroscopy analysis of various bioorganic compounds(TPA,TF and TP)of leaf extract before and after bioreduction of salt solutions(H2PtCl6·6H2O and HAuCl4·3H2O:AgNO3).Significant reduction was observed in the concentrations of various bioorganic compounds after bioreduction of salt solutions(Table 2).This observation suggested the major role of water soluble phenolic acids and flavonoids in the redox reaction,as a main electron donor and played an important part in biofabrication of nanoparticles.UV-Visible spectroscopy analysis of these phytoconstituents revealed that bioorganic compounds of leaf extract act synergistically during the biosynthesis and stabilization of nanoparticles.Biosynthesized nanoparticles also possess negative surface charge(ζ potential)due to these adsorbed bioorganic compounds.The thermogram pattern of both biosynthesized nanoparticles in TGAanalysis also revealed the capping potential of bioorganic compounds.
In addition to this,significant differences observed in RPC and TAC of leaf extract before and after bioreduction of salt solutions,also proved that the antioxidant potential of bioorganic compounds played a major role in bioreduction of salt solution(Table 3).It is also proved earlier that the antioxidant activity of biological extracts is usually linked to their phenolic acids and flavonoid contents[37].
3.3.DPPH·and ABTS·+scavenging activity of nanoparticles at nano-bio interface
DPPH·is used as a representative compound for lipid radical,which contains a nitrogen radicalat the centre and exhibited an intense purple color.Antioxidant compounds interact with DPPH·,by transferring either electrons or hydrogen atoms and thus neutralizes free radical characteristic of DPPH·[38].Similarly,ABTS·+scavenging is an exceptional method for defining the antioxidant activity of chain-breaking or hydrogen-donating antioxidant compounds(scavengers of lipid peroxyl radicals)[39].
It was observed that in the presence of biofabricated nanoparticles(Pt-NPs and AuAg-NPs),the color of DPPH·gradually transformed from deep purple to pale yellow.This observation indicates that the investigated nanoparticles have electron/hydrogen transfer ability towards the free radical and convert it to DPPH-H.Similarly,a gradualchange was also observed in the color of ABTS·+from bluish-green to colorless in the presence of investigated biofabricated nanoparticles.Positive DPPH·and ABTS·+assay demonstrated that biosynthesized nanoparticles scavenged DPPH·and ABTS·+radicals in an almost linear manner and follow a similar trend.Mass response bar chart ofD.regiaderived nanoparticles for scavenging of DPPH·and ABTS·+is shown in Fig.7(a)-(b).

Table 2 Calorimetric assay of TPA,TF and TP of D.regia leaf extract

Table 3 Calorimetric assay of RPC and TAC of D.regia leaf extract

Fig.7.Mass response bar chart of free radical scavenging of DPPH·by(a)Pt-NPs and AuAg-NPs;ABTS·+by(b)Pt-NPs and AuAg-NPs.
Interestingly,the ABTS·+scavenging activity of nanoparticles was found consistently higher than that obtained for DPPH·scavenging for all applied mass of the materials.The SC50(mass of applied material required to scavenge 50%of DPPH·and ABTS·+)values of Pt-NPs and AuAg-NPs were calculated from the mass response bar chart and mentioned in Table 4.
Free radical scavenging activity of biofabricated nanoparticles was also compared with their biological resources on mass basis(Table 4).As leafextract ofD.regiais a combination of various bioorganic compounds of different molar weight and surface adsorption of these compounds at the surface of nanoparticles is con firmed from UV-Visible spectroscopy,TGA and ζ potential analysis.Therefore,comparison of free radical scavenging activity is only possible through mass basis.Surprisingly,it was found that the biofabricated nanoparticles exhibited comparatively better DPPH·and ABTS·+scavenging activity thanD.regia.This was due to the fact that several antioxidant polyphenolic compounds ofD.regiaact synergistically,during the biofabrication of the nanoparticles.These compounds were adsorbed onto the active surface of the nanoparticles.This was also suggested by ζ potential,FTIR and EDX analysis.

Table 4 Comparative table for SC50
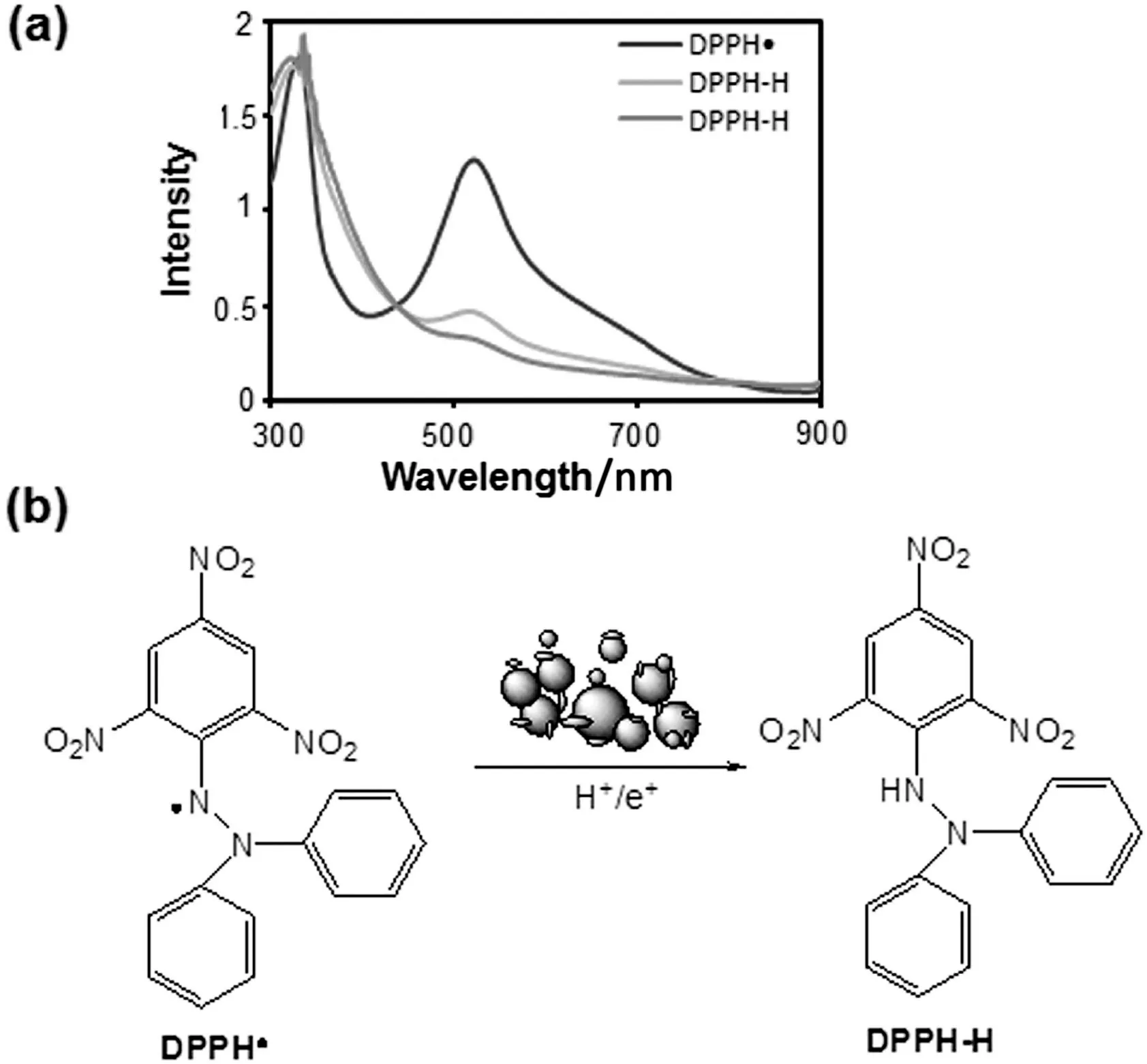
Fig.8.Scavenging of DPPH·to DPPH-H by biofabricated nanoparticles:(a)UV-Visible spectra;(b)possible mechanism.
To evaluate the reaction mechanism of nanoparticle mediated free radical scavenging reaction,wavelength scanning of DPPH·and ABTS·+was carried out in the presence of Pt-NPs and AuAg-NPs.It was observed that in the presence of biofabricated nanoparticles,the peak intensity of DPPH·present at 517 and 333 nm,decreased and replaced with the intermediate wavelengths[Fig.8(a)].This observation suggested that the nanoparticles transfer electron or hydrogen towards the free radical placed at nitrogen atom in DPPH·and convert it to DPPH-H[Fig.8(b)].
Similarly,ABTS·+specific peaks were observed at 414,645,734 and 815 nm in UV-Visible spectra[Fig.9(a)].Addition of biosynthesized nanoparticles(Pt-NPs and AuAg-NPs)in ABTS·+,a new peak developed at 340 nm,suggesting the formation of ABTS.This observation suggested the electron transfer ability of nanoparticles towards ABTS·+[Fig.9(b)].However,the exactchemistry behind the scavenging activity of biosynthesized nanoparticles against free radicals at nano-bio interface still needs further investigation.Biofabricated nanoparticles interact and scavenge DPPH·and ABTS·+due to ambient electrostatic field and large surface area to volume ratio[40-42].This observation was also supported by the observations made earlier for enhanced antioxidant activity of biosynthesized nanoparticles as compared to their biological resources[15,43],where surface adsorbed antioxidant bioorganic compounds aided the generation of electrostatic field on active surface of nanoparticles.
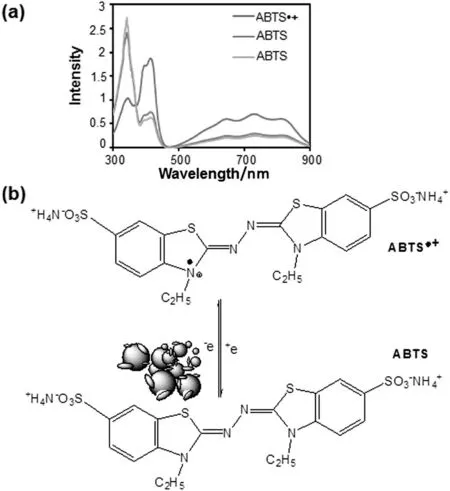
Fig.9.Scavenging of ABTS·+to ABTS by biofabricated nanoparticles:(a)UV-Visible spectra;(b)possible mechanism.
Free radical scavenging activity of various chemically and physically synthesized nanoparticles is also reported recently due to their surface reaction phenomenon[8,44].The chemistry involved in the free radical scavenging activity of biofabricated nanoparticles at nano-bio interface needs further investigation.However,the study of biofabricated nanoparticles in this aspect opens the door for new range of antioxidants,which might lead to a significant impact in the domain of biomedical science and food preservation industry.
4.Conclusions
The current study establishedD.regiaas a potential bio-resource for the biofabrication of distorted spherical,crystalline,stable mono-and bimetallic nanoparticles.Biofabricated nanoparticles have concentration dependent potential for scavenging of ABTS·+and DPPH·.The SC50values of the Pt-NPs and AuAg-NPs are calculated to be 5.39 and 4.45 mg for DPPH·and 5.39 and 4.45 mg for ABTS·+.Both nanoparticles exhibited higher scavenging potential towards ABTS·+.This study of biofabricated nanoparticles as an antioxidant could further lead to a different vision to the potential of biogenic nanoparticles in the domain of biomedical science and food preservation industry.
Acknowledgements
Authors are thankful to SAIF and MEMS IIT Bombay for providing research facility.Electrical Research and Development Association(ERDA)Vadodara is also acknowledged for providing EDX facility.
[1]Z.Wang,Y.Zhu,A simple plasma reduction for synthesis of Au and Pd nanoparticles at room temperature,Chin.J.Chem.Eng.23(6)(2015)1060-1063.
[2]G.Benelli,Plant-mediated synthesis of nanoparticles:a newer and safer tool against mosquito-borne diseases?Asian Pac.J.Trop.Biomed.6(4)(2016)353-354.
[3]J.T.Coyle,P.Puttfarcken,Oxidative stress,glutamate,and neurodegenerative disorders,Science262(5134)(1993)689-695.
[4]J.A.Sonnen,J.C.Breitner,M.A.Lovell,W.R.Markesbery,J.F.Quinn,T.J.Montine,Free radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models,Free Radic.Biol.Med.45(3)(2008)219-230.
[5]Q.Liu,H.Liu,Z.Yuan,D.Wei,Y.Ye,Evaluation of antioxidant activity of chrysanthemum extracts and tea beverages by gold nanoparticles-based assay,Colloids Surf.B92(2012)348-352.
[6]E.Beltran,R.Pla,J.Yuste,M.Mor-Mur,Use of antioxidants to minimize rancidity in pressurized and cooked chicken slurries,Meat Sci.66(3)(2004)719-725.
[7]M.Valko,D.Leibfritz,J.Moncol,M.T.Cronin,M.Mazur,J.Telser,Free radicals and antioxidants in normal physiological functions and human disease,Int.J.Biochem.Cell Biol.39(1)(2007)44-84.
[8]A.Watanabe,M.Kajita,J.Kim,A.Kanayama,K.Takahashi,T.Mashino,Y.Miyamoto,In vitro free radical scavenging activity of platinum nanoparticles,Nanotechnology20(45)(2009)455105.
[9]P.Dauthal,M.Mukhopadhyay,In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles usingPrunus armeniaca(apricot)fruit extract,J.Nanopart.Res.15(2013)1366-1376.
[10]N.J.Reddy,V.D.Nagoorvali,M.Rani,S.S.Rani,Evaluation of antioxidant,antibacterial and cytotoxic effects of green synthesized silver nanoparticles byPiper longumfruit,Mater.Sci.Eng.C34(2014)115-122.
[11]B.Moldovan,L.David,M.Achim,S.Clichici,G.A.Filip,A green approach to phytomediated synthesis of silver nanoparticles usingSambucus nigraL.fruits extract and their antioxidant activity,J.Mol.Liq.221(2016)271-278.
[12]A.H.Labulo,E.T.Adesuji,O.A.Dedeke,O.S.Bodede,C.O.Oseghale,R.Moodley,V.O.Nyamori,E.O.Dare,O.A.Adegoke,A dual-purpose silver nanoparticles biosynthesized using aqueous leaf extract ofDetarium microcarpum:An underutilized species,Talanta160(2016)735-744.
[13]P.Dauthal,M.Mukhopadhyay,Noble metal nanoparticles:Plant mediated synthesis,mechanistic aspects of synthesis and applications,Ind.Eng.Chem.Res.55(36)(2016)9557-9577.
[14]P.Kuppusamy,M.M.Yusoff,G.P.Maniam,N.Govindan,Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report,Saudi Pharm.J.24(4)(2016)473-484.
[15]U.Suriyakalaa,J.J.Antony,S.Suganya,D.Siva,R.Sukirtha,S.Kamalakkannan,P.B.T.Pichiah,S.Achiraman,Hepatocurative activity of biosynthesized silver nanoparticles fabricated usingAndrographis paniculata,Colloids Surf.B102(2013)189-194.
[16]P.Dauthal,M.Mukhopadhyay,Biosynthesis of palladium nanoparticles usingDelonix regialeaf extract and its catalytic activity for nitro-aromatics hydrogenation,Ind.Eng.Chem.Res.52(51)(2013)18131-18139.
[17]P.Dauthal,M.Mukhopadhyay,Phyto-synthesis and structural characterization of catalytically active gold nanoparticles biosynthesized usingDelonix regialeaf extract,3 Biotech.6(2016)118-126.
[18]P.Dauthal,M.Mukhopadhyay,AuPd bimetallic nanoparticles:Single step biofabrication,structural characterization and catalytic activity,J.Ind.Eng.Chem.35(2016)45-53.
[19]C.K.Sathiya,S.Akilandeswari,Fabrication and characterization of silver nanoparticles usingDelonix elataleaf broth,Spectrochim.Acta A128(2014)337-341.
[20]P.Dauthal,M.Mukhopadhyay,Biofabrication,characterization,and possible bioreduction mechanism of platinum nanoparticles mediated by agro-industrial waste and their catalytic activity,J.Ind.Eng.Chem.22(2015)185-191.
[21]M.Oyaizu,Studies on product of browning reaction prepared from glucose amine,Jpn.J.Nutr.44(1986)307-315.
[22]P.Prieto,M.Pineda,M.Aguilar,Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex:Specific application to the determination of vitamin E,Anal.Biochem.269(2)(1999)337-341.
[23]A.Serpen,E.Capuana,V.Fogliano,V.Gokmen,New procedure to measure the antioxidant activity of insoluble food components,J.Agric.Food Chem.55(19)(2007)7676-7681.
[24]M.C.Foti,Use and abuse of the DPPH·radical,J.Agric.Food Chem.63(40)(2015)8765-8776.
[25]S.S.Shankar,A.Rai,A.Ahmad,M.Sastry,Rapid synthesis of Au,Ag,and bimetallic Au core-Ag shell nanoparticles using neem(Azadirachta indica)leaf broth,J.Colloid Interface Sci.275(2)(2004)496-502.
[26]J.Y.Song,B.S.Kim,Biological synthesis of bimetallic Au/Ag nanoparticles using persimmon(Diopyros kaki)leaf extract,Korean J.Chem.Eng.25(4)(2008)808-811.
[27]M.X.Zhang,R.Cui,J.Y.Zhao,Z.L.Zhang,D.W.Pang,Synthesis of sub-5 nm Au-Ag alloy nanoparticles using bio-reducing agent in aqueous solution,J.Mater.Chem.21(2011)17080-17082.
[28]C.Hendrich,J.Bosbach,F.Stietz,F.Hubenthal,T.Vartanyan,F.Träger,Chemical interface damping of surface plasmon excitation in metal nanoparticles:A study by persistent spectral hole burning,Appl.Phys.B Lasers Opt.76(8)(2003)869-875.
[29]Y.Yu,Q.Zhang,X.Lu,J.Y.Lee,Seed-mediated synthesis of monodisperse concave trisoctahedral gold nanocrystals with controllable sizes,J.Phys.Chem.C114(25)(2010)11119-11126.
[30]S.Tokonami,N.Morita,K.Takasaki,N.Toshima,Novel synthesis,structure,and oxidation catalysis of Ag/Au bimetallic nanoparticles,J.Phys.Chem.C114(23)(2010)10336-10341.
[31]G.Zhang,M.Du,Q.Li,X.Li,J.Huang,X.Jiang,D.Sun,Green synthesis of Au-Ag alloy nanoparticles usingCacumen platycladiextract,RSC Adv.3(2013)1878-1884.
[32]Z.Y.Li,J.P.Wilcoxon,F.Yin,Y.Chen,R.E.Palmer,R.L.Johnston,Structures and optical properties of 4-5 nm bimetallic Ag-Au nanoparticles,Faraday Discuss.138(2008)363-373.
[33]B.Xia,F.He,L.Li,Preparation of bimetallic nanoparticles using a facile green synthesis method and their application,Langmuir29(15)(2013)4901-4907.
[34]B.Zheng,T.Kong,X.Jing,T.Odoom-Wubah,X.Li,D.Sun,F.Lu,Y.Zheng,J.Huang,Q.Li,Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism,J.Colloid Interface Sci.396(2013)138-145.
[35]M.S.Shore,J.Wang,A.C.Johnston-Peck,A.L.Oldenburg,J.B.Tracy,Synthesis of Au(core)/Ag(shell)nanoparticles and their conversion to AuAg alloy nanoparticles,Small7(2)(2011)230-234.
[36]T.Serizawa,Y.Hirai,M.Aizawa,Novel synthetic route to peptide-capped gold nanoparticles,Langmuir25(20)(2009)12229-12234.
[37]Y.S.Velioglu,G.Mazza,L.Gao,B.D.Oomah,Antioxidant activity and total phenolics in selected fruits,vegetables,and grain products,J.Agric.Food Chem.46(10)(1998)4113-4117.
[38]G.H.Naik,K.I.Priyadarsini,J.G.Satav,M.M.Banavalikar,D.P.Sohoni,M.K.Biyani,H.Mohan,Comparative antioxidant activity of individual herbal components used in ayurvedic medicine,Phytochemistry63(1)(2003)97-104.
[39]L.P.Leong,G.Shui,An investigation of antioxidant capacity of fruits in Singapore markets,Food Chem.76(1)(2002)69-75.
[40]D.Das,B.C.Nath,P.Phukon,A.Kalita,S.K.Dolui,Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity,Colloids Surf.B111(2013)556-560.
[41]K.L.Niraimathi,V.Sudha,R.Lavanya,P.Brindha,Biosynthesis of silver nanoparticles usingAlternanthera sessilis(Linn.)extract and their antimicrobial,antioxidant activities,Colloids Surf.B102(2013)288-291.
[42]T.Shanmugasundarama,M.Radhakrishnan,V.Gopikrishnana,R.Pazhanimurugana,R.Balagurunathana,A study of the bactericidal,anti-biofouling,cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles,Colloids Surf.B111(2013)680-687.
[43]J.Seralathan,P.Stevenson,S.Subramaniam,R.Raghavan,B.Pemaiah,A.Sivasubramanian,A.Veerappan,Spectroscopy investigation on chemo-catalytic,free radical scavenging and bactericidal properties of biogenic silver nanoparticles synthesized usingSalicornia brachiataaqueous extract,Spectrochim.Acta A118(2014)349-355.
[44]J.P.Saikia,S.Paul,B.K.Konwar,S.K.Samdarshi,Nickel oxide nanoparticles:A novel antioxidant,Colloids Surf.B78(1)(2010)146-148.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
- An innovative trigeneration system using biogas as renewable energy
