A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
Chidchon Sararuk ,Dan Yang ,Guoliang Zhang ,Chunshan Li,*,Suojiang Zhang
1 Key Laboratory of Ionic Liquid Clean Process,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2 University of Chinese Academy of Sciences,Beijing 100190,China
1.Introduction
PCs/γ-Al2O3is a very popular catalyst applied in many reaction such as aldol condensation[1,2],and transesterification[3]due to the fact that aluminium oxide has gained significant importance in catalysis owing to their surface area which usually uses as a commercial supporter.The addition of alkali and alkaline earth such as Li,Na,K,and Cs has obtained basicity property[4].Phosphorus was investigated as a resource for increasing acidic property and reducing the amount of coking of catalyst in many reactions[5,6].Particularly,the combination of these components is well known as a bifunctional catalyst.Furthermore,zirconium represented as active material after its addition during synthesis can lead to the formation of Lewis and Brönsted acid sites along with basic sites[7].Other than acidic and basic properties,its crystalline phase and physical properties are key factors for catalytic properties;synthesis controlling is very important.
So far,many techniques including co-precipitation[6,7],sol-gel[8],hydrothermal[9],and impregnation[3]have been developed to synthesize catalysts.Previously,considerable attention was focuse d on the preparation method through co-precipitation and sol-gel method.Meanwhile,there was no study on the impregnation method.Impregnation has several advantages which includes its relative simplicity,rapidity,and capability for depositing the precursor at high metal loadings.In addition,it's an easier way and suitable for large scale preparation.Many attempts to prepare catalysts have been reported,while no study on experiment condition to obtain this catalyst was found.Impregnation method consists of three main steps:impregnating step,drying step,and calcination step.The calcination step is the important step.The major factor in this step is calcination temperature which effected to chemical transformation,crystalline structure and catalyst properties[10,11].
The aim of this work was a simple synthesis of zirconium modified PCs/γ-Al2O3with fixed P and Cs content in various calcination temperatures and Zr content through the step incipient-wetness impregnation method.TG-DTA,XRD,FT-IR,and NH3-and CO2-TPD were used for the thermal behavior study,phase identification of a crystalline material,functional group,and acidity-basicity on the sample surface,respectively.
2.Experimental
2.1.Materials
The γ-Al2O3spheres were obtained from a commercial company with an average particle diameter of 1 mm.Cesium carbonate[Cs2CO3;99.0%],diammonium hydrogen phosphate[(NH4)2HPO4,99.0%],and zirconium nitrate pentahydrate[Zr(NO3)4·5H2O,99%]which were A.R.grade were utilized in this work.
2.2.Sample preparation
A series of ZrPCs/γ-Al2O3were prepared by steps of incipient-wetness impregnation method of γ-Al2O3with aqueous solution of cesium carbonate,diammonium hydrogen phosphate and zirconium nitrate pentahydrate.The loading amounts of Cs,P,and Zr were reported as nominal mass percentages.The Cs and P contents were fixed at 10 wt%and 3 wt%,respectively.The loading amount of Zr was added at different contents(0.5 wt%,1 wt%,3 wt%,5 wt%,10 wt%,and 20 wt%).Each step,the impregnating supports were incubated at room temperature for 6 h and then dried in an oven at 100 °C for 12 h.The ZrPCs/γ-Al2O3samples were calcined in the air flow for 5 h at a constant heating rate of 5 °C·min-1with different calcination temperatures.
2.3.Characterization methods
Thermogravimetric analysis(TG-DTA)was performed on a SII TG/DTA6300 analyzer(Japan).The samples were heated from room temperature up to 1000 °C with a heating rate of 10 °C·min-1 under air flow 200 ml·min-1.The XRD patterns of the samples were recorded by a PANalytical diffractometer operated at an accelerating voltage of 40 kV and an emission of 40 mA with Cu Kα radiation(λ =0.15418 nm).Angle(2θ)was measured in steps of 10°min-1between 5°and 90°.FT-IR spectra were performed with Nicolet 380 spectrometer.Total acidity and basicity of the samples were measured with an Autochem II 2920 apparatus Micrometrics(USA).Samples were placed in a quartz tube pretreated by flowing helium at 120°C for 30 min.For acidity measurement,a mixture of 10%NH3in helium flowed through the sample at 50°C for 90 min,while basicity was used as a mixture of 10%CO2in helium.Then,helium was passed through a sample at 50°C for 30 min until the baseline had been stabilized.The NH3and CO2desorption signal was registered by a thermal conductivity detector(TCD)under helium flowing at heating rate 10 °C·min-1up to 700 °C.
3.Results and Discussion
3.1.Characterization of the initial reagents
TG-DTA spectra of Cs2CO3,and Zr(NO3)4·5H2O are depicted in Fig.1.Based on TG-DTA of Cs2CO3curve in Fig.1(A),the thermos analytical analysis was observed at 2 endothermic peaks.First,the small peak showed at 191°C due to water desorption and decomposition of impurities with slightly mass loss[12].The second peak was presented at787°C.Meanwhile,the mass loss was started with a distinct rate,due to the melting temperature of Cs2CO3at 610°C which was attributed to the decomposition of Cs2CO3[13]as shown in Eq.(1).

Fig.1(B)presented the TG/DTA profile of Zr(NO3)4·5H2O.The metal nitrate hydrate,Zr(NO3)4·5H2O showed 2 mainly successive endothermic peaks,and 1 exothermic peak.Thermal decomposition of this compound started above 100°C.It was showed a small endothermic peak at 139 °C,and a slightly mass loss~5.3%which due to water desorption.The second peak which was presented at 193°C due to the dehydration of Zr(NO3)4·5H2O lose five molecules of water,which leads to the formation of Zr(NO3)4and then the anhydrous nitrate was decomposed below 400°C,and it might occur through an intermediate nitrite or oxide species with a variable valence for metal ions as shown in Eqs.(2)and(3),respectively[14].Furthermore,the exothermic peak was observed at 470°C,indicating that the transition form of ZrO2amorphous to the crystalline phase is accompanied[15,16].
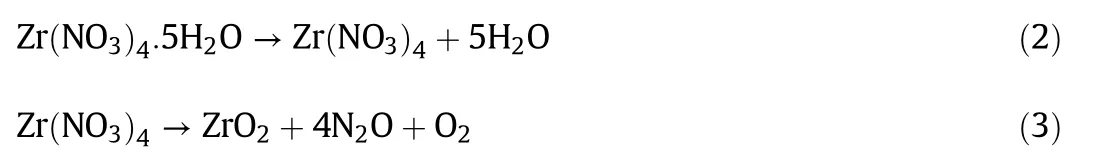
3.2.Crystal structure of ZrPCs/γ-Al2O3
The XRD pattern of 20%ZrPCs/γ-Al2O3samples dried at 100 °C and calcined with different temperatures(450 °C,550 °C,650 °C and 750°C)for 5 h in air flow is shown in Fig.2(A).The small diffraction pattern peaks at 2θ =19.3°,37.5°,39.3°,45.7°,60.5°and 66.6°were typical of the Al2O3cubic phase(XRD PDF#50-0741).Further,there was clearly showed small peaks at 2θ =28.2°and 37.6°,indicated to cesium oxide(CsO2)phase(XRD PGF#26-0395)which occurred basicity property[13,20].There was a small diffraction peak at 2θ=43.1°on the sample calcined at 550°C,indicating that the ZrO2started generating on the sample surface which conformed with exothermic peak in TG/DTA profile in Fig.1(B).Further increase in calcination temperature to 650°C obviously presented sharp peaks of the ZrO2phase(XRD PDF#50-1089)at broadened peaks 2θ=30.3°,35.2°,43.1°.50.4°,and 60.2°which accorded to tetragonal phase zirconia(t-ZrO2)in the literature[18,19].The intensity of peaks slightly increased,due to the increasing of crystalline structure.It may be considered that amorphous and semicrystalline of zirconia appeared at temperature below 650°C[15,19].
Furthermore,the intensity of the peak increased with the increasing of calcination temperature,due to the Al0.1Zr0.9O1.95cubic phase(XRD PDF#53-0559)which exhibited at broadened peaks 2θ =30.3°,35.1°,50.5°,60°,and 63°.Owing to the alumina support compounded with zirconia intermediate compound which showed in Eq.(4).In addition,the intensity of ZrO2and Al0.1Zr0.9O1.95peaks increased with increasing Zr content as illustrated in Fig.2(B).The diffraction pattern peaks of Al2O3slightly shifted with the increasing of the Zr content,indicating that the distortion of Al2O3structure was emerged by Zr atoms.
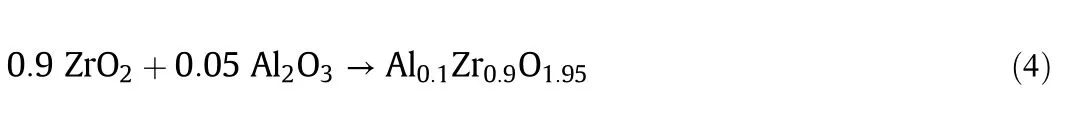

Fig.1.TG/DTA curves of pure(A)CsCO3 and(B)Zr(NO3)4·5H2O compound.

Fig.2.XRD patterns of(A)the 20%ZrPCs/γ-Al2O3 samples calcined with different temperatures;(B)the xZrPCs/γ-Al2O3 samples calcined at 750 °C with different Zr contents.

Fig.3.FT-IR spectra of the 20%ZrPCs/γ-Al2O3 samples calcined with different temperatures.
Fig.3 shows the FT-IR spectra of the ZrPCs/γ-Al2O3samples.Dried sample(100°C)presented three more peaks at 2420,2046,and 1552 cm-1as compared to the calcined samples.It may due to the interaction of metal ion with carbonate ions of initial reagent[21,22].These three peaks disappeared after the samples were calcined at a higher temperature,owing to the decomposition of cesium carbonate reagent accorded with the distinctly mass loss of TG curve in Fig.1(A).A broad absorption was observed in the range of 4000-3000 cm-1which might be due to the OH-stretching vibration of the metal,phosphorus,and alumina support[6,17].The spectrum which was generally dominated by an intense symmetric peak at 3460 cm-1,was attributed with the hydroxyl group on alumina and zirconia.All the samples exhibited bending vibration band around 1640 cm-1due to presence of H-O-H which indicated the absorbed water molecule[6,23].The peak at approximately 1385 cm-1corresponded to the stretching vibration of N-O in the nitrate,indicating its incomplete decomposition during 450°C calcination process[24].Whereas,the band at 1084,823,and 595 cm-1indicated the metal and oxygen bond of Al-O,Cs-O,and Zr-O,respectively[3,24,25].
3.3.Acidity-basicity properties of ZrPCs/γ-Al2O3
The acidic and basic properties of the ZrPCs/γ-Al2O3samples were measured by NH3-and CO2-TPD.The total acidity and basicity were obtained from the area under the peaks in the TPD profiles as shown in Fig.4(A)and 4(B),respectively.NH3-and CO2-TPD profiles demonstrated all sample desorption peaks at low temperature(T<200°C).Thus,all samples presented weak acidic and basic sites.
Table 1 summarized the total acidity and basicity of ZrPCs/γ-Al2O3samples with different Zr contents.It can be seen that the total acidity and basicity increased as the content of Zr increased which due to the formation of ZrO2and exhibited acid sites on the surface[25].Furthermore,the total basicity clearly presents three times higher than the total acidity.
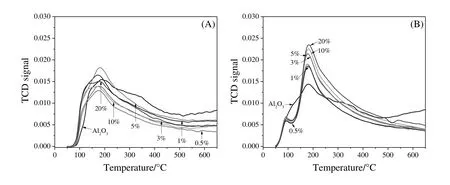
Fig.4.TPD profile of the xZrPCs/γ-Al2O3 samples calcined at 550 °C with different Zr contents(A)NH3 and(B)CO2.

Table 1 Total acidity and basicity of the ZrPCs/γ-Al2O3 with different Zr contents calcined at550 °C
4.Conclusions
The structure and chemical transformation of ZrPCs/γ-Al2O3is significantly influenced by calcination temperature.The alumina support and amorphous zirconia were exhibited at low temperatures.Moreover,the ZrO2was formed during heating process from 450 °C to 550 °C due to the decomposition and dehydration reactions and disappear.Then the ZrO2crystal phases were clearly observed when the temperature raised up to 650°C.Due to the interaction of the zirconia intermediate compound with γ-Al2O3support,the new crystal phase of Al0.1Zr0.9O1.95was obtained.Influence of zirconium adding content effected on the acidic and basic properties.The acidity and basicity surface increased with the addition of amount of zirconium.
Acknowledgements
One of the authors,Chidchon Sararuk,acknowledges the Chinese Academy of Sciences(CAS)and The World Academy of Sciences(TWAS)for awarding the CAS-TWAS President's fellowship to carry out the research.
[1]G.Zhang,H.Zhang,D.Yang,C.LI,Z.Peng,S.Zhang,Catalysts,kinetics and process optimization for the synthesis of methyl acrylate over Cs-P/γ-Al2O3,Catal.Sci.Technol.6(2016)6417-6430.
[2]C.Sararuk,D.Yang,G.Zhang,C.Li,S.Zhang,One-step aldol condensation of ethyl acetate with formaldehyde over Ce and P modified cesium supported alumina catalyst,J.Ind.Eng.Chem.46(2017)342-349.
[3]H.Amani,Z.Ahmad,B.H.Hameed,Highly active alumina-supported Cs-Zr mixed oxide catalysts for low-temperature transesterification of waste cooking oil,Appl.Catal.A Gen.487(2014)16-25.
[4]B.Li,R.Yan,L.Wang,Y.Diao,Z.Li,S.Zhang,Synthesis of methyl methacrylate by aldol condensation of methyl propionate with formaldehyde over acid-base bifunctional catalysts,Catal.Lett.143(2013)829-838.
[5]R.Liu,T.Wang,C.Liu,Y.Jin,Highly selective and stable CsPW/Nb2O5catalysts for dehydration of glycerol to acrolein,Chin.J.Catal.34(2013)2174-2182.
[6]A.Hamza,N.Nagaraju,Amorphous metal-aluminophosphate catalysts for aldol condensation of n-heptanal and benzaldehyde to jasminaldehyde,Chin.J.Catal.36(2015)209-215.
[7]Y.Wei,Y.Li,Y.Tan,J.Zhou,Z.Wu,Y.Liu,A facile route for one-pot synthesis of short-channeled bimetallic Zr-Al-SBA-15,Mater.Lett.141(2015)145-148.
[8]L.E.Davies,N.A.Bonini,S.Locatelli,E.E.Gonzo,Characterization and catalytic activity of zirconium dioxide prepared by sol-gel,Lat.Am.Appl.Res.35(2005)23-28.
[9]R.Si,Y.-W.Zhang,C.-X.Xiao,S.-J.Li,B.-X.Lin,Y.Kou,C.-H.Yan,Non-template hydrothermal route derived mesoporous Ce0.2Zr0.8O2nanosized powders with blue-shifted UV absorption and high CO conversion activity,Phys.Chem.Chem.Phys.6(2004)1056-1063.
[10]G.Zhang,Z.Peng,C.Li,A study of thermal behavior of cesium phosphate,J.Therm.Anal.Calorim.124(2016)1063-1070.
[11]M.Laspéras,H.Cambon,D.Brunel,I.Rodriguez,P.Geneste,Cesium oxide encapsulation in faujasite zeolites effect of framework composition on the nature and basicity of intrazeolitic species,Microporous Mater.7(1996)61-72.
[12]S.Yuvaraj,L.Fan-Yuan,C.Tsong-Huei,Y.Chuin-Tih,Thermal decomposition of metal nitrates in air and hydrogen environments,J.Phys.Chem.B107(2003)1044-1047.
[13]N.Gorodylova,P.Šulcová,M.Bosacka,E.Filipek,DTA-TG and XRD study on the reaction between ZrOCl2·8H2O and(NH4)2HPO4for synthesis of ZrP2O7,J.Therm.Anal.Calorim.118(2014)1095-1100.
[14]A.Keshavaraja,N.E.Jacob,A.V.Ramaswamy,Thermal decomposition of coprecipitated oxide hydrates of zirconium and manganese,Thermochim.Acta254(1995)267-275.
[15]M.Lasperas,I.Rodriguez,D.Brunel,H.Cambon,P.Geneste,Effect of the framework composition on the nature and the basicity of intrazeolitic cesium oxides.Correlation activity/basicity,Stud.Surf.Sci.Catal.97(1995)319-326.
[16]S.Damyanova,P.Grange,B.Delmon,Surface characterization of zirconia-coated alumina and silica carriers,J.Catal.168(1997)421-430.
[17]G.Li,W.Li,M.Zhang,K.Tao,Characterization and catalytic application of homogeneous nano-composite oxides ZrO2-Al2O3,Catal.Today93(2004)595-601.
[18]T.Klimova,M.L.Rojas,P.Castillo,R.Cuevas,J.Ramírez,Characterization of Al2O3-ZrO2mixed oxide catalytic supports prepared by the sol-gel method,Microporous Mesoporous Mater.20(1998)293-306.
[19]V.S.De Portilla,The nature of hydrogen bonds and water in legrandite by IR spectroscopy,Am.Mineral.61(1976)95-99.
[20]M.Dixit,M.Mishra,P.A.Joshi,D.O.Shah,Physico-chemical and catalytic properties of Mg-Al hydrotalcite and Mg-Al mixed oxide supported copper catalysts,J.Ind.Eng.Chem.19(2013)458-468.
[21]H.Song,Y.Sun,X.Jia,Hydrothermal synthesis of iron phosphate microspheres constructed by mesoporous polyhedral nanocrystals,Mater.Charact.107(2015)182-188.
[22]J.Yan,C.Zhang,C.Ning,Y.Tang,Y.Zhang,L.Chen,S.Gao,Z.Wang,W.Zhang,Vapor phase condensation of methyl acetate with formaldehyde to preparing methyl acrylate over cesium supported SBA-15 catalyst,J.Ind.Eng.Chem.25(2015)344-351.
[23]D.Sarkar,D.Mohapatra,S.Ray,S.Bhattacharyya,S.Adak,N.Mitra,Synthesis and characterization of sol-gel derived ZrO2doped Al2O3nanopowder,Ceram.Int.33(2007)1275-1282.
[24]A.R.Hajipour,H.Karimi,Synthesis and characterization of hexagonal zirconium phosphate nanoparticles,Mater.Lett.116(2014)356-358.
[25]Y.Li,J.Feng,D.Li,Preparation and characterization of spherical mesoporous ZrO2-Al2O3composites with high thermal stability,Sci.China Chem.54(2011)1032-1038.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
- An innovative trigeneration system using biogas as renewable energy
