Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
Min Xia *,Chunsong Ye Kewu Pi,Defu Liu ,Andrea R.Gerson
1 School of Power and Mechanical Engineering,Wuhan University,Wuhan 430072,Hubei,China
2 School of Resource and Environmental Engineering,Hubei University of Technology,Wuhan 430068,Hubei,China
3 Blue Minerals Consultancy,Wattle Grove,Tasmania 7109,Australia
1.Introduction
As a result of industrialization,significant discharge of heavy metals into the environment has taken place[1]often resulting in accumulation in water and soil,followed by causing serious pollution and deleterious effects on human health[2].
Chromium,one of the most toxic heavy metals,can exist in trivalent(Cr(III))and hexavalent(Cr(VI))oxidation states.Its pollution in waterways is mainly derived from electroplating,tanning,mining and fertilizer manufacture[3].Chromium toxicity and behavior in aqueous systems are oxidation state dependent.The toxicity of Cr(VI)has been claimed to be up to 300 times that of Cr(III)[4].However,it has also been reported that excessive intake of Cr(III)depresses the immune system and may lead to necrosisviainteraction with micro filaments,mitochondria,lysosome and nucleolus[5].Contact with chromium Cr(III)for a long time would incur skin allergy even worse cancer.Therefore,minimization of Cr(III)levels in aqueous from the source discharge is essential for elimination of Cr(III)pollution.The maximum allowable concentration of chromium in drinking water that is regarded as harmless by the US Environmental Protection Agency is 0.1 mg·L-1,although the World Health Organization defines the critical concentration threshold as 0.01 mg·L-1[2].
From environmental protection point of view,an effective strategy for heavy metals removal should be imminently implemented.To date,various physico-chemical methods have been developed to remove aqueous heavy metals including precipitation,membrane,ion exchange,electrolysis and oxidation-reduction approaches[6].However,all these methods either involve high operating costs or may produce large volumes of solid wastes[7].For these reasons,adsorption approach using waste materials would be a promising technology in heavy metals removal in terms of the potential of an effective,simple and cheap alternative.Pérez Marínet al.[2]investigated the orange waste to adsorb Cr(III)from aqueous solution.The result showed the maximun Cr(III)adsorption derived from Langmuir isothermal model could reach 1.44 mmol·g-1.Meanwhile,a study on heavy metal(Cu(II))removal using rice husk was reported by Elhafezet al.[8]with the attainment efficiency for 25.6 mg·g-1,a considerably higher than that of 11 mg·g-1for commercial activated carbon cloth[9].In addition,other industrial wastes including zeolite[10],eggshell[11],bentonite[12],olive stone[13]and coir pitch[14]have already been demonstrated to have the capacity to retain heavy metals.
Fly ash(FA)is the major industrial waste from coal combustion.Due to the increasing demand for energy its annual output is increasing rapidly.A growing number of utilizations for this waste have been found such as in cement manufacture or as a soil amendment.Additionally,the high aluminum content of fly ash can be an important resource[15].However,the utilization rate of fly ash is far less than the output rate[16].Reportedly,in China the utilization rate of fly ash is approximately 70%with the output approximately 580 million tons by 2015[17].To the extent that most of the unutilized fly ash(approximately 174 million tons)is inevitably dumped into the environment,leading to secondary environment pollution.Consequently,the development of additional approaches for the disposal of fly ash has attracted interest[18].Previous studies showed fly ash could be used as high-efficiency adsorbent for pollutants removal in both gaseous and aqueous applications[17].Due to high specific surface area and porosity[19,20],FA therefore means potentially as a material for heavy metal uptake and in practice has already been successfully applied in Cr(VI),Pb(II),Cu(II),Ni(II)and Cd(II)[21-23]removal.Beyond that,to the author's knowledge,there has been little study of the application of fly ash for removal of aqueous Cr(III),especially modified fly ash for Cr(III)removal enhancement is still missing.
Previously,Magnesium was introduced onto the granular activated carbon surface with the form of MgO and was used to remove Zn(II)and Cd(II).The results revealed the high removal efficiency for these metal ions[24].However,the preparation process needed to calcine at high temperature from hydrous magnesium oxide,resulting in lots of energy loss and making it environmental unfriendly.Therefore,it is necessary to find a feasible strategy[25].Hydrous magnesium oxide has also been reported to adsorb heavy metals from wastewater[26].Therefore, fly ash can be modified by hydrous magnesium oxide to adsorb Cr(III),which can use the advantages of both fly ash and magnesium hydroxide fully.In view of this,a new material,hydrous magnesium oxide coated fly ash(MFA)was synthesized and potentially used for the study of the uptake of aqueous Cr(III).This composite was characterized using Fourier-transform infrared(FTIR)spectroscopy,X-ray diffraction(XRD),scanning electron microscopy(SEM)and energy dispersive X-ray spectroscopy(EDS).A response surface methodology(RSM)was carried out to reveal and comprehend the effect of pH,MFA dosage and initial Cr(III)concentration on the removal process as well as to assess the feasibility of this new adsorbent for Cr(III)removal from effluent.Specially,the possible mechanism for Cr(III)adsorption onto MFA was discussed.
2.Experimental
2.1.Materials and chemicals
The fly ash used in this study was obtained from a power plant located in Hubei,China,its chemical compositions are presented in Table 1.A simulated solution containing 1.0 g·L-1of Cr(III)was prepared by dissolving 5.124 g of CrCl3·6H2O in 1 L of distilled water.Distilled water was used throughout all experiments.Working solutions of different initial Cr(III)concentrations were obtained by serial dilution of the simulated solution.All commercial reagents,purchased from Sinopharm Chemical Reagent Co.,Ltd.,Shanghai,China,were of analytical grade with the purity between 96%and 99%.
2.2.Synthesis of hydrous magnesium oxide-coated fly ash composite
Twenty grams of 96-μm fly ash was first activated using 200 ml of 2 mol·L-1HCl solution at 30 °C for 24 h.Previous studies indicated acid etching(HCl,H2SO4or the mixture)for fly ash or similar material(kaolinite)had been positive effect on fractionation of amorphous materials and dredge channels to increase specific surface area along with the amount of active sites,therefore,giving rise to higher adsorption capability[27-30].Then,the suspension was vacuum filtered through a 0.45-μm membrane,washed with distilled water and dried in an oven at80°C.Hydrous magnesium oxide coated fly ash composite was made by the addition of20 g of the HCl activated fly ash into 200 ml of 0.49 mol·L-1magnesium chloride solution at 30 °C.After vigorous stirring for 24 h,250-ml ammonia solution(25 wt%)was added dropwise.With continuous reaction for 24 h at ambient temperature,the hydrous magnesium oxide coated fly ash(MFA)was centrifuged,washed with distilled water several times and then dried at 80°C for later use.
2.3.Characterization and analysis technique
Surface morphology was imaged using scanning electron microscopy(SEM)(FEI,Quanta 200)with energy dispersive X-ray spectroscopy(EDS)for elemental quantification.X-ray diffraction(XRD)(PANalytical,X'Pert Pro)was carried out to identify the crystalline phases present.Functional groups were identified using Fourier-transform infrared(FTIR)(Nicolet,5700)spectroscopy using the compressed KBr pellet method.The pH of the point of zero charge(pHpzc)of the MFA was determined according to the pH drift method[31](Thermo Orion,230A+),and is defined as the point where the curve pHfinalversuspHinitialcrosses the line pHfinal=pHinitial.Cr(III)concentration was measured using an atomic absorption spectroscope(PinAAcle,900F)equipped with Cr hollow-cathode lamps.The specific surface area of fly ash was determined using methylene blue adsorption test method previously described[32].
2.4.Response surface methodology(RSM)
Response surface methodology(RSM)is generally carried out to investigate the effect of several independent variables.It is often used in process design,improvement and optimization.This approach is practical as it employs experimental data and thus contains the interactive effects of variables on overall process performance[33]and systematically appraise corresponding significance levels.As a result of these advantages,RSM has been recently and consistently applied to optimize process parameters in many water treatment fields,such as electro coagulation[34],traditional coagulation- flocculation[35],membrane[36],advanced oxidation[37],also including adsorption process[38].In previous studies,Iqbalet al.[38]investigated the shoes waste as adsorbents to treat cadmium Cd(II)from simulated aqueous solution using RSM for optimization of operating variables,the results indicated it could predict the response with good accuracy and reliability.Similarly,Srivastavaet al.[39]research on Co(II)adsorption by means of NiO nanoparticles also suggested the methodological feasibility of RSM for statistical model construction in adsorption process.With a comprehensive consideration of Cr(III)removal process,the Box-Behnken design(BBD)was employed for RSM modeling using three crucial variables,namely MFA dosage,initial pH and initial Cr(III)concentration.The detailed descriptions on variable factors with the coded and actual values are presented in Table 2.For this design,a total of seventeen experiments consisting of thirteen different combined coded levels and four repeated central coded levels were needed with a randomized experimental rank in order to minimize the influence of uncontrolled factors.Specially,the Design Expert8.0.6 software was performed for either BBD matrix/experimental data analysis or mathematics modeling.Additionally,blank tests using the same Cr(III)containing solution without any addition of adsorbent were conducted to evaluate any adsorption of Cr(III)on the glass surface during incubation.Eachexperiment was repeated in at least triplicate at 25°C to ensure reproducibility and reliability.
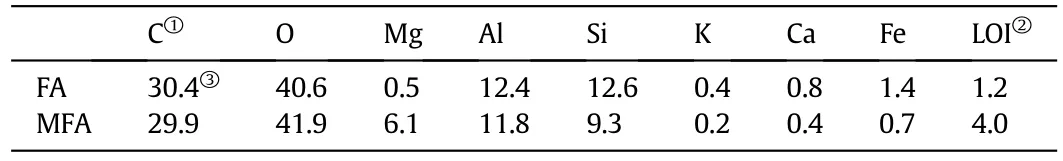
Table 1 Elemental compositions(wt%)of FA and MFA
To predict the optimum condition for Cr(III)removal and to express the interaction between dependent and independent variables,the mathematical quadratic model shown in Eq.(1)is obtained using the Design Expert software:
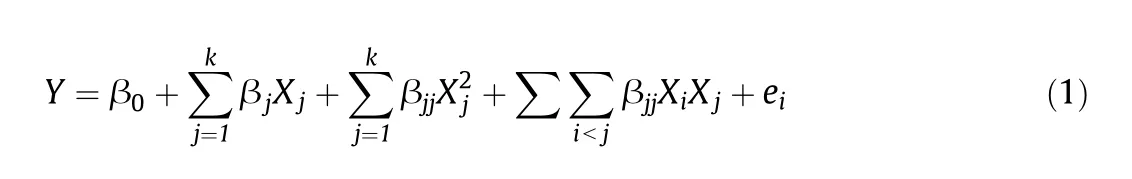
Yis the estimated response;β0represents the constant;βi,βjjand β0refer to the coefficients of linear,quadratic and interactive items,respectively,andXis the dimensionless coded value of the independent variable.Analysis of variance(ANOVA)is applied to evaluate the applicability of this response surface quadratic model and the significance of each item in the equation.
Metal removal efficiency,R(%)for different conditions is calculated using the general definition:

C0andCeare the initial and equilibrium concentrations of Cr(III)(mg·L-1),respectively.
3.Results and Discussion
3.1.Characterization
Table 1 summarizes the evolution of semiquantitative elemental compositions on FA and MFA as determined by energy dispersive X-ray spectroscopy(EDS).As suggested by these data,the FA is mainly composed of silica,aluminum,iron oxide and is classified as class F according to standard specifications of ASTM C618.Compared to FA,MFA shows more content of surface metal oxide,which is attributed to the successful introduction of hydrous magnesium oxide,where percentage composition of element magnesium increases from its initial value 0.5%up to 6.1%.Higher metal oxide would prefer to improve the adsorption capacity due to have a responsibility to the generation of variable charges[40,41].It was reported by Linet al.[40]the maximum amount of methylene blue(MB)uptake could be efficiently enhanced(two times higher)using the FA coated with hydrous metal oxides(hydrous zirconia and hydrous iron oxide).Nevertheless,other research suggests silicon oxide could also be involvement in the metal ions(Cd(II),Pb(II),Zn(II))adsorption process[42].On the other hand,1.2%of residual carbon content is found in FA through the measure of loss on ignition(LOI),which is indicative of the impact of residual carbon for Cr(III)retention should be very tiny and consequently excluded[43,44].Besides,it is worth noting LOI of MFA(4.0%)is abnormally increased.With respect to the variation of which in comparison to FA,it can be reasonably explained by the decomposition of hydrous magnesium oxide into magnesium oxide at strong heat(1000°C in this study),which the start of mass loss commences at 270°C[45].
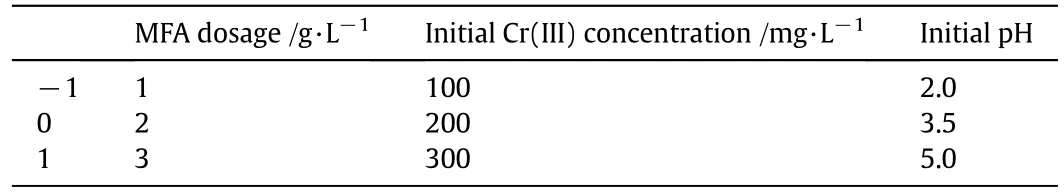
Table 2 The levels and ranges of independent variables for RSM experiments
The XRD patterns of MFA and FA are shown in Fig.1(a).The major diffraction peaks for FA result from mullite(Al6O13Si2,JCPDS Card No.02-0431),quartz(SiO2,JCPDS Card No.02-0471)and amorphous phase[40](diffuse scattering halo 2θ between 20°-30°,not shown in Fig.1(a)due to deduct the background using Jade 5.0 software).After activation by acid and modification by magnesium hydrate,further diffraction peaks for brucite(Mg(OH)2,JPCDS Card No.44-1482[46]at 2θ of 18.17°(001),36.64°(101),48.90°(102),56.32°(110),59.80°(111)and 65.41°(103)are observed in the diffraction pattern from MFA,while matters of the bulge peak for amorphous phase in FA is mostly dissolved accordingly(the weakened of diffuse scattering halo)due to acid etching.This result is consistent with the previous studies reported by Liet al.[28]and Zhanget al.[30],for using FA as adsorbent and Alextraction materials,respectively.

Fig.1.(a)XRD patterns of FA and MFA and(b)FTIR spectra of FA,MFA and MFA after treatment of Cr(III)-containing solution.
To determine which functional groups are responsible for metal uptake FTIR was carried out.Fig.1(b)depicts the FTIR spectra,in the range of 400-4000 cm-1,for FA and MFA prior to and after Cr(III)uptake.The FTIR spectra of FA exhibits adsorption peaks at 3420 cm-1and 1630 cm-1corresponding to the stretching and bending vibrations of--OH from adsorbed water molecular.The strong absorption peak at 1093 cm-1can be assignable to the antisymmetric stretching variation of Si--O--Al network with the band at 463 cm-1arose from the bending vibration of O--Si--O in SiO4tetrahedron.Similar results have been narrated in the published literatures[28,47].The peaks centered at 558 cm-1and 830 cm-1are assigned to the stretching vibration of Al--O in[AlO6]and[AlO4],respectively.The weak absorption peak at 916 cm-1is indicative of the Al--OH bending vibration in[AlO6][23].
In the FTIR spectrum of MFA further absorption peaks are observed.The sharp strong peak at3693 cm-1is due to the Mg--OH stretching vibration with the bending vibrations at 1440 cm-1from Mg--O and 1550 cm-1from--OH,both in Mg(OH)2.Upon uptake of Cr(III)some changes are observed.The absorption peaks of 1440 cm-1and 1550 cm-1shift to 1384 cm-1and 1478 cm-1,respectively,this is mainly resulted from the energy bond in the functional groups(i.e.,--OH)presenting on the surface of MFA[48].In addition,the peak intensity at 3693 cm-1drastically decreases.It appears that the--OH group of Mg(OH)2is the major functional group involved in Cr(III)adsorption onto MFA.
The morphology differences between FA to MFA are illustrated in Fig.2.It is clearly observed FA is spheroidal with a relatively smooth surface[Fig.2(a)].In contrast,MFA[Fig.2(b)]has a rough and uneven surface composing of small exfoliated flakes,suggesting the successful loading of hydrous magnesium oxide onto the surface of FA.In addition,a few filiform-like structures are quite apparent.Similar structures have been observed by[49]on adsorption of fluorinion onto FA modified by magnesium chloride,which is defined as the fusion of magnesium and FA(magnesium-aluminum-silicate(MgAl2Si4O12)and pyrope(Mg3Al2(SiO4)3)).
3.2.RSM analysis
3.2.1.Analysis of variance(ANOVA)
The final experimental design matrix and response results for Cr(III)removal efficiency under different experimental conditions are listed in Table 3.

Table 3 BBD design matrix for three variables with response values of Cr(III)removal efficiency
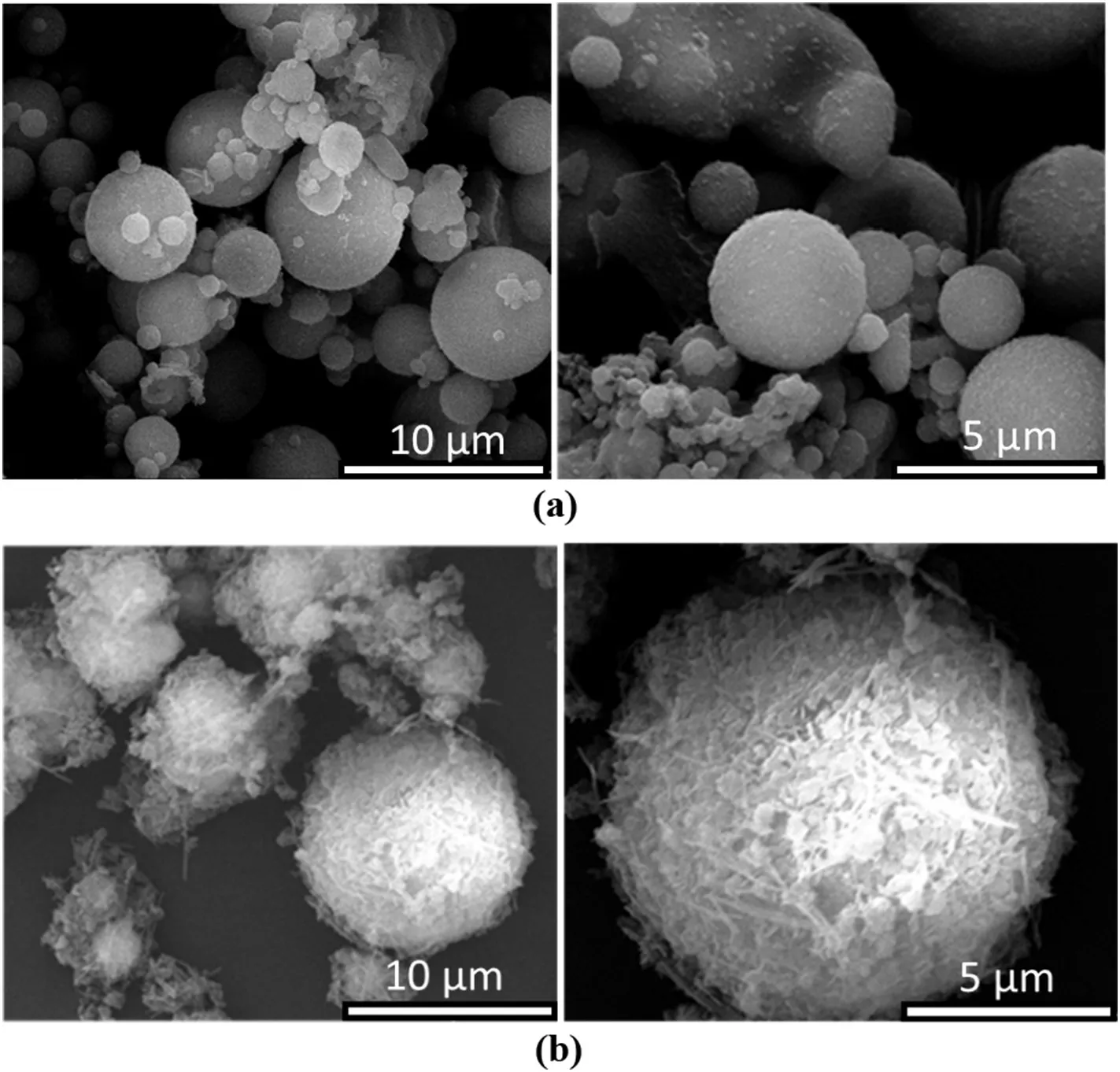
Fig.2.SEM images of(a)FA and(b)MFA.
The mathematical quadratic model in terms of coded factors obtained from RSM modeling is:

whereAis the MFA dosage(g·L-1);Bis the concentration of Cr(III)(mg·L-1)andCis the pH.ANOVA of the regression parameters derived to model Cr(III)adsorption is shown in Table 4.A highF-value of 38 together with a low probability value(Prob>F<0.001)demonstrate that the response surface quadratic model is highly significant.The significant model terms are those with the value(Prob>F)less than 0.05[50].As seen from Table 4,A,B,C,AB,AC,A2andC2are the significant terms that affect Cr(III)adsorption.Since insignificant model terms(i.e.,BC,B2)have little influence on the contribution to Cr(III)removal,these are not important to the response surface model construction.

Table 4 Analysis of variance(ANOVA)of quadratic model for the significant terms and relevant summary statistics,A-MFA dosage(g·L-1);B-initial Cr(III)concentration(mg·L-1);C-pH
In order to completely verify the models' reliability and accuracy,summary statistics of adequate precision,determinationR2and adjustedR2have been evaluated.Adequate precision measures the range of the predicted response relative to its associated error,i.e.a signal to noise ratio,for which a value of greater than 4 is desirable and con firms the applicability of the model for navigation of the design space[51].The adequate precision of 16 obtained indicates an adequate signal to noise ratio.TheR2value of 0.97 obtained implies that 97%of the variability in the response could be explained using this empirical model.However,only taking an individual value ofR2(even though theR2value close to 1)into account would not intuitively evaluate whether the model is a good regression or not,since more amounts of variables are included in model building,higherR2value can be obtained consequentially.In this case,adjustedR2should be synchronously considered.It is shown from Table 4,both high and close values betweenR2and adjustedR2are obviously observed,in turn supporting the good fit of the model to the experimental data[52].Summarily,all these summary statistics indicate the statistical model is validated from the statistical point of view and can perfectly simulate the Cr(III)adsorption performance on MFA.
3.2.2.Effect of variables on Cr(III)adsorption
The three dimensional(3D)response surface plots with their corresponding contour plots for Cr(III)uptake using the quadratic model generated are shown in Fig.3.Fig.3(a)shows the effect of initial pH and MFA dosage on Cr(III)removal efficiency for the initial Cr(III)concentration of 200 mg·L-1.The surfaces of this plot are steep implying the interactive effect is significant,in agreement with ANOVA analysis(Table 4).According to the corresponding contour plot Cr(III)removal increases with pH increase up until pH 3.20 at the MFA dosage of 3.0 g·L-1,above which it almost plateaus.
Fig.3(b)shows the effect of MFA dosage on Cr(III)removal efficiency as a function of initial Cr(III)concentration at pH 3.5.As for Fig.3(a)the 3D plot surface is steep.Above the MFA dosage of 2 g·L-1for the initial Cr(III)concentration of200 mg·L-1,the Cr(III)removal efficiency levels off.The effect of initial concentration of Cr(III)is also reflected in Fig.3(b).The Cr(III)removal efficiency decreases with increase of initial Cr(III)concentration,especially at small MFA amount.The range of Cr(III)removal efficiencies is greater at high dosage of MFA than at low dosage across the range of initial Cr(III)concentrations examined,with values between 0 and 95%at 1.0 g·L-1MFA compared to 95%-100%at 2.5 g·L-1MFA[contour plot Fig.3(b)].
3.3.Optimization
Optimized conditions for Cr(III)removal efficiency were predicted using the Design Expert software within the range of conditions studied.The model predicted Cr(III)removal efficiency of 100%for pH,initial Cr(III)concentration and MFA dosage of 4.11,126 mg·L-1and 1.57 g·L-1,respectively.The value of 98%derived experimentally matches the predicted value well.This value compares Cr(III)removal efficiency of 20%when using FA under the same conditions.However,this does suggest some interaction between the aqueous chromium species and the FA surface.The finding of present study is compared with those of published work by using various waste materials for Cr(III)removal,the overview related to the maximum Cr(III)adsorption capacitiesQmis summarized in Table 5.As it can be seen in Table 5,the adsorption capacity of MFA under the optimum parameters is fairly high(78.6 mg·g-1,value would be more higher if inferred from Langmuir isothermal model),significantly superior or comparative to other waste adsorbents except for eggshell,indicating it can used as a cost-effective adsorbent for the treatment of effluent containing Cr(III).
3.4.Cr(III)removal mechanism
On the basis of the results described,a mechanism for Cr(III)removal is proposed.It is known that changes in pH affect not only the characteristics and availability of metal ions in solution but also the functional groups responsible for adsorption[57].The presence of aqueous Cr(III)-hydroxide is a function of pH[43,58]as is the degree of ionization of the surface active MFA groups.Fig.4 presents the evolution of Cr(III)speciesversuspH calculated by Visual MINTEQ 3.0 software.Visual inspection from Fig.4,seven types of Cr(III)species are in existence across the pH 2.0-13.0.When pH is below 4.0,Cr3+is the dominant solution chromium specie,whereas when pH is greater than 4.0(to approximately 6.5),Cr(OH)2+dominates.As pH value surpasses 5.0,Cr(OH)3(aq)first occurs and the fraction increases gradually with the decrease of acidity until reaching to the maximum at pH 9.0.Cr(III)removal would be predominant by metal hydroxide precipitation with the substitution of adsorption behavior at the pH 6.5-11.5.As a result,pH 2-5 are undertaken in the batch experiments,so that for eliminating the impact of chemistry disposition.In this pH range,a series of other species such as Cr3(OH)45+,Cr2(OH)24+,Cr(OH)2+are also found but in a small fraction.
At low pH where Cr(III)is present in solution as Cr3+,the low percentage of Cr(III)adsorption onto MFA may be explained by strong competition of protons with Cr3+for active surface binding sites because of higher ionic mobility of H3O+[56]and the strong electrostatic repulsion between Cr3+and the protonated MFA[11].On increasing pH,the Cr(III)removal efficiency increases significantly due to weakened surface adsorption of protons resulting in increased Cr(III)adsorption either as Cr3+or Cr(OH)2+.However,the pHpzcvalue of 10.3 indicates the surface MFA will be positively charged across all the pH conditions studied,which implies the electrostatic contribution from electrostatic adsorption for Cr(III)removal would be unconsidered and chemisorptions might dominate instead.According to the results of FTIR analysis,the--OH groups of Mg(OH)2from fly ash are the major functional group involved in Cr(III)adsorption.The adsorption may be achieved through the ligand exchange of--OH groups from MFA and the--H of--OH from Cr(III)hydrate,orvice versa.Besides,the huge specific surface area(27.2 m2·g-1)from substrate fly ash also has the responsibility to increase the adsorption capacity.

Fig.3.3D graphs and contour plots illustrating(a)effect of pH and MFA dosage,(b)effect of initial Cr(III)concentration and MFA dosage on Cr(III)removal efficiency.
At low MFA dosage the available active sites will be fewer and the removal efficiency is determined by the dosage for a given pH and initial Cr(III)concentration.As continuously increasing MFA dosage further sites become available due to the improvement of MFA surface area,resulting in increased Cr(III)adsorption.However,perpetuallyincreasing the dosage of MFA does not lead to greater Cr(III)uptake for a given Cr(III)concentration when excess active sites are available.This trend is in accordance with that in some previous studies using FA as adsorbent for either organics or inorganics adsorption.Anet al.[59]reported the removal efficiency of sulfonated humic acid increased with the increasing dosage of two kinds of FA(Shand and BD)until the dosage was higher than 0.75 g·L-1for Sand and 4 g·L-1for BD,the removal percentage almost remained unchangeable thereafter.Likewise,Mohan and Gandhimathi[60]reported the critical dosage for heavy metals elimination was 2 g·L-1,the excess dosage of FA would have little effect on removal efficiency.
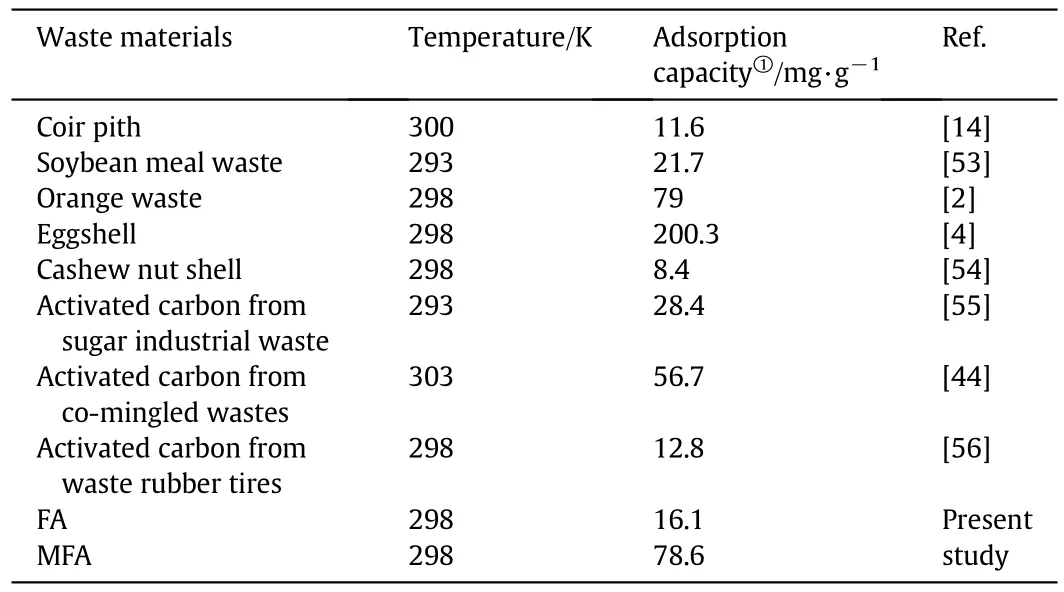
Table 5 Comparative overview of the Cr(III)adsorption capacities from different waste materials
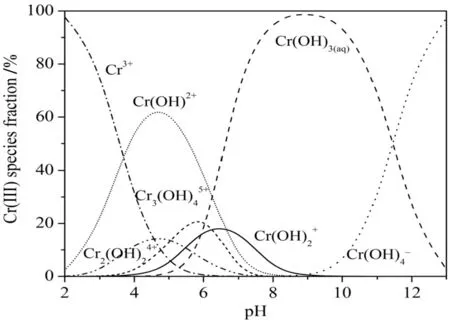
Fig.4.Calculated distribution of Cr(III)species as a function of pH with the constant concentration in aqueous solution.
4.Conclusions
Cr(III)is a toxic pollutant and its removal from aqueous environmental systems has become a priority.A new material,hydrous magnesium oxide coated fly ash(MFA)has been synthesized to evaluate its effectiveness as a substrate for aqueous Cr(III)adsorption.pH,MFA dosage and initial Cr(III)concentration were varied according to Box-Behnken design to obtain optimal adsorption using the response surface methodology.All these variables were found to have significant effect on Cr(III)removal efficiency with the interaction between pH-MFA dosage,and MFA dosage-Cr(III)initial concentration being strong.The analysis of variance shows the mathematical quadratic model derived from the Cr(III)adsorption measurements was reliable.This model was applied to predict optimal conditions of pH(4.11),MFA dosage(1.57 g·L-1)and initial concentration(126 mg·L-1)for Cr(III)removal from aqueous solution to give 98%efficiency.The mechanism for Cr(III)adsorption appears to be predominately associated with surface binding to the functional group--OH from hydrous magnesium oxide with the possibility of a significantly smaller contribution of Cr(III)bound directly to the FA components of the MFA.The results of this study have crucial implications in the case of pollution control in related Cr(III)discharge industries.Further studies regarding thermodynamics,kinetics and real Cr(III)wastewater are desired to prove and obtain a more theoretical foundation for better recognizing the interaction nature between Cr(III)and MFA.
Acknowledgements
The authors are thankful for the support of Wuhan University,China for supply of equipment and chemicals needed to complete this work.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2017.11.008.
[1]O.S.Amuda,A.A.Giwa,I.A.Bello,Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon,Biochem.Eng.J.36(2007)174-181.
[2]A.B.Pérez Marín,M.I.Aguilar,V.F.Meseguer,J.F.Ortuño,J.Sáez,M.Lloréns,Biosorption of chromium(III)by orange(Citrus cinensis)waste:batch and continuous studies,Chem.Eng.J.155(2009)199-206.
[3]I.Y.El-Sherif,S.Tolani,K.Ofosu,O.A.Mohamed,A.K.Wanekaya,Polymeric nanofibers for the removal of Cr(III)from tannery waste water,J.Environ.Manag.129(2013)410-413.
[4]S.Elabbas,L.Mandi,F.Berrekhis,M.N.Pons,J.P.Leclerc,N.Ouazzani,Removal of Cr(III)from chrome tanning wastewater by adsorption using two natural carbonaceous materials:eggshell and powdered marble,J.Environ.Manag.166(2016)589-595.
[5]M.Suwalsky,R.Castro,F.Villena,C.P.Sotomayor,Cr(III)exerts stronger structural effects than Cr(VI)on the human erythrocyte membrane and molecular models,J.Inorg.Biochem.102(2008)842-849.
[6]Y.Wen,Z.Tang,Y.Chen,Y.Gu,Adsorption of Cr(VI)from aqueous solutions using chitosan-coated fly ash composite as biosorbent,Chem.Eng.J.175(2011)110-116.
[7]M.C.Basso,E.G.Cerrella,A.L.Cukierman,Lignocellulosic materials as potential biosorbents of trace toxic metals from wastewater,Ind.Eng.Chem.Res.41(2002)3580-3585.
[8]S.E.A.Elhafez,H.A.Hamad,A.A.Zaatout,G.F.Malash,Management of agricultural waste for removal of heavy metals from aqueous solution:adsorption behaviors,adsorption mechanisms,environmental protection,and techno-economic analysis,Environ.Sci.Pollut.Res.24(2016)1397-1415.
[9]K.Kadirvelu,C.Faur-Brasque,P.L.Cloirec,Removal of Cu(II),Pb(II),and Ni(II)by adsorption onto activated carbon cloths,Langmuir16(2000)8404-8409.
[10]E.Erdem,N.Karapinar,R.Donat,The removal of heavy metal cations by natural zeolites,J.Colloid Interface Sci.280(2004)309-314.
[11]K.Chojnacka,Biosorption of Cr(III)ions by eggshells,J.Hazard.Mater.121(2005)167-173.
[12]L.Yuan,Y.Liu,Removal of Pb(II)and Zn(II)from aqueous solution by ceramisite prepared by sintering bentonite,iron powder and activated carbon,Chem.Eng.J.(2013)432-439.
[13]G.Blázquez,F.Hernáinz,M.Calero,M.A.Martín-Lara,G.Tenorio,The effect of pH on the biosorption of Cr(III)and Cr(VI)with olive stone,Chem.Eng.J.148(2009)473-479.
[14]H.Parab,S.Joshi,N.Shenoy,A.Lali,U.S.Sarma,M.Sudersanan,Determination of kinetic and equilibrium parameters of the batch adsorption of Co(II),Cr(III)and Ni(II)onto coir pith,Process Biochem.41(2006)609-615.
[15]N.Nayak,C.R.Panda,Aluminium extraction and leaching characteristics of Talcher thermal power station fly ash with sulphuric acid,Fuel89(2010)53-58.
[16]T.K.Naiya,S.K.Das,Removal of Cr(VI)from aqueous solution using fly ash of different sources,Desalin.Water Treat.57(2016)5800-5809.
[17]Z.T.Yao,X.S.Ji,P.K.Sarker,J.H.Tang,L.Q.Ge,M.S.Xia,Y.Q.Xi,A comprehensive review on the applications of coal fly ash,Earth-Sci.Rev.141(2015)105-121.
[18]D.G.Grubb,M.S.Guimaraes,R.Valencia,Phosphate immobilization using an acidic type F fly ash,J.Hazard.Mater.76(2000)217-236.
[19]M.Ahmaruzzaman,A review on the utilization of fly ash,Prog.Energy Combust.36(2010)327-363.
[20]M.S.Oh,D.D.Brooker,E.F.De Paz,J.J.Brady,T.R.Decker,Effect of crystalline phase formation on coal slag viscosity,Fuel Process.Technol.44(1995)191-199.
[21]G.Gupta,N.Torres,Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent,J.Hazard.Mater.57(1998)243-248.
[22]K.He,Y.Chen,Z.Tang,Y.Hu,Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash,Environ.Sci.Pollut.Res.23(2016)2778-2788.
[23]Q.Zhou,C.Yan,W.Luo,Polypyrrole coated secondary fly ash-iron composites:novel float able magnetic adsorbents for the removal of chromium(VI)from wastewater,Mater.Des.92(2016)701-709.
[24]H.Yanagisawa,Y.Matsumoto,M.Machida,Adsorption of Zn(II)and cd(II)ions onto magnesium and activated carbon composite in aqueous solution,Appl.Surf.Sci.256(2010)1619-1623.
[25]B.Lei,H.D.Eman,P.S.Shahid,Proton-conducting solid oxide fuel cell(SOFC)with Y-doped BaZrO3electrolyte,Electrochem.Commun.80(2017)20-23.
[26]K.Wang,J.Zhao,H.Li,X.Zhang,H.Shi,Removal of cadmium(II)from aqueous solution by granular activated carbon supported magnesium hydroxide,J.Taiwan Inst.Chem.Eng.61(2016)287-291.
[27]W.Gao,S.Zhao,H.Wu,W.Deligeer,S.Asuha,Direct acid activation of kaolinite and its effects on the adsorption of methylene blue,Appl.Clay Sci.126(2016)98-106.
[28]F.Li,W.Wu,R.Li,X.Fu,Adsorption of phosphate by acid-modified fly ash and palygorskite in aqueous solution:experimental and modeling,Appl.Clay Sci.132-133(2016)343-352.
[29]S.Wang,Y.Boyjoo,A.Choueib,A comparative study of dye removal using fly ash treated by different methods,Chemosphere60(2005)1401-1407.
[30]J.B.Zhang,S.P.Li,H.Q.Li,M.M.He,Acid activation for pre-desilicated high-alumina fly ash,Fuel Process.Technol.151(2016)64-71.
[31]K.Pi,M.Xia,P.Wu,M.Yang,S.Chen,D.Liu,A.R.Gerson,Effect of oxidative modification of activated carbon for the adsorption behavior of nicotine,J.Ind.Eng.Chem.31(2015)112-117.
[32]K.Saakshy,A.B.Singh,A.K.Gupta,Sharma, fly ash as low cost adsorbent for treatment of effluent of handmade paper industry-kinetic and modelling studies for direct black dye,J.Clean.Prod.112(2016)1227-1240.
[33]M.H.Isa,E.H.Ezechi,Z.Ahmed,S.F.Magram,S.R.M.Kutty,Boron removal by electrocoagulation and recovery,Water Res.51(2014)113-123.
[34]K.Pi,Q.Xiao,H.Zhang,M.Xia,A.R.Gerson,Decolorization of synthetic methyl orange wastewater by electrocoagulation with periodic reversal of electrodes and optimization by RSM,Process.Saf.Environ.92(2014)796-806.
[35]A.Y.Zahrim,A.Nasimah,N.Hilal,Coagulation/ flocculation of lignin aqueous solution in single stage mixing tank system:modeling and optimization by response surface methodology,J.Environ.Chem.Eng.3(2015)2145-2154.
[36]Z.Šereš,N.Maravić,A.Takači,I.Nikolić,D.Šoronja-Simović,A.Jokić,C.Hodur,Treatment of vegetable oil refinery wastewater using alumina ceramic membrane:optimization using response surface methodology,J.Clean.Prod.112(Part 4)(2016)3132-3137.
[37]A.V.Schenone,L.O.Conte,M.A.Botta,O.M.Alfano,Modeling and optimization of photo-Fenton degradation of 2,4-D using ferrioxalate complex and response surface methodology(RSM),J.Environ.Manag.155(2015)177-183.
[38]M.Iqbal,N.Iqbal,I.A.Bhatti,N.Ahmad,M.Zahid,Response surface methodology application in optimization of cadmium adsorption by shoe waste:a good option of waste mitigation by waste,Ecol.Eng.88(2016)265-275.
[39]V.Srivastava,Y.C.Sharma,M.Sillanpää,Application of response surface methodology for optimization of Co(II)removal from synthetic wastewater by adsorption on NiO nanoparticles,J.Mol.Liq.211(2015)613-620.
[40]L.Lin,Y.Lin,C.Li,D.Wu,H.Kong,Synthesis of zeolite/hydrous metal oxide composites from coal fly ash as efficient adsorbents for removal of methylene blue from water,Int.J.Miner.Process.148(2016)32-40.
[41]J.Zhang,Y.Zhou,M.Jiang,J.Li,J.Sheng,Removal of methylene blue from aqueous solution by adsorption on pyrophyllite,J.Mol.Liq.209(2015)267-271.
[42]M.Visa,L.Isac,A.Duta,Fly ash adsorbents for multi-cation wastewater treatment,Appl.Surf.Sci.258(2012)6345-6352.
[43]R.Leyva-Ramos,L.Fuentes-Rubio,R.M.Guerrero-Coronado,J.Mendoza-Barron,Adsorption of trivalent chromium from aqueous solutions onto activated carbon,J.Chem.Technol.Biotechnol.62(1995)64-67.
[44]S.I.Lyubchik,A.I.Lyubchik,O.L.Galushko,L.P.Tikhonova,J.Vital,I.M.Fonseca,S.B.Lyubchik,Kinetics and thermodynamics of the Cr(III)adsorption on the activated carbon from co-mingled wastes,Colloids Surf.A Physicochem.Eng.Asp.242(2004)151-158.
[45]A.Genovese,R.A.Shanks,Structural and thermal interpretation of the synergy and interactions between the fire retardants magnesium hydroxide and zinc borate,Polym.Degrad.Stab.92(2007)2-13.
[46]Z.Luo,J.Yang,H.Ma,M.Liu,X.Ma,Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia,Hydrometallurgy157(2015)188-193.
[47]E.Eren,Removal of copper ions by modified Unye clay,Turkey,J.Hazard.Mater.159(2008)235-244.
[48]M.F.M.Din,M.Ponraj,W.P.Low,M.A.Fulazzaky,K.Iwao,A.R.Songip,Removal rate of organic matter using natural cellulose via adsorption isotherm and kinetic studies,Water Environ.Res.88(2016)118-130.
[49]X.Xu,Q.Li,H.Cui,J.Pang,L.Sun,H.An,J.Zhai,Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres,Desalination272(2011)233-239.
[50]S.Yahyapour,A.Golshan,A.H.Ghazali,Removal of total suspended solids and turbidity within experimental vegetated channel:optimization through response surface methodology,J.Hydro Environ.Res.8(2014)260-269.
[51]A.A.L.Zinatizadeh,A.R.Mohamed,M.D.Mashitah,A.Z.Abdullah,M.H.Isa,Optimization of pre-treated palm oil mill effluent digestion in an up- flow anaerobic sludge fixed film bioreactor:a comparative study,Biochem.Eng.J.35(2007)226-237.
[52]L.Mohajeri,H.A.Aziz,M.H.Isa,M.A.Zahed,A statistical experiment design approach for optimizing biodegradation of weathered crude oil in coastal sediments,Bioresour.Technol.101(2010)893-900.
[53]A.Witek-Krowiak,D.H.Reddy,Removal of microelemental Cr(III)and cu(II)by using soybean meal waste-unusual isotherms and insights of binding mechanism,Bioresour.Technol.127(2013)350-357.
[54]G.F.Coelho,C.R.T.Tarley,J.Casarin,H.Nacke,M.A.Francziskowski,Removal of metal ions cd(II),Pb(II),and Cr(III)from water by the cashew nut shell Anacardium Occidentale L,Ecol.Eng.73(2014)514-525.
[55]N.F.Fahim,B.N.Barsoum,A.E.Eid,M.S.Khalil,Removal of chromium(III)from tannery wastewater using activated carbon from sugar industrial waste,J.Hazard.Mater.136(2006)303-309.
[56]V.K.Gupta,I.Ali,T.A.Saleh,M.N.Siddiqui,S.Agarwal,Chromium removal from water by activated carbon developed from waste rubber tires,Environ.Sci.Pollut.Res.20(2013)1261-1268.
[57]S.Al-Asheh,F.Banat,R.Al-Omari,Z.Duvnjak,Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data,Chemosphere41(2000)659-665.
[58]J.Rivera-Utrilla,M.Sánchez-Polo,F.Carrasco-Marín,Adsorption of 1,3,6-Naphthalenetrisulfonic acid on activated carbon in the presence of Cd(II),Cr(III),and Hg(II)importance of electrostatic interactions,Langmuir19(2003)10857-10861.
[59]C.J.An,S.Q.Yang,G.H.Huang,S.Zhao,P.Zhang,Y.Yao,Removal of sulfonated humic acid from aqueous phase by modified coal fly ash waste:equilibrium and kinetic adsorption studies,Fuel165(2016)264-271.
[60]S.Mohan,R.Gandhimathi,Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent,J.Hazard.Mater.169(2009)351-359.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- An innovative trigeneration system using biogas as renewable energy
