Thermodynamic behaviors of Cu in interaction with chlorine,sulfur,phosphorus and minerals during sewage sludge co-incineration☆
Jingyong Liu *,Chao Liu Guang Sun Wuming Xie Xiao'e Dang ,Jiahong Kuo Kenlin Chang 3,Jian Sun Shuiyu Sun Musa Buyukada ,Fatih Evrendilek
1 School of Environmental Science and Engineering,Institute of Environmental Health and Pollution Control,Guangdong University of Technology,Guangzhou 510006,China
2 Shaanxi Key Laboratory for Gold and Resources,School of Metallurgical Engineering,Xi'an University of Architecture and Technology,Xi'an 710055,China
3 Institute of Environmental Engineering,National Sun Yat-Sen University,Kaohsiung 80424,China
4 Department of Environmental Engineering,Abant Izzet Baysal University,Bolu 14052,Turkey
1.Introduction
In response to increasingly growing human population,there has been a paralleling increase in the standards applied for wastewater discharge,the wide spread development of wastewater treatment plants and technologies,and the quantity of sewage sludge as a byproduct of wastewater treatment plants.Properties of sewage sludge such as high moisture content,huge volume,bad odor,toxic and harmful substances,and ease of degradation pose significant public health risks[1,2].Given the recent treatment and pollution control technologies and policies,(co-)incineration of municipal sewage sludge has been considered to be one of the most feasible options for its disposal.Coincineration with coal may be applied to reduce investment costs and environmentally harmful wastes as well as to enhance resource recovery,reuse and recycling[3].However,sewage sludge may also increase emissions of inorganic and organic air pollutants during the co-incineration due to its heavy metal(HM)content,thus restricting the development of environmentally benign co-combustion technologies[4].
Copper(Cu)concentration of sewage sludge may vary greatly with sources,locations,and time.For example,the mean Cu concentration was estimated at(63.7± 72)mg·kg-1for commercial composts with 2.6%sewage sludge in China[5],(95.8 ± 157)mg·kg-1for soils amended with sewage sludge in Turkey[6],and 410 mg·kg-1for sewage sludge in Sweden[7].The previous studies reported 182.5 mg·kg-1to be the mean Cu concentration of sewage sludge in China[8].According to the guideline ofDischarge Standard of Pollutants for Municipal Wastewater Treatment Plant(GB18918-2002),this exceeded the pollutant limit values of agricultural sludge applications for basic and acid soils by 2.3%and 7.1%,respectively.Sludge as a large source of phosphorus(P),chlorine(Cl)-containing flocculants in wastewater treatment,Cl-containing conditioners in deep dewatering of sludge,and sulfur(S)-containing fire coals add further P,Cl,and S to the co-incineration systems[9,10].Therefore,Cl,S and P interact with Cu in the sludgeoriented co-incineration systems.In addition,the main slag components of the sludge/waste/coal co-incineration include SiO2,Al2O3,and CaO which in turn allow for adsorption of heavy metals in different forms[11].These minerals precipitate in the competing reactions between HMs and Cl,S,and P during co-incineration,thus changing the migration and transformation paths of HMs including Cu[12].To the best of our knowledge,there exist no studies on migration and transformation behavior ofCu when coupled with interactions between Cl,S,and P and the minerals such as SiO2,Al2O3and CaO.
The multiple migration pathways of HMs during sludge coincineration are a function of incinerator types,their operation variables(e.g.incineration temperature,residence time,and combustion atmosphere)[13,14],initial sludge compositions,and Cl and/or S contents of mixed wastes[15,16].Cl-containing compounds can react with Cu easily,thus forming metal chlorides and Cu emissions in large quantities[17,18].At low temperatures,metal sulfides can substitute metal chlorines or metal oxides,thus inhibiting Cu volatilization.However,at temperatures above 800°C,the presence of volatile metal sulfides makes S compounds enhance Cu volatilization[19].During the co-incineration,P-containing compounds can form phosphate compounds with strong heat stability,thus inhibiting Cu volatilization and emission[20,21].In the complex sludge co-incineration systems coupled with Cl,S,and P,Cu migration and transformation are affected by both the form of Cu and the competing reaction between Cl and S.This reaction is also influenced by the existence and forms of P and the minerals.However,there still exist gaps in the current knowledge accumulation about the roles and relative contributions of P and the minerals in these reactions.
Currently,the simulated grate,and fluidized bed incinerators are usually employed to track the migration and transformation behaviors of HMs in the laboratory[22-29].Thus far,due to the limitations of the partial detection technologies,the migration and transformation behaviors of Cu across the entire range of temperatures during sludge coincineration have not been obtained accurately using experiments under various interaction conditions.In addition,Cu compounds act during the co-incineration as the catalysts in the formation of PCDD/Fs at low temperatures ranging from 300 °C to 500 °C,under the presence of Cl[30].As an important catalyst for the generation of dioxins and Cl source,CuCl2is more active than KCl,CaCl2and FeCl3[31].In addition,a previous incineration experiment showed that when 0.07%CuCl2·H2O was added to simulated waste,the PCDD/Fs production increased by 30%[30].Therefore,it is necessary to accurately estimate and monitor chemical interactions among Cl,S,P,and Cu across the entire temperature range and process.
In so doing,the present study predicts the thermal conversion behavior of Cu in interaction with Cl,S,and P using the thermodynamic equilibrium software(FACT sage 6.3).Also,this study quantifies effects of the coupling of Cl,S,and P with the minerals of SiO2,CaO,and Al2O3on migration and thermodynamic equilibrium behaviors of Cu.This can in turn pave the way for effective predictions of Cu emission,thus hindering the formation of dioxins during sludge co-incineration.
2.Materials and Methods
2.1.Sampling strategy
Sewage sludge used in the experiments were sampled from four large sewage treatment plants in Guangzhou named KFQ,DTS,LJ,and LD whose specific properties were listed in Table 1[32,33].DTS,LJ,and LD are the sewage treatment plants on a larger scale that adopt the conventional active biomass sludge treatment technology,and thus,are representative of the typical sludge and plant characteristics.Their sludge treatment capacity accounts for over 60%of the total sewage treatment capacity in Guangzhou.To compare municipal sewage sludge composition in detail,sludge samples from a Guangzhou paper mill(ZZ)and a sewage treatment plant(ZQ)in the city of Zhaoqing were also collected.
Elemental C,N,H,O and S contents of sludge samples were detected using an elemental analyzer(Elementar Vario EL III,Germany).While Cl content was determined using the methods of water leaching and nitrate titration,mineral contents(SiO2,CaO,Al2O3,and Fe2O3)were measured using an X ray fluorescence spectrometer(Rigaku 100e,Japan).All results are shown in Table 2[32,33].

Table 2 Composition of elements and minerals in sewage sludge(%)[32,33]
To accurately reflect the HM contents of the municipal sludge samples in China,this study used their time-series data from 2006 to 2013 and estimated the geometric average value of Cu content as the initial value during the simulation,as can be seen in Table 3[8].

Table 3 Concentration of Cu in sewage sludge in China[8]
2.2.Thermodynamic method
The equilibrium constant method and the Gibbs minimum free energy method are usually employed to deal with the chemical equilibrium of complex systems.The FACT sage 6.3 software applied in this study is based on the Gibbs minimum free energy method.The composition and concentration of each component were obtained under isothermal and isobaric conditions,taking the Gibbs free energy minimization as the equilibrium criterion,and using the Lagrange undetermined coefficient method.In this study,it is assumed that air,sludge and other original reaction substances are added into the sludge coincineration system at a certain temperature and pressure for complex chemical reactions to occur.When the system reaches equilibrium,the Gibbs free energy of the entire system reaches the minimum.In this way,the composition and concentration of various gaseous substances and solid materials within the system can be estimated(Fig.1).
The equilibrium system used in predicting the speciation and concentration of Cu in the sludge co-incineration system is a simple system in which the sludge samples were assumed to only contain the major elements(C,H,O,N,S,and Cl)and the minerals,while the flue gas isan ideal model consisting of only the gaseous and pure condensation phases.When the chemical thermodynamic equilibrium model is applied,model predictions are significantly affected by interactions between the trace metals,all possible reactions among the trace metals,and combinations between the melting trace metals and compounds.Therefore,the chemical thermodynamic method leads to large deviations from experimental results under some conditions.The FACT sage calculation system has been gradually developed into a more robust system so as to contain multiple trace metals,all the major elements,minor elements,and minerals in sludge as well as so as to consider interactions between the trace metals,and all possible reactions,in making predictions of the ideal systemversusthe actual system similar.

Table 1 Description of wastewater treatment plant(WWTP)[32,33]

Fig.1.Scheme of equilibrium system by MINGSYS in sewage sludge co-incinerator(Me denotes metal).
The system was established to represent sludge(co-)incineration in an atmosphere the composition of which is similar to that of the burner tube.The sludge used in the model simulation was assumed to consist of C,H,N,O,Cl and S(Table 2).The equilibrium calculations were performed in the range of 400 to 1800 K(127 to 1527°C),and the total pressure was 1.0 MPa(1.013×105Pa).The temperature step was 100 K,and the excess air ratio(λ)was set from 0.6 to 1.2 for the calculations.The air was assumed to be composed(in mole fractions)of 79%N2and 21%O2.
During the simulations,the initial parameters included the four elements(C,H,O and N)of sludge and air,the four minerals(CaO,SiO2,Al2O3,and Fe2O3),Cu(182.5 mg·kg-1)(Table 3),and the three representative elements(Cl,S,and P).The contents of Cl,S,and P were set to 0.3%,3%and 3%,respectively,according to the actual coincineration commonly used in the simulations[12].Conditions and parameterization for the model simulations were listed in Table 4.
It should be emphasized that although the chemical thermodynamics calculation can simulate the thermal conversion and distribution of Cu under various conditions,the calculation based on the Gibbs free energy minimum principle has the following limitations[34].First,due to lack of thermodynamic data,chemical reactions between all the Cu compounds and each mineral were not considered in the calculationprocess.This in turn led to the mismatch between theoretical and experimental data.Nevertheless,the trend of the model estimates conformed to that of experiment values.Second,the thermodynamic equilibrium modeling could not well predict the experimental results due to implicit initial conditions of the model.In addition,some minor parameters difficult to obtain might be ignored that could affect the predictions of Cu.Despite the above limitations,the simulations of chemical thermodynamic equilibrium still offer insights for Cu controls over the actual sludge co-incineration system.

Table 4 Model parameterization under 79%N2-21%O2 atmosphere
3.Result and Discussion
3.1.Thermal migration and conversion behaviors of single and coupled Cl,S,and P
3.1.1.Migration and conversion behaviors of Cl
Fig.2 shows changes in Cl concentration with C,N,H,and O,with λ values of0.6,0.8,1.0 and 1.2,respectively(“s”and “g”in Fig.2 represent the solid and gaseous products,respectively,throughout the article).Cl mainly existed in the forms of NH4Cl,HCl and Cl2at a temperature range of 400 to 1800 K(Fig.2).Under the reducing atmosphere(λ=0.6-0.8),Cl was easily combined with H to form NH4Cl and HCl.At low temperatures(400-500 K),both NH4Cl and HCl existed;however,as the temperature increased,NH4Cl was gradually decomposed and converted completely into HCl at 500 K.Under the oxidizing atmosphere(λ=1-1.2)[Fig.2(c)and(d)],Cl existed mainly in the forms of HCl and Cl2[35].As the temperature rose,Cl2was gradually converted into and completely became HCl at 700 K.As is evident from the above,Cl was mainly released in the form of HCl(g)under both reducing and oxidizing atmospheres within the incineration temperature range of 1000 to 1200 K.
3.1.2.Migration and conversion behaviors of S
S mainly existed in the forms of H2S(g),SO2(g),S2(g),SO3(g)and H2SO4(g)at a temperature range of400 to 1800 K(Fig.3).Under the reducing atmosphere(λ=0.6-0.8),S was in the form of H2S(g)mainly within a temperature range of 400 to 1100 K.At 1200 K,H2S(g)was gradually converted into SO2(g)and S2(g).Under the oxidizing atmosphere(λ=1-1.2)[Fig.3(c)and(d)],S was mainly in the forms of SO2(g),S2(g),SO3(g)and H2SO4(g)[35].Given the atmospheric condition with λ of 1.2 as the baseline,S was in the form of H2SO4(g)between 400 and 600 K,while H2SO4(g)was gradually converted into SO3(g)with the increased temperature.At 950 K,all S turned into SO2(g).Thus,S was mainly emitted in the form of H2S(g)under the reducing atmosphere and in the forms of SO2(g)and SO3(g)under the oxidizing atmosphere.
3.1.3.Migration and conversion behaviors of P
During the incineration temperature between 400 and 1800 K,P appeared mainly in the forms of(NH4)H2PO4(g),(P2O3)2(g),PO2(g),(P2O5)2(g)and H3PO4(s)(Fig.4).Under the reducing atmosphere(λ=0.6-0.8),the major P form was(NH4)H2PO4(g)between 400 and 500 K and(P2O3)2between 500 and 1700 K.Under the oxidizing atmosphere(λ=1-1.2)[Fig.4(c)and(d)],P was found mainly in the forms of(P2O3)2,PO2,(P2O5)2and H3PO4[35].Considering the atmospheric condition with λ of 1.2 as the baseline,P turned into H3PO4(s)at low temperatures of 400 to 600 K.With the increased temperature,(P2O3)2(g)dominated.At temperatures above 1400 K,P turned into PO2(g).Thus,P was found mainly as(P2O3)2(g)under the reducing atmosphere and as(P2O5)2(g)under the oxidizing atmosphere within the normal range of the incineration temperature.
Changes in the concentration of Cl,S,and P are shown in Fig.5[35]when the sludge samples contained only C,N,H,and O and were coupled with Cl,S,and P.During the sludge co-incineration with λ of 1.2 and under all the combinations of Cl,S,and P(Cl+P,S+Cl,P+S,and Cl+S+P)the conversion characteristics of Cl,S,and P remained the same as with the single-sludge incineration system.This case can be attributed to the excessive contents of H and O in the incineration system.Thus,within the normal range of the incineration temperature under the oxidizing atmosphere(λ=1.2)with the presence of single Cl,S,and P or their combination systems,Cl,S,and P were mainly emitted in the forms of HCl(g),SO2(g)and(P2O5)2(g),respectively.

Fig.2.Thermodynamic equilibrium distribution of chlorine during sludge co-incineration under(a)λ=0.6;(b)λ=0.8;(c)λ=1.0;(d)λ=1.2.

Fig.3.Thermodynamic equilibrium distribution of sulfur during sludge co-incineration under(a)λ=0.6;(b)λ=0.8;(c)λ=1.0;(d)λ=1.2.
3.2.Reactions between single minerals and Cu with or without single and coupled Cl,S,and P
3.2.1.Reactions between single minerals and Cu without Cl,S,and P
The migration and conversion characteristics of Cu are presented in Fig.6(a)when the sludge samples contained only C,N,H,and O and a single mineral without Cl,S,and P.At temperatures from 400 to 1200 K,Cu was mainly in the form of CuO(s)in the sludge samples.At temperatures above 1200 up to 1400 K,CuO(s)was converted into Cu2O(s).At 1300 K,Cu existed only as Cu2O(s).At temperatures above 1400 K,Cu2O(s)gradually turned into Cu(g)and CuO(g),with Cu(g)as the major component(≥95%).

Fig.4.Thermodynamic equilibrium distribution of phosphorus during sludge co-incineration under(a)λ=0.6;(b)λ=0.8;(c)λ=1.0;(d)λ=1.2.

Fig.5.Thermodynamic equilibrium distribution of chlorine,sulfur and phosphorus with the interaction of chlorine,sulfur and phosphorus during sludge co-incineration:(a)Cl+P;(b)S+Cl;(c)P+S;(d)Cl+S+P[35].
With Al2O3only,changes in Cu concentration are shown in Fig.6(b).The temperature range for CuO(s)shrunk by 100 K(400 to 1100 K).At temperatures above 1100 K,CuO(s)started to turn into Cu2O(s)which in turn reacted with Al2O3,thus producing Cu2Al2O4(s)with a wide range of temperature(1100-1700 K),and a strong thermal stability.The presence of Al2O3had no effect on the concentrations of Cu(g)and CuO(g).
Fig.6(c)shows changes in Cu concentration with Fe2O3only.A comparison of Fig.6(a)and(c)pointed to a decrease in the temperature window for CuO(s)by 500 K(400 to 700 K).At temperatures above 700 K,CuO(s)began to react with Fe2O3to produce CuO(Fe2O3)(s)with a wide temperature range(700-1700 K),and a strong thermal stability.Fe2O3had no effect on Cu(g)concentration and did not produce CuO(g).

Fig.6.Effects of single minerals[(a)without minerals;(b)Al2O3;(c)Fe2O3;(d)CaO or SiO2 or TiO2]on thermodynamic equilibrium distribution of Cu without chlorine,sulfur and phosphorus during sludge co-incineration.
Fig.6(d)shows changes in Cu concentration with a single mineral such as CaO,SiO2,or TiO2only.The comparison of Fig.6(a)versusFig.6(d)indicated no effect by a single mineral on the concentration and speciation of Cu(g).In conclusion,Fe2O3and Al2O3reacted with Cu inhibiting its volatilization,whereas CaO,TiO2,and SiO2had no significant effect.
Previous simulations showed that the addition of CaO had little influence on the chemical sorption of Cu,and the sorption of CaO resulted from physicalsorption[23]which was in line with our results.The influence of the minerals differed significantly on the distribution and conversion of Cu which has important implications for seeking the most suitable adsorbents to control Cu emissions[36].
3.2.2.Reactions between single minerals and Cu with single Cl,S,and P
3.2 主题活动“达尔文计划” 在开展本次活动前,学生需要具有的前概念是: 适应辐射、达尔文进化论的观点和自然选择作用的机理等。教师出示达尔文和华莱士基于自然选择是如何发生的四条假设(种群中的个体互不相同;性状从亲代传递给子代;有些个体未能存活并繁殖和存活和繁殖不是由运气决定的),并携带镊子、筷子、橡皮糖、坚果和330mL的塑料矿泉水空瓶若干。
3.2.2.1.Reactions between minerals and Cu with Cl.With Cl,Cu was mainly found as CuCl2(s),CuO(s),CuCl(g),(CuCl)3(g)and Cu(g)[Fig.7(a)].With Fe2O3,CuO(Fe2O3)(s)was formed between 700 and 1300 K,while the temperature range for CuO(s)was narrowed down,and the volatilization proportion of(CuCl)3(g)was reduced[Fig.7(b)].With CaO,Cu appeared only as CuO(s)between 400 and 1100 K,without the formation of CuCl2(s)which may be due to the fact that Cl is easily combined with Ca[Fig.7(c)].The presence of the single minerals did not affect Cu concentration[Fig.7(d)].
Comparing Figs.6(c)and 7(b)indicated that Cu was in the form of CuCl2(s)at low temperatures between 400 and 500 K with Cl,while Fe2O3tended to narrow down the temperature window for CuO(s)(400-800 K)and CuO(Fe2O3)(s)(700-1300 K).At temperatures above 1000 K,CuCl(g)and(CuCl)3(g)substituted Cu(g),thus enhancing Cu volatility[Fig.7(b)].CaO shortened the temperature window for CuO(s)by 200 K.Likewise,at temperatures above 1000 K,CuCl(g)and(CuCl)3(g)substituted Cu2O(s)and Cu(g),thus increasing Cu volatility[Figs.6(d)and 7(c)].With the single minerals,Cl tended to shorten the temperature window for CuO(s)and inhibit the formation of Cu2O(s)and Cu2Al2O4(s).Also,the formation of CuCl(g)and(CuCl)3(g)enhanced Cu volatility at high temperatures[Fig.7(d)].
Cl weakened the adsorption of Al2O3,CaO and Fe2O3on Cu,thus resulting in the failure of partial adsorbents,but had no effect on the adsorption of SiO2and TiO2.Consistent with our findings,the previous studies indicated that Cl in the sludge co-incineration system enhanced the ability of Cu to react firstly with Cl2or HCl to produce volatile CuCl(g)and(CuCl)3(g),which in turn rendered the solid adsorbents not able to combine with Cu[37,38].The added chlorides appeared to affect the removal of Cu in a positive manner.Cu was either volatile in the form of the chloride trimer(CuCl)3and the monomer CuCl[39]or reacted first to form CuCl2,followed by reduction to CuCl[40].Another experimental investigation[41]carried out on a tube furnace was also in agreement with this finding.
3.2.2.2.Reactions between minerals and Cu with S.With S,Cu was found in the forms of CuSO4(H2O)(s),CuSO4(s)and CuO(s)from 400 to 500 K,450 to 950 K and 950 to 1250 K,respectively(Fig.8).As the temperature rose,CuO(s)was gradually converted into Cu2O(s),and when the temperature was above 1400 K,Cu(g)appeared[Fig.8(a)].With CaO,the temperature windows for both CuSO4(s)and CuO(s)decreased,and(CuO)(CuSO4)(s)appeared[Fig.8(b)].With Fe2O3,there was a slight change in the temperature window for CuSO4(s),while(CuO)(Fe2O3)(s)appeared in a wider temperature range of 900 to 1600 K,and no Cu2O(s)was formed during the entire process[Fig.8(c)].With Al2O3,(CuO)(s)appeared in a narrower temperature range,whereas(CuO)(Fe2O3)(s)was found in a wider temperature range of 900 to 1600 K,with no observed Cu2O(s)in the entire process[Fig.8(d)].In conclusion,Fe2O3and Al2O3significantly changed the migration and distribution of Cu,while CaO had a slight effect,with SiO2and TiO2having no effect.

Fig.7.Effects of single minerals[(a)without minerals;(b)Fe2O3;(c)CaO;(d)Al2O3 or SiO2 or TiO2]on the thermodynamic equilibrium distribution of Cu with chlorine during sludge coincineration.

Fig.8.Effects of single minerals[(a)without minerals,SiO2,TiO2;(b)CaO;(c)Fe2O3;(d)Al2O3]on the thermodynamic equilibrium distribution of Cu with sulfur during sludge co-incineration.
3.2.2.3.Reactions between minerals and Cu with P.With P,the single minerals had no effect on Cu migration and conversion(Fig.9).This situation differed greatly in the incineration system with Cl and S,thus indicating that Cu preferred to react with Cl and S.During the sludge co-incineration,the elements of Cl,S,and P had different effects on reactions between the single minerals and Cu.For example,Cl and S exerted the greatest effect,while P had a very limited impact.With Cl,Fe2O3and CaO exerted the greatest effect on Cu concentration,whereas Al2O3,SiO2,and TiO2had no effect.S rendered Al2O3,Fe2O3,and CaO most influential but SiO2and TiO2slightly influential.In a comparison of various sorbents such as silica,alumina,kaolin,copiapite,and limestone in terms of their Cd capture efficiency in a thermogravimetric reactor,Uberoiet al.[42]based capture mechanism of these sorbents on the chemical or reaction adsorption but not on the physical absorption.Therefore,adding sorbents(e.g.,lime,limestone,kaolin,and iron)to the sludge can inhibit Cu emissions to some extent.

Fig.9.Effects of single minerals on the thermodynamic equilibrium distribution of Cu with phosphorus during sludge co-incineration.
3.2.3.Reactions between single minerals and Cu with coupled Cl,S,and PThe coupling of Cl,S,and P during the sludge co-incineration complicates the migration and conversion behaviors of Cu.The simulations carried out in the present study(Figs.10 to 11)assumed the presence of single minerals,the coupled combinations of Cl,S,and P,and the presence of the four major elements of the sludge samples.With the coupling of Cl and P,the reactions between the single minerals and Cu were affected by Cl only not by the mineral type.In other words,the reactions between the single minerals and Cu under the coupling of Cl and P were not related to P but were controlled by Cl(Fig.10).
As far as the couplings of Cl and S or Cl,S,and P are concerned,the reactions between the single minerals and Cu were affected by the coupling of Cl and S but by the minerals(Fig.11).S effect dominated greatly between 400 and 1000 K,while Cl effect dominated between 1000 and 1800 K.As for the couplings of Cl and S or Cl,S,and P,the reactions between the single minerals and Cu were affected by both Cl and S that inhibited Cu volatilization.The coupling of S and P caused the reactions between the single minerals and Cu to be affected by only S(Fig.12).S participated in the competition reaction between Cl and the minerals through the following reaction:2R-Cl+SO2+0.5O2+H2O→R2SO4+2HCl,thus forming sulfate(R2SO4)with a high melting point,and reducing the contact chance of the minerals and Cl[43].

Fig.10.Effects of single minerals on the thermodynamic equilibrium distribution of Cu with chlorine and phosphorus during sludge co-incineration.

Fig.11.Effects of single minerals on the thermodynamic equilibrium distribution of Cu with chlorine and sulfur during sludge co-incineration.
3.3.Reactions between coupled minerals and Cu with coupled Cl,S,and P
The coupled minerals during the sludge incineration change the migration and conversion pathways of Cu.Our simulations in this study assumed the thermal dynamic equilibrium concentration of Cu(Fig.13),the coupled minerals(SiO2+CaO+Al2O3),the coupling combinations of Cl,S,and P,and the presence of C,N,H,and O in the sludge samples.With the coupled minerals[Fig.13(a)],Cu concentration was affected by only Al2O3,and Cu2Al2O4(s)was detected between 1100 and 1700 K.Cl[Fig.13(b)]and S[Fig.13(c)]played an important role in the conversion of Cu under the coupled minerals.(CuCl)3(g)appeared between 800 and 1300 K;however,once the temperature was over 1000 K,CuCl(g)began to form,thus rendering the sorbentAl2O3invalid.No Cu2Al2O4(s)was detected during the entire process,and Clenhanced Cu volatilization.S decreased the temperature range for CuO(s)by 100 K,increased the formation temperature for Cu2Al2O4(s)by 100 K and inhibited Cu volatilization[Fig.13(c)].However,P had no significant effect on Cu concentration in this incineration system[Fig.13(d)].Cu existed mainly in the forms of CuO(s),Cu2O(s),Cu(g)and CuO(g)during the entire process[Fig.5(c)].
The coupling of Cl and S exerted a great effect on Cu concentration in the same incineration system[Fig.13(e)].The migration and conversion behaviors of Cu were affected mainly by S between 400 and 900 K and by Cl from 900 to 1800 K.The coupling of Cl and P also changed Cu concentration in this incineration system[Fig.13(f)].At temperatures from 400 to 500 K,CuCl2(g)was observed,while between 500 and 1800 K,the migration and conversion of Cu were affected mainly by Cl but not by P.Across the entire temperature range,the couplings of Cl+S,Cl+P,and Cl+S+P caused the reactions between the coupled minerals and Cu to be invalid.
The couplings of P+S[Fig.13(g)]and Cl+S+P[Fig.13(f)]significantly affected Cu concentration in the incineration system.In the coupling of P+S,the existence temperature range narrowed down for CuSO4and decreased for Cu2O(s)by 100 K,while CuO(CuSO4)(s)disappeared between 400 and 800 K.Across the entire temperature range,Cu concentration was affected mainly by S.As for the coupling of Cl+S+P,the migration and conversion patterns of Cu were affected by S between 400 and 1000 K and by Cl from 900 to 1800 K,with P having no effect.
In conclusion,the coupled Cl+S+P had a dramatic influence on the reactions between the single or coupled minerals and Cu.S dominated the reaction at low temperatures,thus inhibiting Cu volatilization.However,Cl was most influential at high temperatures,thus enhancing Cu volatilization,with P having the weakest effect.
The thermodynamic analysis can be reasonably used to predict volatilization tendency and thermal-chemical behaviors of Cu at high temperatures and to qualitatively and quantitatively explain its general behavior in the presence of single or coupled Cl,S,P,and minerals during the sludge co-incineration.Thermodynamic analysis can help to devise pollution controls strategies in the sludge co-incineration.Previous simulation results indicated that the use of solid sorbents appears to be promising for reducing the emissions of metal vapors in the high temperature flue gases[36].Cu2O(s),Cu2Al2O4(s)and CuFe2O4(s)can formviachemical reactions between Cd compounds and Fe2O3-and Al2O3-containing minerals during co-incineration.Adsorption efficiency of the sorbents is influenced by many factors such as operating temperature,Cl and S contents,minerals in the feed,and the nature of the sorbents.There still remains a more comprehensive study on the formation of solid complex compounds and the effect of specific conditions in order to answer more specific questions[36].
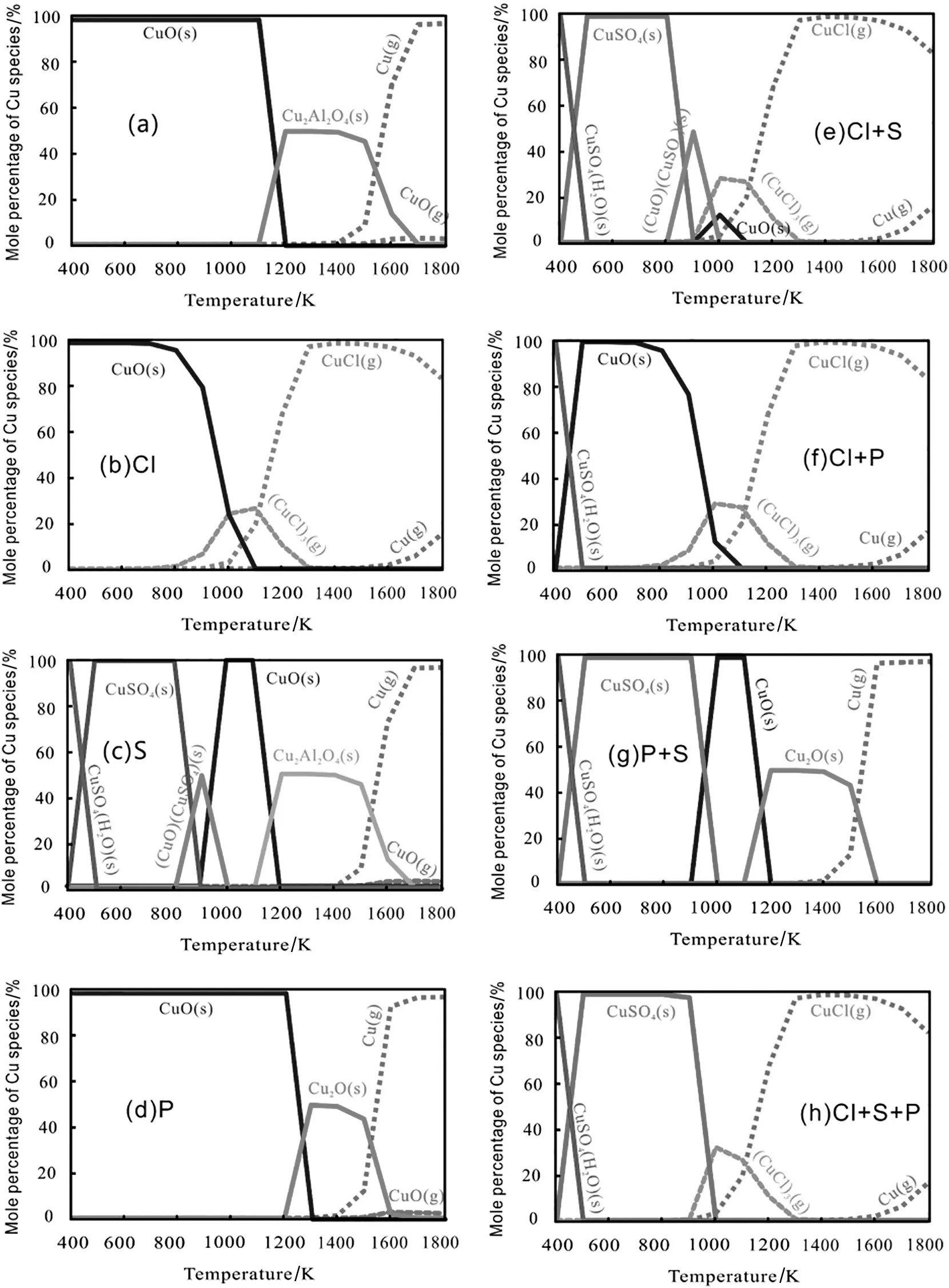
Fig.13.Effects of coupled SiO2+CaO+Al2O3 on the thermodynamic equilibrium distribution of Cu with Cl,S,P,Cl+S,S+P,Cl+S,Cl+P+S during sludge co-incineration:(a)SiO2+CaO+Al2O3+Cu;(b)SiO2+CaO+Al2O3+Cu+0.3%Cl;(c)SiO2+CaO+Al2O3+Cu+3%S;(d)SiO2+CaO+Al2O3+Cu+3%P;(e)SiO2+CaO+Al2O3+Cu+3%S+0.3%Cl;(f)SiO2+CaO+Al2O3+Cu+3%P+0.3%Cl;(g)SiO2+CaO+Al2O3+Cu+3%P+3%S;(h)SiO2+CaO+Al2O3+Cu+3%P+0.3%Cl+3%S.
4.Conclusions
(1)From the complex sludge co-incineration system,Cl,S,and P were emitted mainly in the forms of HCl(g),SO2(g)and(P2O5)2(g)under the oxidizing atmosphere and HCl(g),H2S(g)and(P2O3)2(g)under the reducing atmosphere,respectively.With the coupled Cl,S,and P,no competing reactions between the gases were observed.
(2)With the single minerals,Cl wreaked the reaction of Cu with Al2O3,CaO,and Fe2O3,while S delayed the reaction of Cu with Fe2O3and Al2O3,with P having no effect.
(3)With the coupled Cl+S+P system,the reactions between the single minerals and Cu were affected by both Cl and S in response to the couplings of Cl+S,and Cl+S+P which in turn inhibited Cu volatilization.The reactions between the single minerals and Cu were affected only by Cl in response to the coupling of Cl+P which in turn enhanced Cu volatilization.The reactions between the single minerals and Cu were affected only by S in response to the coupling of S+P.
(4)With the coupled minerals(SiO2+CaO+Al2O3),Cl or Cl and P rendered the reactions between the coupled minerals and Cu invalid.The systems of S,S+Cl,and Cl+S+P enabled the migration and conversion behaviors of Cu to be controlled by S at low temperatures and by Cl at high temperatures.Across the entire temperature range of the sludge co-incineration,P had no effect on the reactions between the coupled minerals and Cu.
Acknowledgments
We are grateful to Jun Lüforhis enthusiastic help with the numerical simulations.
[1]L.Huang,J.Liu,Y.He,S.Sun,Thermodynamics and kinetics parameters of cocombustion between sewage sludge and water hyacinth in CO2/O2atmosphere as biomass to solid biofuel,Bioresource.Technol.218(2016)631-642.
[2]G.Yang,G.Zhang,H.Wang,Current state of sludge production,management,treatment and disposal in China,Water Res.78(2015)60-73.
[3]Z.Pavlík,J.Fořt,M.Záleská,M.Pavlíkováa,A.Trníka,Energy-efficient thermal treatment of sewage sludge for its application in blended cements,J.Clean.Prod.112(2016)409-419.
[4]X.Zhao,G.Jiang,A.Li,Y.Li,Technology,cost,a performance of waste-to-energy incineration industry in China,Renew.Sust.Energ.Rev.55(2016)115-130.
[5]F.Ding,Z.He,S.Liu,S.Zhang,F.Zhao,Q.Li,Heavy metals in composts of China:historical changes,regional variation,and potential impact on soil quality,Environ.Sci.Pollut.Res.24(3)(2017)3194-3209.
[6]G.Yaylalı-Abanuz,Heavy metal contamination of surface soil around Gebze industrial area,Turkey,Microchem.J.99(1)(2011)82-92.
[7]J.Amneklev,A.Augustsson,L.Sörme,B.Bergbäck,Monitoring urban copper flows in Stockholm,Sweden:implications of changes over time,J.Ind.Ecol.21(4)(2016)903-912.
[8]G.Guo,T.Chen,J.Yang,G.Zheng,D.Gao,Regional distribution characteristics and change trend of heavy metals in municipal sewage sludge in China,Acta Sci.Circum.34(10)(2014)2455-2461.
[9]Q.Zhang,H.Liu,P.Liu,H.Hu,H.Yao,Pyrolysis characteristics and kinetic analysis of different dewatered sludge,Bioresour.Technol.170(2014)325-330.
[10]R.Li,Z.Zhang,Y.Li,W.Teng,W.Wang,T.Yang,Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge,Chemosphere141(2015)57-61.
[11]H.Zhou,J.Sun,A.Meng,Q.Li,Y.Zhang,Effects of sorbents on the partitioning and speciation of Cu during municipal solid waste incineration,Chin.J.Chem.Eng.22(11-12)(2014)1347-1351.
[12]J.Luan,R.Li,Z.Zhang,Y.Li,Y.Zhao,Influence of chlorine,sulfur and phosphorus on the volatilization behavior of heavy metals during sewage sludge thermal treatment,Waste Manag.Res.31(10)(2013)1012-1018.
[13]M.Roy,A.Dutta,K.Corscadden,P.Havard,L.Dickie,Review of biosolids management options and co-incineration of a biosolid-derived fuel,Waste Manag.31(11)(2011)2228-2235.
[14]T.Peng,C.Lin,M.Wey,Study of the low-temperature two-stage fluidized bed incineration:influence of the second-stage sand bed operating conditions on pollutant emission,Appl.Therm.Eng.75(2015)592-599.
[15]J.Liu,J.Fu,X.Ning,S.Sun,Y.Wang,W.Xie,S.Huang,S.Zhong,An experimental and thermodynamic equilibrium investigation of the Pb,Zn,Cr,Cu,Mn and Ni partitioning during sewage sludge incineration,J.Environ.Sci.35(2015)43-54.
[16]J.Liu,S.Huang,S.Sun,X.Ning,R.He,X.Li,T.Chen,G.Luo,W.Xie,Y.Wang,Z.Zhuo,J.Fu,Effects of sulfur on lead partitioning during sludge incineration based on experiments and thermodynamic calculations,Waste Manag.38(2015)336-348.
[17]G.Fraissler,M.Joller,H.Mattenberger,T.Brunner,I.Obernberger,Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination,Chem.Eng.Process.Process Intens.48(1)(2009)152-164.
[18]N.Saqib,M.Bäckström,Trace element partitioning in ashes from boilers firing pure wood or mixtures of solid waste with respect to fuel composition,chlorine content and temperature,Waste Manag.34(12)(2014)2505-2519.
[19]D.Verhulst,A.Buekens,P.Spencer,G.Eriksson,Thermodynamic behavior of metal chlorides and sulfates under the conditions of incineration furnaces,Environ.Sci.Technol.30(1)(1995)50-56.
[20]J.Aubert,B.Husson,N.Sarramone,Utilization of municipal solid waste incineration(MSWI) fly ash in blended cement:part 1:processing and characterization of MSWI fly ash,J.Hazard.Mater.136(3)(2006)624-631.
[21]P.Ndiba,L.Axe,T.Boonfueng,Heavy metal immobilization through phosphate and thermal treatment of dredged sediments,Environ.Sci.Technol.42(3)(2008)920-926.
[22]Z.X.Tan,H.B.Li,X.L.Wang,X.G.Jiang,J.H.Yan,Species transformation of trace elements and their distribution prediction in dyestuff residue incineration,Chin.J.Chem.Eng.15(2)(2007)268-275.
[23]H.Zhou,J.Sun,A.H.Meng,Q.H.Li,Y.G.Zhang,Effects of sorbents on the partitioning and speciation of Cu during municipal solid waste incineration,Chin.J.Chem.Eng.22(11-12)(2014)1347-1351.
[24]L.S.Sun,S.Abanades,J.D.Lu,G.Flamant,D.Gauthier,Volatilization of heavy metals during incineration of municipal solid wastes,J.Environ.Sci.16(4)(2004)635-639.
[25]J.Chen,M.Wey,Y.Lin,The adsorption of heavy metals by different sorbents under various incineration condition,Chemosphere37(13)(1998)2617-2625.
[26]J.Kuo,C.Lin,M.Wey,Effect of particle agglomeration on heavy metals adsorption by Al-and Ca-based sorbents during fluidized bed incineration,Fuel Process.Technol.92(10)(2011)2089-2098.
[27]M.Lin,X.Ning,X.Liang,P.Wei,Y.Wang,J.Liu,Study of the heavy metals residual in the incineration slag of textile dyeing sludge,J.Taiwan.Inst.Chem.Eng.45(4)(2014)1814-1820.
[28]J.Soria,D.Gauthier,G.Flamant,R.Rodriguez,G.Mazza,Coupling scales for modeling heavy metal vaporization from municipal solid waste incineration in a fluid bed by CFD,Waste Manag.43(2015)176-187.
[29]W.P.Linak,J.O.L.Wendt,Toxic metal emissions from incineration:mechanisms and control,Prog.Energy Combust.Sci.19(2)(1993)145-185.
[30]I.Halonen,J.Tarhanen,P.Ruokojärvi,K.Tuppurainen,J.Ruuskanen,Effect of catalysts and chlorine source on the formation of organic chlorinated compounds,Chemosphere30(7)(1995)1261-1273.
[31]J.Y.Ryu,J.A.Mulholland,M.Takeuchi,D.H.Kim,T.Hatanaka,CuCl2catalyzed PCDD/F formation and congener patterns from phenols,Chemosphere61(9)(2005)1312-1326.
[32]J.Liu,S.Sun,Y.Xu,M.Xie,T.Chen,M.Chen,Heavy metal characteristics in sewage sludge and its potential ecological risk assessment for agriculture use in Guangzhou,Acta Sci.Circum.29(12)(2009)2545-2556.
[33]J.Y.Liu,S.Y.Sun,Comprehensive evaluation of municipal sewage sludge combustion characteristics and its combustion kinetics model in Guangzhou,Acta Sci.Circum.32(8)(2012)1952-1961.
[34]J.Y.Liu,J.W.Fu,X.E.Dang,J.Sun,S.Y.Sun,J.H.Kuo,Y.J.Wang,Thermal conversion of conditioner and its effect on the volatilization of heavy metals during the dewatering sludge incineration,Acta Sci.Circum.36(7)(2016)2540-2556.
[35]J.Y.Liu,L.M.Huang,J.C.Chen,W.M.Xie,X.E.Dang,J.H.Kuo,J.Sun,K.L.Chang,S.Y.Sun,Effects of the interactions of chlorine,sulfur,phosphorus and minerals during sewage sludge co-incineration on the migration and transformation of Cd,Acta Sci.Circum.36(12)(2016)4407-4420.
[36]Y.Zhang,Y.Chen,A.Meng,Q.Li,H.Cheng,Experimental and thermodynamic investigation on transfer of cadmium influenced by sulfur and chlorine during municipal solid waste(MSW)incineration,J.Hazard.Mater.153(1-2)(2008)309-319.
[37]T.Ho,H.Lee,H.Chu,J.Hopper,W.Bostick,Metal capture by sorbents during fluidized-bed combustion,Fuel Process.Technol.39(1-3)(1994)373-388.
[38]S.Vassilev,C.Braekman-Danheux,P.Laurent,T.Thiemann,A.Fontana,Behavior,capture and inertization of some trace elements during combustion of refusederived char from municipal solid waste,Fuel78(10)(1999)1131-1145.
[39]G.Fraissler,M.Jöller,H.Mattenberger,T.Brunner,I.Obernberger,Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination,Chem.Eng.Process.48(1)(2009)152-164.
[40]Y.Li,R.Li,H.Zhang,L.Wang,X.Ke,Kinetics study and influence of Ca/Si on volatilization of CuCl2in thermal process,Int.Conf.Energy Environ.Technol.3(2009)380-384.
[41]S.Durlak,P.Biswas,J.Shi,Equilibrium analysis of the effect of temperature,moisture and sodium content on heavy metal emissions from municipal solid waste incinerators,J.Hazard.Mater.56(1-2)(1997)1-20.
[42]M.Uberoi,W.Punjak,F.Shadman,Kinetics and mechanism of alkali removal from flue gas by solid sorbents,Prog.Energy Combust.Sci.16(1990)205-211.
[43]T.Yang,X.Kai,Y.Sun,Y.He,R.Li,The effect of coal sulfur on the behavior of alkali metals during co- firing biomass and coal,Fuel90(7)(2011)2454-2460.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
