Osmotic concentration of succinic acid by forward osmosis:Influence of feed solution pH and evaluation of seawater as draw solution
Jeng Yih Law ,Abdul Wahab Mohammad *
1 Department of Chemical and Process Engineering,Faculty of Engineering and Built Environment,Universiti Kebangsaan Malaysia,43600 UKM,Bangi,Selangor,Malaysia
2 Section of Chemical Engineering Technology,Universiti Kuala Lumpur,Malaysian Institute of Chemical&Bioengineering Technology,Lot 1988,Kawasan Perindustrian Bandar Vendor,Taboh Naning,78000 Alor Gajah,Melaka,Malaysia
1.Introduction
Succinic acid,also known as but anedioic acid,is one of the most important commodities which serve as precursor or starting material of many industrially valuable chemicals in food,chemical,and pharmaceutical industries[1,2].Succinic acid has a great potential in the production of biodegradable polymers.For example,it can be used as a monomer to produce polybutylene succinate(PBS)which has similar physical properties as polyethylene terephthalate(PET)[3].Being recognized as one of the most promising platform chemicals,the global market demand of succinic acid is expected to strongly increase to an estimated market size of>700000 tons per year by 2020[3,4].
Succinic acid can be producedviatwo different pathways:petrochemical pathway through chemical process and biological pathway through fermentation process[2,5].At present,the production of succinic acid in industry is still dominated by petrochemical pathway using petroleum as raw material[4,6].However,due to the arising concerns of unpredictable crude oil prices,scarcity of crude oil supply and resources,costly catalysts,increasing consumer awareness on the food and pharmaceutical products,and concerns on environmental problems,alternative production pathwayviamicrobial fermentation process has generated lots of attention[4,6,7].It is worth noting that succinic acid was identified as one of the top bio-based building block chemicals in 2004 and 2010 reports[3].
The downstream processing of fermentation-based process remains challenging.In the last decade,many efforts have been made in developing efficient recovery technologies for the separation and purification of bio-based succinic acid.Example of such processes includes electrodialysis,ion-exchange chromatography,acidification,evaporation,crystallization,precipitation,esterification,pressure-driven membrane filtration,and reactive extraction[6,8-12].The major challenges in succinic acid recovery have been widely reported in a number of literatures[7,13].One of the major obstacles in the fermentative production of succinic acid is the low product concentration[9,13].Dilute broth solution produced during fermentation process has been a continuing challenge in bioprocess industries.Hence,it is desirable to explore the potential techniques for separating or eliminating water from the dilute broth solution.
An emerging membrane technology namely forward osmosis(FO)has been considered for the enrichment or concentration of broth solution.FO utilizes osmotic pressure gradient as the driving force to drive the migration of water molecule from the feed solution towards the draw solution(DS)across a semi-permeable membrane.DS is referred to as the higher osmotic pressure solution compared to the feed solution,and is used to induce the permeation of water molecule in FO process[14].FO technology offers prominent advantages compared to the existing concentration and dewatering methods.Unlike thermal-driven and pressure-driven processes such as evaporation,distillation,and reverse osmosis(RO),FO has very low energy requirement,low membrane fouling propensity,and is harmless to thermal-sensitive products[15-18].These apparent benefits have attracted much attention on the potential use of FO technology.Among the potential applications of FO include seawater or brackish water desalination[19-21],wastewater treatment[22-24],pharmaceutical enrichment[15],power generation[25],and bioproducts enrichment[16,26].To date,there is still very little published data on FO concentration of organic acids.Choet al.[16]demonstrated the potential application of FO technology in the downstream processing of fermentation-based process.FO was integrated as the final stage unit to concentrate the butyric acid solution.More recently,Abousnina and Nghiem[27]investigated the removal of acetic acid from produced water,and observed that feed solution pH was a key factor affecting acetate rejection.
In general,pH neutralization is required during fermentation for controlling the pH of fermentation broth,and consequently,the production of succinic acid are found in salt form rather than the free succinic acid[9,28].The downstream process for succinic acid production often includes acidification by an addition of H2SO4or HCl for the conversion of succinate salt into a free acid form[12,28].We note that the integration of FO unit can be performed in two different routes attributed to the process sequence variation concerning FO and acidification.When acidification is performed at the earlier stage of recovery process thereby releasing free succinic acid,the feed to the FO unit is therefore in the undissociated free acid form.On the contrary,when FO process is performed prior to acidification,the feed to the FO unit shall be in salt form.In view of that,feed solution pH plays an important role in selecting the appropriate integration route.
A variety of phenomena need to be thoroughly explored and understood before osmotically driven FO process can achieve broad acceptance.Hence,the goal of this study is to systematically investigate the influence of feed solution pH so as to gain insights into the transport mechanisms of succinic acid concentration by FO process.The water flux and bidirectional transport of succinate and chloride anions were quantified to facilitate the understanding of the transport mechanisms involving dicarboxylic acid.Furthermore,a direct application of real sea water as DS was evaluated at increasing feed solution osmotic pressure.The readily available seawater source opens possibilities for direct use and discharge of a DS.
2.Methodology
2.1.FO membrane and chemicals
The asymmetric membrane(CTA-ES)was supplied by Hydration Technology Innovations(HTI,Albany,OR,USA)as flat sheets.It is made of cellulose triacetate polymer layer with embedded polyester support screen.This commercial CTA membrane consists of a dense active layer and is specially designed for osmotically driven processes.The thickness of the membrane is less than 50 μm[14].The contact angle of the active layer was 62.8°± 3.9°,which is moderately hydrophilic[29].The hydrophilic nature of CTA ensures proper wetting of membrane and thus reducing internal concentration polarization(ICP)and increasing the water flux in FO process[30].The mean effective membrane pore radius was estimated to be 0.37 nm(equivalent to the mean effective membrane pore size of 0.74 nm)[29].
Succinic acid supplied by Acros Organics(New Jersey,USA)was used to prepare the feed solution for FO process.The pH of feed solution was adjusted to the intended value using sodium hydroxide(NaOH,R&M Chemicals,Malaysia)or hydrochloric acid(HCl,R&M Chemicals,Malaysia).Succinic acid of 20 g·L-1was employed,unless otherwise specified.NaCl(Merck,Darmstadt,Germany)and seawater were used as the DS in this study.All solutions employed in the study were prepared by dissolving the solutes in ultrapure(UP)water obtained from lab water system(arium®pro ultrapure water systems,Sartorius,Germany).For seawater experiments,the feed containing UP water or succinic acid ranging from 10 to 40 g·L-1were used.The pH of succinic acid was adjusted to 6.90.Seawater as DS was collected directly from Pantai Bagan Lalang beach(Sepang,Selangor,Malaysia)along the Strait of Malacca.The seawater was left for at least48 h for the precipitation of large insoluble solutes(i.e.sand)prior to use.The seawater was used as DS after being pre- filtered by vacuum filtration(Whatman cellulose nitrate membrane filters,plain,sterile,0.45μm pore size,47 mmcircle).
2.2.Bench-scale FO experimental set-up
A bench-scale FO experimental setup was employed in the study.Unlike other membranes separation process which can either be deadend or cross flow filtration,FO process is typically operated in cross flow mode.A FO cell(Sterlitech CF042 Cell)with channel outer dimensions measuring 12.7 cm×10 cm×8.3 cm and effective membrane area of 42 cm2was utilized.
The commercial CTA membrane was placed in the center of FO cell separating feed and draw channels.The orientation of the membrane is arranged in FO mode(AL-FS orientation)in which active layer(dense selective layer)of the membrane facing the feed channel.The complete FO cell was clamped together by four bolts.Two peristaltic pumps equipped with speed controller(BT600-2J,LongerPump,China)were used to circulate the feed and DS on each side of the membrane channel in closed loop counter-current mode.The initial volumes of feed and DS were 1 L and 0.6 L,respectively,unless otherwise indicated.All experimental tests were performed at room temperature(25°C±1°C).To mitigate the impact of dilution of the DS,the duration of each test was set to 2.5 h[31].In order to measure the variation of solution mass,the DS tank was rested on a digital mass balance(GF-6100,A&D Company Limited,Japan).
2.3.Analytical methods
A Thermo Scientific HPLC system(Dionex UltiMate 3000,Thermo Scientific,USA)equipped with a solvent rack(SR-3000),HPLC pumps(UltiMate 3000 Series),autosamplers(WPS-3000),column compartment(TCC-3000),and a RI detector(RefractoMax521)was used to analyze the concentration of succinic acid(succinate ion).The operation of HPLC system was performed by Chromeleon Console software.Rezex ROA column(300 mm×7.8 mm;Phenomenex,USA)which is specially used to analyze organic acid was employed in the study.The temperature of column oven was maintained at 60°C.The sulfuric acid mobile phase(0.0025 mol⋅L-1)was maintained at the flow rate of 0.6 ml·min-1.The concentration of inorganic chloride anion was quantified using ion chromatography(858 Professional Sample Processor,882 Compact IC Plus,Metrohm,Switzerland).All samples were filtered through a 0.22-μm membrane syringe filter prior to HPLC and IC analysis.For seawater DS,the increasing ion concentration in the UP water feed was measured using conductivity meter(Mi306,Martini,Romania).Analytical results were used to investigate the behavior of forward solute transport and reverse solute leakage in FO process.
2.4.Measurements and calculations
In the osmotic-driven FO process,mass transfer can be found in two directions.The migration of water molecule from the feed solution towards the DS is driven by the osmotic pressure gradient across the membrane.Reverse draw solute permeation is an undesired transport of draw solute in an opposite direction from the DS compartment joining the feed solution.Despite the water permeation,other elements in the feed solution may also diffuse through the semi-permeable membrane generating another undesired forward transport phenomenon in FO process.
The water flux(Jw)in FO process was determined by measuring the mass increment of DS and was calculated using Eq.(1).

where Δmis the mass variation of DS over a time interval of Δt,ρ is the density of water,andAis the effective membrane area.
Reverse draw solute permeation is commonly known as draw solute leakage.The reverse solute flux(Jds)can be calculated using a species mass balance equation[26]:
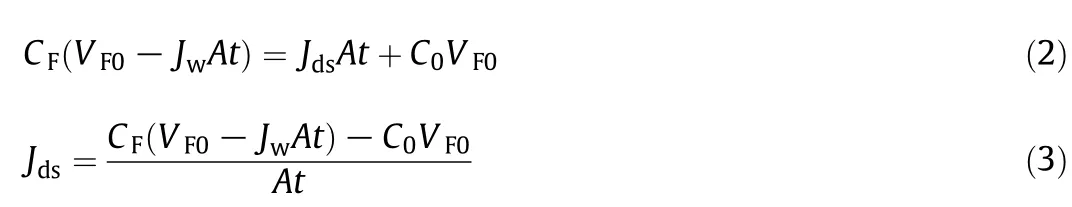
where CFis the measured draw solute concentration in the feed solution aftertexperiment duration,VF0is the initial volume of feed solution,Jwis the average water flux,Ais the effective membrane area,andC0is the initial draw solute concentration in the feed solution.
Ideally,the semi-permeable membrane rejects the transport of feed solutes but allows water migration from the feed solution to the DS compartment.In the actual FO performance,the migration of water molecule is accompanied by the forward transport of dissolved solutes.The feed solute transport during the FO process can be evaluated using the solute rejection equation.Considering the dilution effect of DS compartment,the calculated feed solute rejection is higher than the actual rejection performance.Hence,an adjustment on the final concentration of feed solute can be performed by taking into account of the dilution factor:

whereCfs(t)is the corrected final concentration of feed solute in the DS compartment,Cfs(DC)is the measured final concentration of feed solute in the DS compartment,VDS(t)is the final volume of the DS,andVPis the permeate volume of water to the DS.Subsequently,the feed solute rejection(RFO)was calculated using Eq.(5)as below:

whereCfs(o)is the initial feed solute concentration in the feed solution compartment.
3.Results and Discussion
3.1.Osmotic pressure gradient and water permeability
To investigate the water permeability in FO process,the osmotic pressure of both feed and DS must be considered,since both have significant impacts on FO performance.The osmotic pressures with respect to solution concentration were simulated using OLI Stream Analyzer 9.2.2(OLI Systems Inc.,Morris Plains,NJ,USA).The concentration of biobased succinic acid as reported in majority literatures was not higher than 35 g·L-1[1,13].Fig.1(a)shows the osmotic pressure of succinic acid and sodium succinate at different concentrations.Succinic acid reacts with NaOH by donating two protons to form disodium succinate(also known as sodium succinate).Since sodium succinate is not available from the software database,the osmotic pressure of sodium succinate was calculated using Van't Hoffequation.According to the figure,linear relationships were obtained between the osmotic pressure and the solution concentration.Interestingly,sodium succinate exhibited greater osmotic pressure compared to the succinic acid solution.Based on Van't Hoff equation,the Van't Hoff factor is a dimensionless constant related to the degree of dissociation of the solute.The predicted Van't Hoff factor for sodium succinate is 3 as it dissociates into three ions.It is clear that the ionic form of sodium succinate solution will induce higher osmotic pressure.The net driving force of FO which is defined as osmotic pressure gradient(Δπ)between the feed solution and NaCl DS is presented in Fig.1(b).In all cases,higher osmotic pressure gradients were observed for succinic acid compared to sodium succinate solution.The variations of osmotic pressure gradient at elevating feed concentrations were not significant for 3 mol·L-1and 5 mol·L-1NaCl DS even though a gradual decrease of osmotic pressure gradients were observed.However,this is not the case for 1 mol·L-1NaCl DS which generated 47.87-atm osmotic pressure.Considering the influence of concentration polarization phenomenon externally in the bulk solutions and internally inside the asymmetric FO membrane,the effective osmotic pressure gradient was generally lower than estimated[14,32].
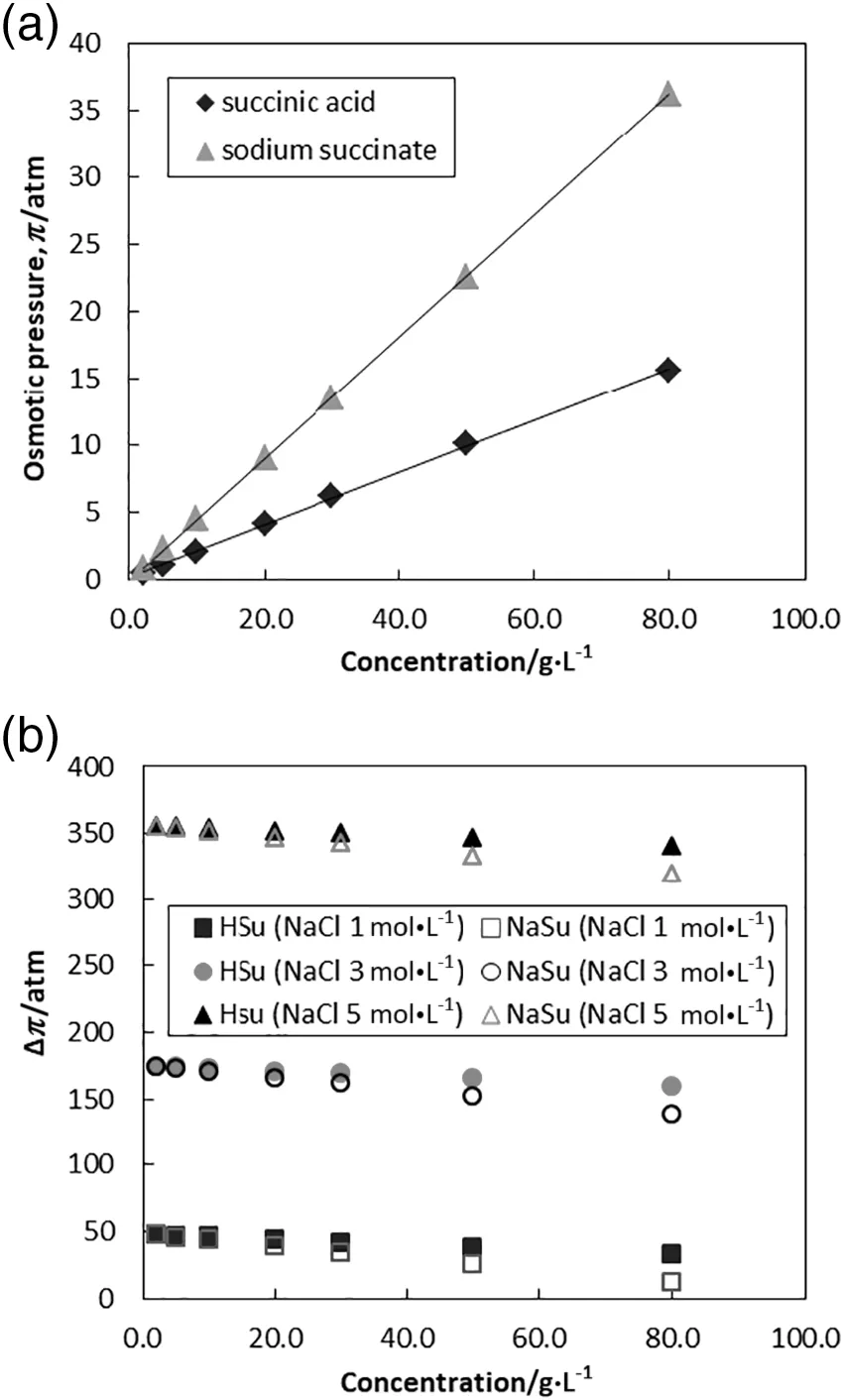
Fig.1.(a)Variation of osmotic pressure of succinic acid and sodium succinate solutions at various concentration.Calculation made at ambient temperature(25°C).(b)Osmotic pressure gradient(Δπ)as a function of succinic acid(HSu)and sodium succinate(NaSu)concentration at different NaCl concentrations.(1 atm=101325Pa).
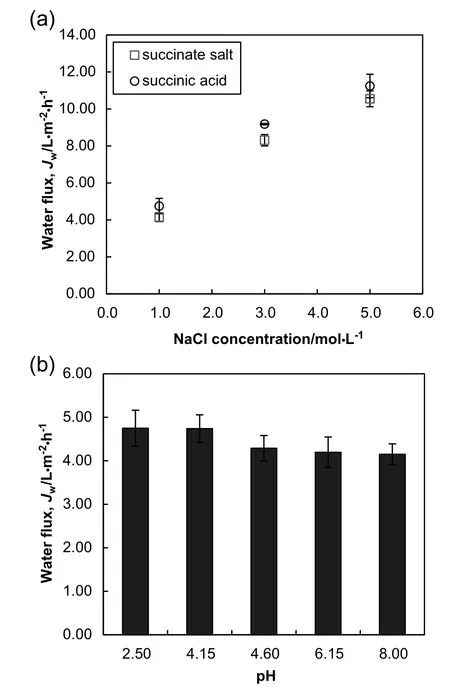
Fig.2.(a)Water flux performance of succinate salt and succinic acid feed solutions at increasing NaCl DS concentration.(b)Water flux performance using 1 mol·L-1 NaCl DS at various feed solution pH.
The performance of water permeability was evaluated in water flux.Succinic acid feed solution at pH 8.00 was termed succinate salt in this context.Fig.2(a)shows the experimental water flux of succinate salt(pH 8.00)and succinic acid(pH 2.50)feed solutions at different NaCl DS concentrations.According to the figure,notably higher water fluxes were attained at higher NaCl concentrations.Comparing the succinate salt and succinic acid feed solutions,water flux differences of no greater than 1 L·m-2·h-1have been observed.Since succinate salt generated relatively higher osmotic pressure than succinic acid,lower water flux was shown by succinate saltfeed solution.This was due to the relatively lower osmotic pressure gradient across the membrane as shown in Fig.1(b).It was found that higher DS concentrations(i.e.5 mol·L-1)exhibited less flux improvements than expected.In the AL-FS orientation,dilutive internal concentration polarization(ICP)occurred in the porous support layer and reduced the effective osmotic pressure gradient across the membrane[33-35].The ICP effect became increasingly dominant at high DS concentration,resulting in non-linear flux performance.Fig.2(b)presents the average water flux atvarious feed solution pH.Results revealed that increasing the pH of feed solution gradually decreased water flux across the membrane.The osmotic pressure of succinic acid was enhanced by the increase of ionization and degree of dissociation at higher pH value resulting in reduced water flux performance.Due to the fact that the pH control during fermentation was within a pH range of3.0-7.6,with neutral pH being the most commonly used by bio-based succinic acid producers,the pH selection in this work was not extended to beyond 8.0[4].
3.2.Succinate rejection and forward transport behavior
Fig.3.(a)Succinate rejection and forward succinate transport as a function of feed solution pH.(b)Succinate rejection of succinate salt feed solution(pH 8.00)as a function of NaCl concentration.
Forward transport of succinate feed solute in FO process is highly undesirable,as it can lead to the loss of succinate solute,resulting in a decrease of the final succinic acid yield.In view of that,it is important to examine the succinate rejection performance during the FO process.Fig.3(a)shows the effect of pH on the succinate rejection and forward succinate transport.Forward succinate transport was calculated using Eq.(4).High succinate rejection indicates low transport of succinate solute from feed to DS andvice versa.It was found that the succinate rejection increased as the feed solution pH increased indicating lower succinate leakage to the DS compartment.Likewise,the improvement of succinate rejection was shown by the decrease of forward succinate transport.This transport phenomenon can be explained by the dissociation equilibria of succinic acid and the rejection mechanism of CTA FO membrane.Unlike the carboxylic acids that contain only one carboxyl functional group(i.e.butyric acid,acetic acid),succinic acid is a dicarboxylic acid with pKa1and pKa2of 4.21 and 5.64,respectively.The two steps dissociation equilibria of succinic acid are represented by Eqs.(6)and(7).

At low pH condition of typically less than its pKa1value,the free acid(C2H4C2O4H2)or undissociated form of succinic acid molecules could penetrate the active layer of CTA membrane.As can be seen,13.74 g·L-1of succinate transport corresponding to 31.3%of succinate rejection was found at pH 2.50.The free acid molecules behaved like uncharged molecules and hence the rejection of succinate solute was mainly governed by molecular size sieving mechanism[36].As the feed solution pH increased to 4.15(slightly below its pKa1value),the succinate transport decreased considerably to 6.38 g·L-1corresponding to 66.1%succinate rejection.This result can be explained by two rejection mechanisms:size exclusion and electrostatic repulsion.The first step dissociation of succinic acid[Eq.(6)]gradually increased the ionic form of succinic acid and thus,resulting in a mixture of monovalent succinate anion(C2H4C2O4Hˉ)and undissociated succinic acid molecule in the feed solution[28].Subsequently,stronger rejection performance was promoted by the larger effective molecular size of hydrated succinate anion compared to that of succinic acid through size exclusion[37].Apart from that,monovalent succinate anion was also rejected by the negatively charged membrane surface layer.Generally,the CTA membrane which contains acetyl and hydroxyl functional groups was presumed non-ionogenic due to the conversion of polymer functional groups to esters during the formation process[38,39].The negative zeta potential of CTA membrane across a wide range of pHs(isoelectric point~4)is likely to be related to the adsorption behavior of hydroxide ions on the membrane surface[38,40,41].
Further improvementon the succinate transport(3.94 g·L-1)with a rejection of 78.5%was obtained at the pH of 4.60,which fell in between the pKa1and pKa2values.The feed solution at this stage contained primarily monovalent succinate anion with minimal free acid form of succinic acid,and hence,greater rejection was observed at this pH.Interestingly,when the pH of the feed solution was adjusted to greater than its pKa2value,strong succinate rejection(>99.0%)was achieved indicating a negligible amount of succinate loss.According to Eq.(7),the second step succinic acid dissociation is the deprotonation of monovalent succinate anion producing divalent anion(C2H4C2of bi-succinate salts.Hence,divalent succinate anion existed predominantly in the feed solutions at pH 6.15 and 8.00.At pH above 8.00,100%succinic acid was in its ionic form[28].Stronger rejection of succinate was promoted by the larger-sized hydrated succinate anions dominating the feed solution.In addition,the divalent succinate anions also contributed to the stronger electrostatic repulsion mechanism and as a consequence,dramatically reduced the forward solute transport across the FO membrane.This interesting observation was consistent with the results previously reported by Xueet al.[31]who examined the nutrient retentions by CTA membrane.They reported further strengthening of phosphate rejection under the speciation transformation from H2PO4ˉtowhich was attributed to the electrostatic repulsion between the negatively charged ions and the membrane.Similar rejection mechanism had also been reported in some nanofiltration processes[42,43].Based on the observation in this work,it can be concluded that the rejection of succinate by CTA membrane at varying feed solution pH increased in the following order of chemical form:C2H4C2O4H2<C2H4C2O4Hˉ<C2H4C2.
The solute rejection performance was further investigated at increasing osmotic pressure driving force.The effect of DS concentration on the rejection of succinate is depicted in Fig.3(b).The results revealed that there was a slight decrease in the succinate rejection at higher NaCl concentration.At high pH condition(pH>>pKa2),rejections of greater than 98.9%were obtained for all selected NaCl concentrations.It is interesting to note that while water flux substantially increased as the NaCl concentration increased from 1 to 5 mol·L-1[Fig.2(a)],the CTA membrane could effectively retain the divalent succinate anions to a high degree.
3.3.Reverse solute flux
Reverse solute flux(Jds)in this work is the quantitative measurement of chloride anion(Cl-)leakage in the opposite direction to the feed compartment.To maintain solution electroneutrality,coupled sodium cation(Na+)was also transported from DS to feed side.Fig.4(a)presents the reverse chloride flux at different feed solution pH.At 1 mol·L-1NaCl DS,a gradual increase of reverse chloride flux was observed.Chloride fluxes ranging from 1.938 to 2.994 g·m2·h-1were obtained over the entire pH range.To illustrate further,the reverse permeation was examined at different NaCl concentrations as presented in Fig.4(b).For both feed solutions(pH 2.50 and 8.00),relatively higher chloride fluxes were found at increasing NaCl concentration.The results are in good agreement with those of previous studies[44,45].The permeation of draw solute in FO process can be closely related to the solute diffusion behavior as described in Fick's law[45].The increment of NaCl initial concentration from 1 mol·L-1to 3 and 5 mol·L-1resulted in an increase of concentration gradient between the feed solution and DS and thus leading to a more pronounced reverse chloride flux.
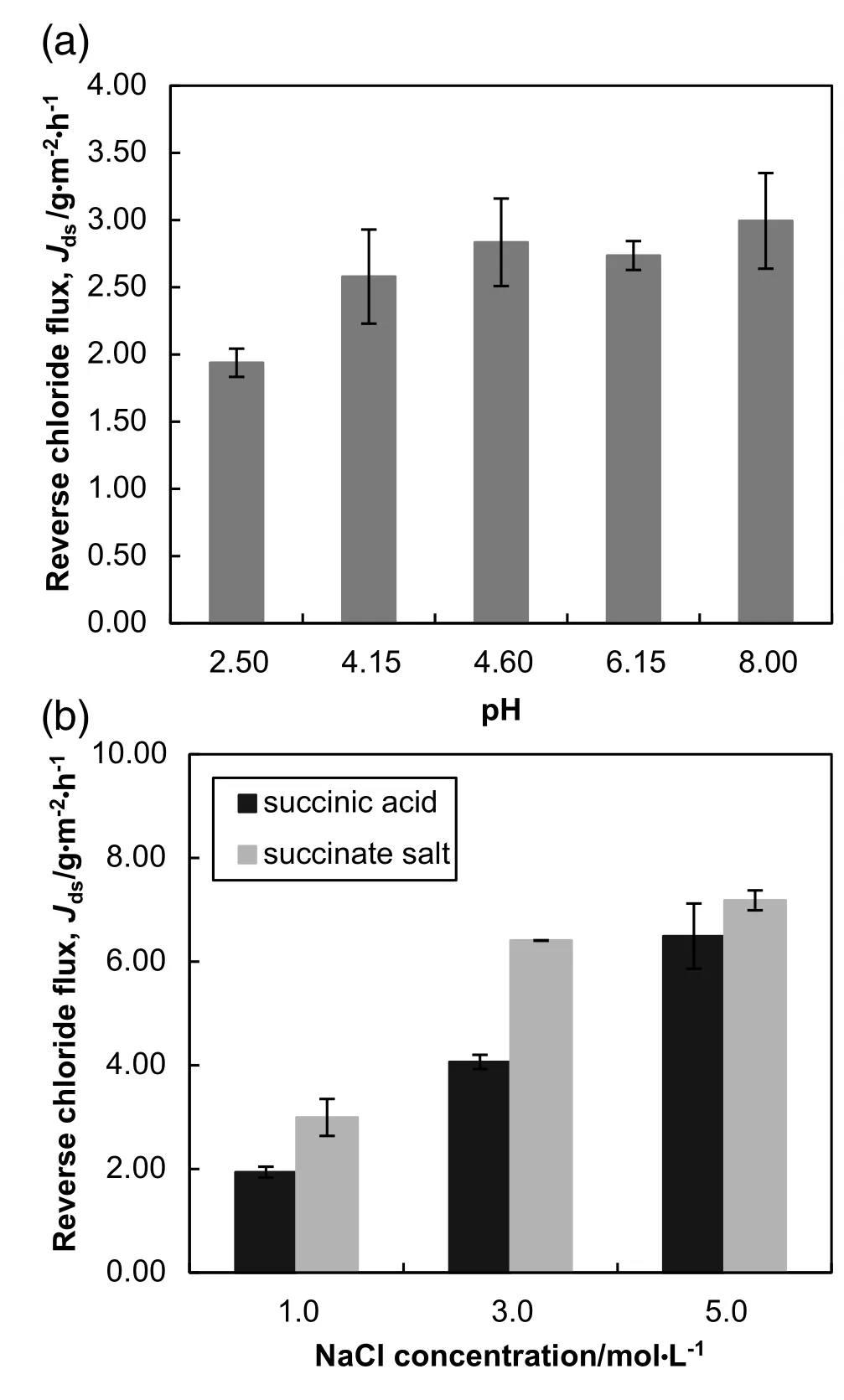
Fig.4.(a)Reverse chloride flux using 1 mol·L-1 NaCl DS at various feed solution pH.(b)Reverse chloride flux of succinate salt and succinic acid feed solutions at increasing NaCl DS concentration.
Furthermore,the results revealed that the chloride leakage was relatively higher for the succinate salt feed solution at increasing NaCl concentration[Fig.4(b)].This is attributed to the competitive transport relating to forward and opposite solute transport.At pH 2.50,the undissociated or neutral form of succinic acid dominated the feed solution and exhibited higher forward solute transport(lower succinate rejection)compared to the ionic form of succinic acid at pH 8.00.It is likely that higher forward solute transport may have hindered the reverse solute permeation since the two fluxes occur in opposite direction.Further evidence of this is shown in the results from the comparison between Figs.3(a)and 4(a).The competitive nature of simultaneous forward and reverse diffusion of solutes in FO process had been highlighted in several literatures[29,36,46].
3.4.Evaluation of FO performance using seawater as DS
The regeneration or replenishment of diluted DS plays a substantial role in FO process.Since seawater is abundantly available in most of the countries including Malaysia,the potential of seawater as DS should be explored.This section evaluated the potential and limitation of seawater as DS.The experimental observation on the water permeability in a stepwise increase of succinic acid concentrations is depicted in Fig.5.Four different feed concentrations(10,20,30 and 40 g·L-1)were tested at pH 6.90.The initial volumes of both feed and DS were 1 L.Baseline experiment using UP water as feed solution was conducted to which experimental results can be compared.According to Fig.5(a),highest flux of 3.70 L·m-2·h-1was obtained at UP water feed due to its negligible osmotic pressure.Low to moderate water flux was induced by seawater with respect to succinic acid feed.The water flux declined with increasing succinic acid concentration as a result of increased osmotic pressure in the feed.When the feed concentration was increased to 40 g·L-1,the water flow across the membrane was zero.Apparently,the seawater DS was unable to induce sufficient osmotic pressure gradient across the membrane.Water flux as a function of time is illustrated in Fig.5(b).No discernible reduction in the water flux over the duration period was observed.This observation can be attributed to several factors,including insignificant dilution effect of DS due to low/moderate water flux,and minimal membrane fouling.
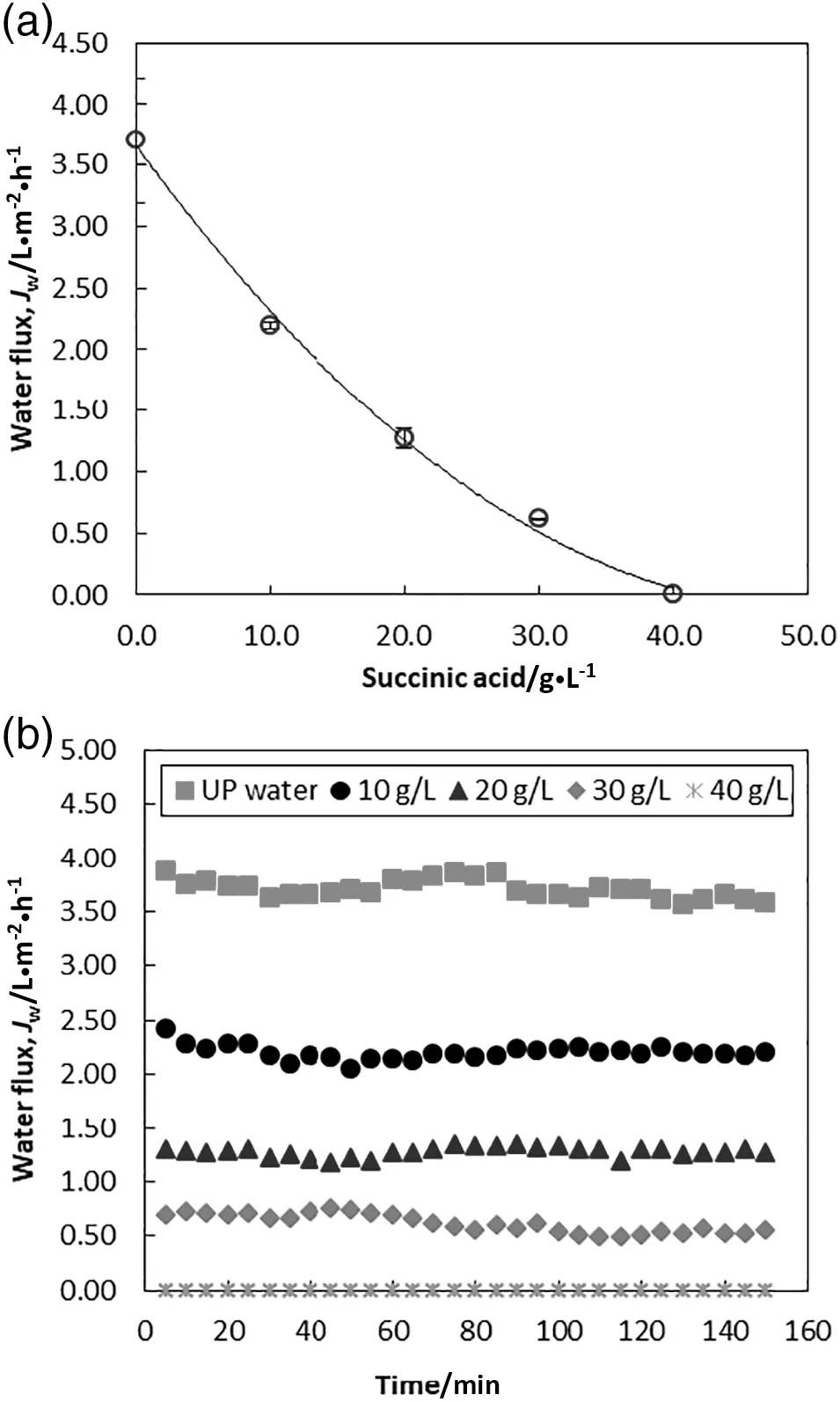
Fig.5.(a)Water flux as a function of feed concentration conducted with seawater DS.(b)Water flux profiles as a function of time at increasing feed concentrations.
The conductivity of the feed solution and the DS was recorded using conductivity meter as shown in Table 1.Typically,the conductivity of a salt solution is proportional to the concentration and hence also proportional to the osmotic pressure[47].Seawater comprises of multiple salt solutes which complicates the estimation of osmotic pressure.By relating conductivity to solution concentration,the water flux limitation can be estimated.As Table 1 shows,an increment of the conductivity from 10.40 to 35.70 mS·cm-1was obtained at increasing initial succinic acid concentration.When the feed solution conductivity was higher than ~30 mS·cm-1,the real seawater with a conductivity of~42.50 mS·cm-1was no longer capable to induce any significant water flux.This observation also indicates that the final succinic acid concentration that can be induced by seawater DS is no greater than 40 g·L-1.Since seawater containing predominantly NaCl,the concentration of the seawater can be estimated by comparing the conductivity of the seawater and the single-solute NaCl solution.According to Fig.6(a),the seawater concentration is equivalent to 0.6 mol·L-1NaCl solution,corresponding to an osmotic pressure of approximately 2.78 MPa,thus generating low medium range osmotic pressure.Nonetheless,the water flux limitation of seawater may be enhanced.Cathet al.[48]employed synthetic seawater as DS and found out that the water flux obtained from the pilot-scale experiment was approximately 20%higher than that of the flux obtained from the bench-scale experiments.Further concentration of the succinic acid solution can be achieved by higher molarity of NaCl solution or other draw solutes.

Table 1 Electrical conductivity for succinic acid(pH=6.90)and real seawater(pH=7.73).All values refer to(25 ± 1)°C
Reverse draw solute permeation of seawater was determined using UP water as the feed solution.Fig.6(b)demonstrates the variation of feed solution conductivity during a 150-min FO process.The increase in the feed solution conductivity can be explained by the reverse salt leakage from the seawater DS that gave rise to a corresponding increase in conductivity.The reverse draw solute permeation was minimal(0.12%)compared to the conductivity of seawater(Table 1).
The effect of succinic acid concentration on succinate rejection was investigated(Fig.7).It is worth noting that high succinate rejection(>97.8%)has been achieved in all cases albeit with higher feed solution concentration.Evidently,the pH of feed solution(above the pKa2of succinic acid)is the main factor governing the forward succinate transport.Superior succinate rejection performances also implied that the influence of succinic acid concentration on succinate transport was not significant.The trends in this work are consistent with the previous study[36].
4.Conclusions
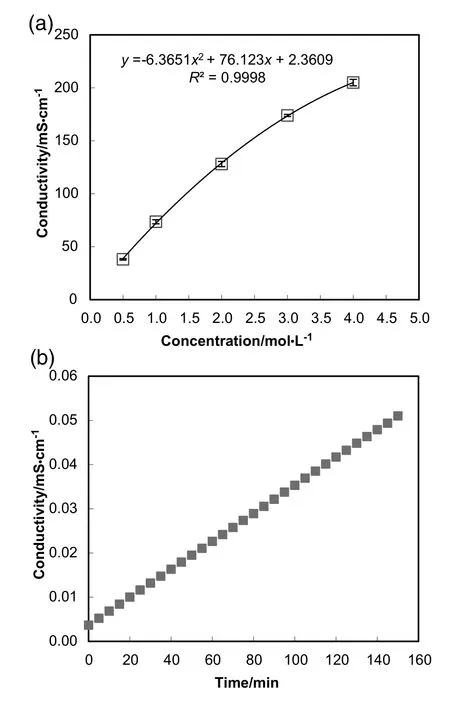
Fig.6.(a)Electrical conductivity for NaCl at varying concentration.All values refer to 25°C.(b)Electrical conductivity as a function of time for UP water feed solution.

Fig.7.Succinate rejection as a function of feed solution concentration using seawater as DS.
The present study underlines the influence of feed solution pH and evaluates the performance of seawater as DS for the concentration of succinic acid,a key ingredient in a number of industrially valuable products.With respect to the water permeability,both the succinate salt and the succinic acid feed solutions responded to the variation in NaCl DS concentration in a similar manner.The gap of water flux between these feed solutions was attributed to higher osmotic pressure shown by ionic form of succinate salt solution thereby reducing the effective driving force.The feed solution pH showed a notable impact on forward succinate transport due to changes in succinic acid speciation with respect to the pH.Strong succinate rejections at higher pH above its pKa2value(5.64)were obtained in all cases,which is noteworthy.Molecular size exclusion and electrostatic repulsion mechanisms are responsible for the enhanced rejection of succinate by CTA membrane.In the absence of electrostatic interaction,the rejection of uncharged succinic acid(<pKa1)was dominated by size exclusion.Reverse chloride flux was strengthened at higher NaCl concentration and that relatively higher chloride leakage was observed when succinate salt feed solution was employed.Evidently,the reverse chloride permeation was influenced by the competitive nature of bidirectional transport of feed and draw solutes in opposite direction.Seawater is a low-cost source of DS which eliminates the need of DS regeneration step.The real seawater with a measured conductivity of~42.50 mS·cm-1was equivalent to 0.6 mol·L-1NaCl solution,thus inducing low to moderate water flux.Seawater as DS featured low reverse draw solute permeation.The diluted seawater containing insignificant amount of organic succinate is possible to be directly discharged into the sea.Further investigation of seawater in combining with other solutes as novel DS is needed to explore its potential in FO technology.
Acknowledgments
The authors wish to gratefully acknowledge the financial support for this work provided by the LRGS/2013/UKM-UKM/PT/03 grant from the Ministry of Education Malaysia.
[1]H.Song,S.Y.Lee,Production of succinic acid by bacterial fermentation,Enzym.Microb.Technol.39(2006)352-361.
[2]H.G.Nam,C.Park,S.H.Jo,Y.W.Suh,S.Mun,Continuous separation of succinic acid and lactic acid by using a three-zone simulated moving bed process packed with Amberchrom-CG300C,Process Biochem.47(2012)2418-2426.
[3]S.Choi,C.W.Song,J.H.Shin,S.Y.Lee,Biorefineries for the production of top building block chemicals and their derivatives,Metab.Eng.28(2015)223-239.
[4]J.H.Ahn,Y.-S.Jang,S.Y.Lee,Production of succinic acid by metabolically engineered microorganisms,Curr.Opin.Biotechnol.42(2016)54-66.
[5]A.A.I.Luth fi,S.F.A.Manaf,R.M.Illias,S.Harun,A.W.Mohammad,J.M.Jahim,Biotechnological route for sustainable succinate production utilizing oil palm frond and kenaf as potential carbon sources,Appl.Microbiol.Biotechnol.101(2017)3055-3075.
[6]H.Song,Y.S.Huh,S.Y.Lee,W.H.Hong,Y.K.Hong,Recovery of succinic acid produced by fermentation of a metabolically engineeredMannheimia succiniciproducensstrain,J.Biotechnol.132(2007)445-452.
[7]A.Cukalovic,C.V.Stevens,Feasibility of production methods for succinic acid derivatives:a marriage of renewable resources and chemical technology,Biofuels Bioprod.Biorefin.2(2008)505-529.
[8]K.-K.Cheng,X.-B.Zhao,J.Zeng,R.-C.Wu,Y.-Z.Xu,D.-H.Liu,et al.,Downstream processing of biotechnological produced succinic acid,Appl.Microbiol.Biotechnol.95(2012)841-850.
[9]A.Orjuela,A.Orjuela,C.T.Lira,D.J.Miller,A novel process for recovery of fermentation-derived succinic acid:process design and economic analysis,Bioresour.Technol.139(2013)235-241.
[10]N.Khairul Zaman,J.Y.Law,P.V.Chai,R.Rohani,A.W.Mohammad,Recovery of organic acids from fermentation broth using nanofiltration technologies:a review,J.Phys.Sci.28(2017)85-109.
[11]C.Wang,Q.Li,H.Tang,D.Yan,W.Zhou,J.Xing,et al.,Membrane fouling mechanism in ultra filtration of succinic acid fermentation broth,Bioresour.Technol.116(2012)366-371.
[12]T.H.T.Nguyen,A.Boontawan,Production of very-high purity succinic acid from fermentation broth using micro filtration and nanofiltration-assisted crystallization,J.Membr.Sci.524(2017)470-481.
[13]P.A.Sosa,C.Roca,S.Velizarov,Membrane assisted recovery and purification of bio-based succinic acid for improved process sustainability,J.Membr.Sci.501(2016)236-247.
[14]T.Y.Cath,A.E.Childress,M.Elimelech,Forward osmosis:principles,applications,and recent developments,J.Membr.Sci.281(2006)70-87.
[15]C.A.Nayak,N.K.Rastogi,Forward osmosis for the concentration of anthocyanin fromGarcinia indicaChoisy,Sep.Purif.Technol.71(2010)144-151.
[16]Y.H.Cho,H.D.Lee,H.B.Park,Integrated membrane processes for separation and purification of organic acid from a biomass fermentation process,Ind.Eng.Chem.Res.51(2012)10207-10219.
[17]B.Mi,M.Elimelech,Organic fouling of forward osmosis membranes:fouling reversibility and cleaning without chemical reagents,J.Membr.Sci.348(2010)337-345.
[18]Y.Wang,H.Yu,R.Xie,K.Zhao,X.Ju,W.Wang,et al.,An easily recoverable thermosensitive polyelectrolyte as draw agent for forward osmosis process,Chin.J.Chem.Eng.24(2016)86-93.
[19]D.Zhao,S.Chen,C.X.Guo,Q.Zhao,X.Lu,Multi-functional forward osmosis draw solutes for seawater desalination,Chin.J.Chem.Eng.24(2016)23-30.
[20]J.Y.Law,A.W.Mohammad,Employing forward osmosis technology through hybrid system configurations for the production of potable/pure water:a review,J.Teknol.79(2017)125-135.
[21]D.Emadzadeh,M.Ghanbari,W.J.Lau,M.Rahbari-Sisakht,T.Matsuura,A.F.Ismail,et al.,Solvothermal synthesis of nanoporous TiO2:the impact on thin- film composite membranes for engineered osmosis application,Nanotechnology27(2016)345702(11pp).
[22]P.Zhao,B.Gao,Q.Yue,P.Liu,H.K.Shon,Fatty acid fouling of forward osmosis membrane:effects of pH,calcium,membrane orientation,initial permeate flux and foulant composition,J.Environ.Sci.(China)46(2016)55-62.
[23]S.Kim,G.-W.Go,A.Jang,Study of flux decline and solute diffusion on an osmotically driven membrane process potentially applied to municipal wastewater reclamation,J.Ind.Eng.Chem.33(2016)255-261.
[24]C.S.Ong,B.Al-anzi,W.J.Lau,P.S.Goh,G.S.Lai,A.F.Ismail,et al.,Anti-fouling doubleskinned forward osmosis membrane with zwitterionic brush for oily wastewater treatment,Sci.Rep.7(2017)6904.
[25]N.Y.Yip,A.Tiraferri,W.A.Phillip,J.D.Schi,L.A.Hoover,Y.C.Kim,et al.,Thin- film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients,Environ.Sci.Technol.45(2011)4360-4369.
[26]X.Zhang,Z.Ning,D.K.Wang,J.C.Diniz da Costa,A novel ethanol dehydration process by forward osmosis,Chem.Eng.J.232(2013)397-404.
[27]R.M.Abousnina,L.D.Nghiem,Removal of dissolved organics from produced water by forward osmosis,Desalin.Water Treat.52(2014)570-579.
[28]Q.Li,D.Wang,Y.Wu,W.Li,Y.Zhang,J.Xing,etal.,One step recovery of succinic acid from fermentation broths by crystallization,Sep.Purif.Technol.72(2010)294-300.
[29]M.Xie,L.D.Nghiem,W.E.Price,M.Elimelech,Comparison of the removal of hydrophobic trace organic contaminants by forward osmosis and reverse osmosis,Water Res.46(2012)2683-2692.
[30]J.R.McCutcheon,M.Elimelech,Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes,J.Membr.Sci.318(2008)458-466.
[31]W.Xue,T.Tobino,F.Nakajima,K.Yamamoto,Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater:effect of membrane properties and feed solution chemistry,Water Res.69(2015)120-130.
[32]A.Achilli,T.Y.Cath,A.E.Childress,Selection of inorganic-based draw solutions for forward osmosis applications,J.Membr.Sci.364(2010)233-241.
[33]Y.Xu,X.Peng,C.Y.Tang,Q.S.Fu,S.Nie,Effect of draw solution concentration and operating conditions on forward osmosis and pressure retarded osmosis performance in a spiral wound module,J.Membr.Sci.348(2010)298-309.
[34]S.Zhao,L.Zou,C.Y.Tang,D.Mulcahy,Recent developments in forward osmosis:opportunities and challenges,J.Membr.Sci.396(2012)1-21.
[35]J.Y.Law,A.W.Mohammad,Multiple-solute salts as draw solution for osmotic concentration of succinate feed by forward osmosis,J.Ind.Eng.Chem.51(2017)264-270.
[36]W.Fam,S.Phuntsho,J.H.Lee,J.Cho,H.K.Shon,Boron transport through polyamidebased thin film composite forward osmosis membranes,Desalination340(2014)11-17.
[37]C.Kim,S.Lee,H.K.Shon,M.Elimelech,S.Hong,Boron transport in forward osmosis:measurements,mechanisms,and comparison with reverse osmosis,J.Membr.Sci.419-420(2012)42-48.
[38]B.D.Coday,T.Luxbacher,A.E.Childress,N.Almaraz,P.Xu,T.Y.Cath,Indirect determination of zeta potential at high ionic strength:specific application to semipermeable polymeric membranes,J.Membr.Sci.478(2015)58-64.
[39]A.Tiraferri,M.Elimelech,Direct quantification of negatively charged functional groups on membrane surfaces,J.Membr.Sci.389(2012)499-508.
[40]R.Zimmermann,U.Freudenberg,R.Schweiß,D.Küttner,C.Werner,Hydroxide and hydronium ion adsorption—a survey,Curr.Opin.Colloid Interface Sci.15(2010)196-202.
[41]A.A.Alturki,J.A.McDonald,S.J.Khan,W.E.Price,L.D.Nghiem,M.Elimelech,Removal of trace organic contaminants by the forward osmosis process,Sep.Purif.Technol.103(2013)258-266.
[42]S.H.Kang,Y.K.Chang,Removal of organic acid salts from simulated fermentation broth containing succinate by nanofiltration,J.Membr.Sci.246(2005)49-57.
[43]P.Y.Pontalier,A.Ismail,M.Ghoul,Mechanisms for the selective rejection of solutes in nanofiltration membranes,Sep.Purif.Technol.12(1997)175-181.
[44]E.Tian,C.Hu,Y.Qin,Y.Ren,X.Wang,X.Wang,et al.,A study of poly(sodium 4-styrenesulfonate)as draw solute in forward osmosis,Desalination360(2015)130-137.
[45]S.Phuntsho,S.Sahebi,T.Majeed,F.Lot fi,J.E.Kim,H.K.Shon,Assessing the major factors affecting the performances of forward osmosis and its implications on the desalination process,Chem.Eng.J.231(2013)484-496.
[46]N.T.Hancock,T.Y.Cath,Solute coupled diffusion in osmotically driven membrane processes,Environ.Sci.Technol.43(2009)6769-6775.
[47]V.Yangali-Quintanilla,Z.Li,R.Valladares,Q.Li,G.Amy,Indirect desalination of Red Sea water with forward osmosis and low pressure reverse osmosis for water reuse,Desalination280(2011)160-166.
[48]T.Y.Cath,N.T.Hancock,C.D.Lundin,C.Hoppe-Jones,J.E.Drewes,A multi-barrier osmotic dilution process for simultaneous desalination and purification of impaired water,J.Membr.Sci.362(2010)417-426.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
