Functional Tricuspid Regurgitation and Ring Annuloplasty Repair
William B. Weir, MD , Matthew A. Romano, MD and Steven F. Bolling, MD
1 Department of Cardiac Surgery, University of Michigan, Ann Arbor, MI, USA
Introduction
Functional tricuspid regurgitation (TR) is recognized as an important clinical condition for which surgical repair is being increasingly applied [1].Functional TR primarily arises from left-sided heart failure due to myocardial or valvular dysfunction, leading to right ventricular (RV) enlargement and asymmetric tricuspid annular dilation. This leads to a vicious cycle of TR, volume overload,and then to worsening TR. Untreated TR can cause signi ficant clinical symptoms, from decreased cardiac output and the development of right-sided heart failure, leading to congestive hepatopathy,ascites, peripheral edema, and excess mortality.Of importance is the development of signi ficant TR years after mitral valve (MV) surgery, with a growing body of literature supporting the notion that acceptance of TR at the time of MV surgery(especially in the presence of tricuspid annular dilation) without intervention may no longer be acceptable. Since reoperations for recurrent TR are especially high-risk procedures, more aggressive treatment of TR at the time of initial surgery may be important [2, 3]. In brief, TR is not reliably alleviated after successful MV surgery. Therefore it is important to understand the surgical anatomy of the tricuspid valve (TV), the geometric distortions that result in functional TR, and therapies for functional TR.
Anatomy of the Tricuspid Valve
The TV complex is analogous to the MV, as coordination of annular, lea flet, chordal, and papillary muscle and RV function are required for effective lea flet coaptation during systole. The relationship of these structures as seen from the surgical perspective is shown in Figure 1. The TV consists of three lea flets: anterior, posterior, and septal. The anterior and posterior tricuspid lea flets both arise from the annulus along the RV free wall. The anterior lea flet is the largest, followed by the posterior and septal lea flets, respectively. The septal lea flet arises from the tricuspid annulus directly above the interventricular septum. There are two named papillary muscles: anterior and posterior. Chordae tendinae tether the anterior and posterior lea flets to the anterior papillary muscle, and the posterior and septal lea flets to the posterior papillary muscle. There is no formal septal papillary muscle. Rather, the interventricular septum anchors chordae to the anterior and septal lea flets. In addition, there are accessory chordal attachments to the moderator band and RV free wall. These multiple and variable chordal attachments are important mediators of TR as they may impair proper lea flet coaptation in the setting of RV dysfunction and dilation [4]. The degree of TR can therefore be dynamic and directly affected by RV preload, afterload, and systolic function.
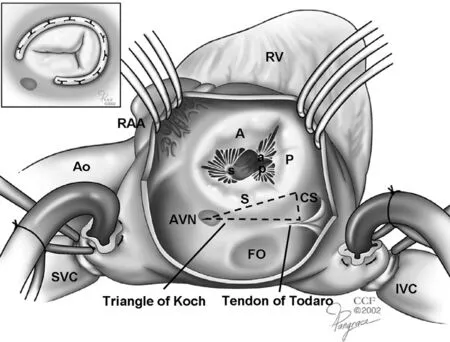
Figure 1 Tricuspid Valve Complex.
Dilation of the tricuspid annulus is the primary mechanism leading to functional TR, and should then be the main target for surgical correction with annular prostheses. Tricuspid annular enlargement occurs primarily along the RV free wall, resulting in lea flet malcoaptation [5]. Conversely, the septal aspect of the tricuspid annulus, analogous to the intertrigonal portion of the mitral annulus, is relatively spared from annular dilation [6]. As a result,tricuspid annular sizing algorithms are traditionally based on the dimension of the base of the septal lea flet [7]. It has also been shown that the tricuspid annulus diameter is dynamic and can change markedly with loading conditions. During the cardiac cycle, there is normally a 19% reduction in annular circumference with atrial systole, and this highlights the critical need to understand the relationship between tricuspid disease and anatomy [8, 9].
The tricuspid annulus has a unique three-dimensional structure, and has important implications for the design of tricuspid annuloplasty rings.Fukuda et al. [8] performed a three-dimensional transthoracic echocardiographic study to better understand this shape and the movement of the tricuspid annulus in healthy individuals and diseased patients. They examined 15 healthy individuals and 16 patients with moderate-severe functional TR. Healthy individuals had a nonplanar, elliptical tricuspid annulus, with the posteroseptal portion being “ lowest” (toward the RV apex) and the anteroseptal portion being “ highest” ( Figure 2).Patients with functional TR generally had a more planar annulus, which was dilated primarily in the septal-lateral direction, resulting in a more circular shape as compared with the elliptical “ egg” shape seen in healthy individuals. Thus the data suggest that uniquely tailored nonplanar tricuspid rings could potentially improve ventricular function and reduce lea flet stress.
Lastly, the atrioventricular (AV) node and bundle of His are important structures that exist anteriorly along the septal aspect of the annulus. This region of the annulus is a surgical “ no touch” zone, and sutures must not be placed in this region to avoid the development of AV conduction block. Open rings that do not contact the AV node are commonly used to reduce potential rhythm disturbances.
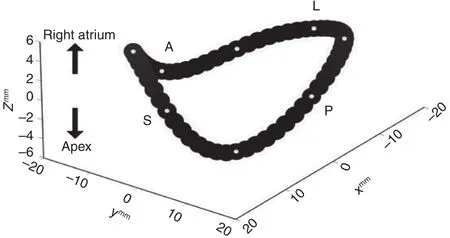
Figure 2 Three-Dimensional Shape of the Tricuspid Annulus.
Current Surgical Approaches to Tricuspid Regurgitation
As with functional MV disease, the goal of surgical correction for functional TR should be the application of a rigid (or semirigid) annular ring to reduce annular diameter with the goal of lea flet coaptation.In addition, preemptive correction of TR should be increasingly applied, as surgical treatment of a leftsided cardiac abnormality does not always result in secondary reduction of TR [1]. As such, Dreyfus et al. [10] propose that at the time of MV repair,the presence of tricuspid annular dilation ( ≥ 70 mm measured intraoperatively in a flaccid heart, equivalent to a 40 mm diameter), even in the absence of signi ficant TR, should be an indication for TV annuloplasty. Their findings also showed that TR increased by at least two grades in 45% of patients who received isolated MV repair without TV repair.This supports the notion that tricuspid dilation is an ongoing, progressive process that often warrants preemptive surgical treatment [10]. Furthermore,preoperative echocardiographic grading of TR is much clearer for those patients with mild or severe disease, but becomes much more difficult to quantify in the moderate disease category. Thus, as current guidelines suggest, any patient with greater than grade 2 TR, or a tricuspid annular diameter of 40 mm or more in any echocardiographic view,should be considered for repair of TR during any left-sided valve surgery.
Ideally the repair should include the application of a rigid (or semirigid) ring for functional TR, which has been shown to offer the most durability over time in multiple series as compared with flexible bands or plication annuloplasty techniques. Proving this, McCarthy et al. [11]reported on 790 patients from 1990 to 1999 who underwent TV annuloplasty for functional TR during concomitant surgery using four tricuspid annular approaches: Carpentier-Edwards semirigid ring; Cosgrove-Edwards flexible band; the DeVega procedure; or Peri-Guard annuloplasty.During the 8-year follow-up period, regurgitation severity increased more rapidly over time with the DeVega and Peri-Guard procedures, while regurgitation severity was stablest across time with the Carpentier-Edwards ring [11]. In a separate series,Tang et al. [12] described 702 patients who underwent TV repair (largely in the setting of concomitant MV surgery), 493 of whom predominantly had a DeVega repair and 209 had an annuloplasty ring placed (54% Carpentier-Edwards, 25% Duran, and 21% Cosgrove-Edwards). At up to 21-year follow up (mean 5.9 years), the long-term survival, eventfree survival, and freedom from recurrent TR were signi ficantly better in the rigid ring group.
An additional important study was published by Navia et al. [13], who reported a cohort of 2277 patients who underwent TV procedures during primarily mitral and aortic operations. Here, a rigid tricuspid annular ring was used in 26% of patients,a flexible ring was used in 46%, DeVega annuloplasty was used in 5.7%, Peri-Guard annuloplasty was used in in 8.1%, a Kay (commissure) procedure was used in 11%, and an edge-to edge lea flet suture technique was used in 3.5%. At the 5-year followup, TR had increased only slightly for isolated rigid prosthesis annuloplasty (12%), but was progressively greater for all other annular procedures( flexible prosthesis 16%, DeVega annuloplasty 24%, Peri-Guard annuloplasty 44%, Kay procedure 19%). Taken together, these key studies highlight the superiority of annuloplasty ring placement in mitigating the risk of recurrent or progressive TR postoperatively ( Figure 3).
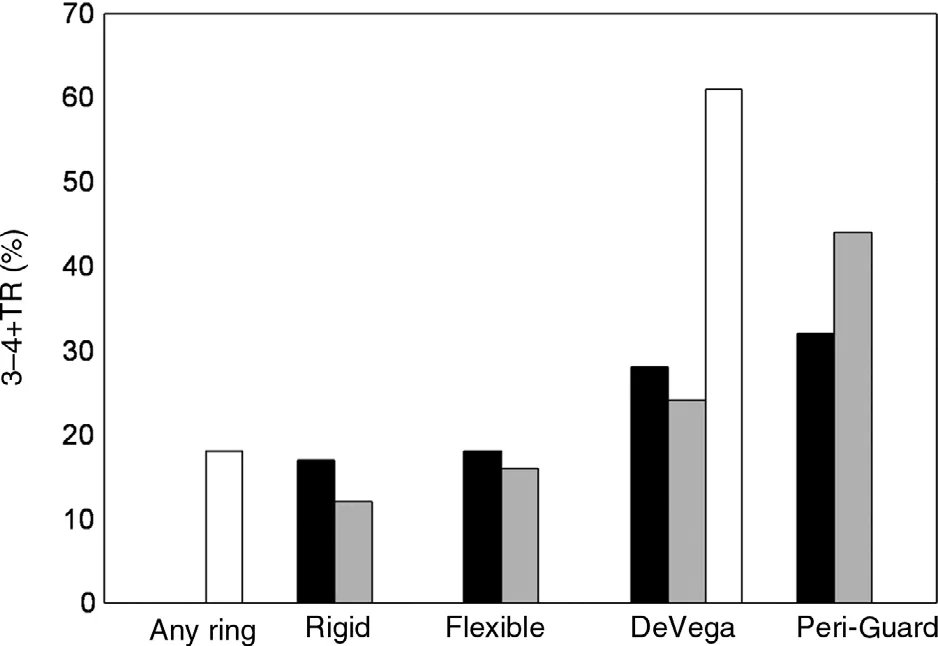
Figure 3 Recurrence of Tricuspid Regurgitation (TR)After Ring and Nonring Annuloplasty.
Surgical Techniques for Functional Tricuspid Regurgitation
Tricuspid Valve Replacement
In general, TV repair is favored over replacement to avoid the risk of thrombotic complications associated with bioprosthetic or mechanical valves. Additionally, TV repair appears to result in improved midterm survival (up to 10 years after surgery) as compared with replacement, although there is no signi ficant difference in valve-related mortality or need for TV reoperation [2]. While the mechanisms for this difference are not fully understood, it is hypothesized that a large rigid prosthesis in a distorted, low-pressure cavity (right ventricle)may result in RV dysfunction and a perioperative low-output state.
Tricuspid Valve Repair
Although ring annuloplasty for functional TR is not technically demanding, careful respect should be shown to surrounding structures. A traditional approach has been to place stitches from the 10 o’ clock position to the 6 o’ clock position,thus avoiding the AV node and the triangle of Koch( Figure 2, inset). Furthermore, and especially in the treatment of functional TR, numerous annular stitches should be used. This helps address the fundamental disease in which the tricuspid annulus is usually underdeveloped as compared with the mitral annulus, and the distracting force from the geometric distortion of the right ventricle must be counteracted with suf ficient sutures. Within the circumference of the tricuspid annulus, these annuloplasty sutures should be taken parallel with and travel in the annulus. A common source of failure of TV annuloplasty is the dehiscence of the ring from inadvertent application of the stitches to the atrial wall or to the lea flet tissue.
Ring Sizing in Functional Tricuspid Regurgitation
While most would agree that rigid TV annuloplasty offers the best solution for functional TR, controversy exists regarding size selection for the TV annuloplasty; some have suggested oversizing the TV annuloplasty for fear of subsequent tricuspid stenosis.
One approach is to use criteria that are triggered by an annular diameter of greater than 40 mm, with a general guideline for sizing being to “ undersize”by at least two ring sizes. Huffman et al. [14] examined patients who underwent MV repair with a rigid complete annuloplasty ring and who simultaneously underwent TV repair with the same-size TV annuloplasty ring. Their data suggests that the same-size ring can be used for TV repair as was used for MV repair, without development of signi ficant tricuspid stenosis or negative effects on right-sided heart function. Speci fically, there was no obvious worsening of RV function, with most patients having normal to only moderately decreased function, in line with their preoperative right-sided heart function. These findings were con firmed by three large clinical trials by Mukherjee et al. [15] (United States), Desai et al. [16] (United States), and Bertrand et al. [17](Europe), which showed that the right ventricle gets better when functional TR is repaired. Moreover,the “ same size” approach seemed to prevent the recurrence of signi ficant TR. Additionally, Gammie et al. [18] showed that the application of a renormalizing tricuspid ring (26– 28 mm) results in very little recurrent TR and no tricuspid stenosis.
Following ring annuloplasty, there are less commonly used surgical techniques for the treatment of functional TR. Posterior annular bicuspidalization is performed by the placing of a pledget-supported mattress suture from the anteroposterior commissure to the posteroseptal commissure along the posterior annulus. Deloche et al. [5] showed that focal posterior TV annuloplasty can be effective in selected cases. Nevertheless, other approaches include anterior lea flet augmentation patching(Dreyfus) or edge-to-edge (Al fieri-type) repairs as described by Castedo et al. [19, 20] and partial purse-string suture techniques to reduce the anterior and posterior portions of the annulus (DeVega-style techniques) [21].
While operating times (cross clamp/bypass) are potentially longer with the addition of TV repair during left-sided surgical procedures, Dreyfus et al.[10] showed no additional increase in 30-day mortality with a TV repair added [10]. Furthermore,across all degrees of TR, Badhwar et al. [22] examined 88,473 patients and found that there is no increased mortality when concomitant TV repair and an MV operation are performed. What is more,Cala fiore et al. [23] demonstrated decreased 30-day mortality, with less recurrent TR and better 5-year survival with the addition of a TV repair in a small retrospective series of 110 patients undergoing MV surgery.
Current Guidelines for the Management of Functional Tricuspid Regurgitation
The American College of Cardiology/American Heart Association 2014 practice guidelines for the surgical treatment of patients with functional TR give a class I indication for TV repair in any patient with severe TR undergoing MV surgery [24]. In patients with less than severe TR, a class IIb recommendation is given for patients undergoing MV surgery if there is pulmonary hypertension or any tricuspid annular dilation greater than 40 mm. The European Society of Cardiology 2012 guidelines take a more aggressive stance, with a class IIa recommendation for TV repair in patients with moderate functional TR and a dilated tricuspid annulus( > 40 mm) in a patient undergoing left-sided surgery [25].
Despite the American College of Cardiology/American Heart Association and European Society of Cardiology guidelines, which support surgical repair of TR at the time of MV surgery in many patients, TV repair currently appears underused.The current surgical volume of TV repair with or without concomitant MV surgery (Society of Thoracic Surgeons National Cardiac Database)averages approximately 5000 operations per year This represents only approximately one-tenth of the more than 60,000 MV operations performed yearly in the United States [18]. This can be due in part to the currently debated decision making on whether or not concomitant TV repair should be performed during MV surgery. Data presented by David et al.[26] on 1171 patients from 1985 through 2005 suggest that TR does not increase with time and that unless it is preoperatively graded as severe, there should be intervention while MV repair is being performed. This conclusion is drawn from near 15-year follow-up data showing only 9% of patients had isolated TR.
Although there are few prospective mortality or functional data to speci fically address this question, there are clear consequences of allowing TR to progress to severe (and potentially cause worsening right-sided heart failure). Thus it would seem logical that earlier intervention for TR, especially in the presence of ongoing right atrial and RV enlargement, would be bene ficial. Attempting to shed light on this debate, Chikwe et al. [27] reported on 645 patients who had MV repairs and underwent functional TV repair because of grade 2+ or greater TR and/or a dilated annulus ( > 40 mm). The results showed the addition of TV annuloplasty was independently associated with less TR and better RV function. There was no increase in operative mortality, but a survival bene fit was not shown in this retrospective study. Likewise, 1-year data from Ward et al. [28] showed that performing concomitant TV repair with a triad annuloplasty correlated with reduced TR, stable RV dimensions, and reduction of symptoms. However, a randomized trial comparing acceptance of TR versus earlier intervention may be required to further answer the greatly debated question of the bene fits of early intervention for TR.
New Directions in Tricuspid Valve Repair
During TV surgery, a surgeon must be comfortable with complex decision making, including cannulation site selection, placement of the right atrial incision, caval snaring, use of active venous suction,the management of intravenous pacing wires, and the handling of TV repair both during cardioplegic arrest and on the beating heart. Additionally, a surgeon must understand the intraoperative evaluation of the quality of a TV repair in terms of assessment while the patient is under anesthesia and the impact on residual or recurrent TR. Unfortunately,concomitant TV surgery remains underused at the time of MV surgery for a variety of reasons, including the lack of randomized mortality data to support aggressive treatment, and the unfounded fear of the additional operating time required to perform TV annuloplasty. Techniques that reduce the morbidity and time required to perform TV annuloplasty could be helpful. In addition, reoperations for recurrent TR are especially high risk, with up to 35% inhospital mortality, and are therefore not routinely offered to patients [3]. One such technique is minimally invasive TV surgery, which was described by Lee et al. [29]. They reported a series of 141 consecutive patients undergoing TV operation using a minithoracotomy without cross-clamping. This was done with a beating heart or by allowing the heart to fibrillate. The repair rate was 61%, and 30-day mortality was low at 2.1%. Secondly, new percutaneous approaches for treating TV disease are on the horizon. The MitraClip (Abbott, Minneapolis,MN, USA) has been used to achieve a percutaneous Al fieri-type edge-to-edge repair of the TV. Lastly,percutaneous annular rings that can be delivered to the MV or TV are also under development, as is a new TV stent, the GATE (NaviGate Cardiac Structures, Laguna Hills, CA, USA). Additionally,at the forefront of tricuspid interventions, there have been several case series describing successful off-label use of transcatheter aortic and pulmonic prostheses for tricuspid valve-in-valve replacement with promising initial results.
In conclusion, concomitant surgical repair of TR at the time of MV surgery should be considered, as this approach has been shown to result in improved perioperative outcomes, functional class, and potentially survival. Any TR with annular dilation cannot simply be ignored when one is performing corrective surgical procedures for mitral regurgitation, as TR does not reliably disappear after successful MV surgery, and reoperations for recurrent TR carry high mortality rates. Furthermore,the addition of TV repair has been shown not to increase operative mortality when MV repair is performed. Consequently, aggressive application of TV repair based solely on tricuspid annular dilation is becoming more common. The application of a rigid or semirigid ring appears to have improved durability, or at least freedom from recurrence of signi ficant TR. All suture-based or other nonring approaches should be avoided. With this understanding, use of TV annuloplasty for functional TR can be maximized.
Conflict of Interest
The authors declare no Conflict of interest.
REFERENCES
1. Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation 2009;119:2718– 25.
2. Singh SK, Tang GH, Maganti MD,Armstrong S, Williams WG, David TE, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease.Ann Thorac Surg 2006;82:1735– 41.
3. Bernal JM, Morales D, Revuelta C, Llorca J, Guti é rrez-Morlote J,Revuelta JM. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498– 503.
4. Silver MD, Lam JH, Ranganathan N, Wigle ED. Morphology of the human tricuspid valve. Circulation 1971;43:333– 48.
5. Deloche A, Guerinon J, Fabiani JN, Morillo F, Caramanian M,Carpentier A, et al. [Anatomical study of rheumatic tricuspid valve diseases: application to the study of various valvuloplasties]. Ann Chir Thorac Cardiovasc 1973;12:343– 9.
6. Ewy G. Tricuspid valve disease. In:Alpert JS, Dalen JE, Rahimtoola SH, editors. Valvular heart disease.3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
7. Yiwu L, Yingchun C, Jianqun Z, Bin Y, Ping B. Exact quantitative selective annuloplasty of the tricuspid valve. J Thorac Cardiovasc Surg 2001;122:611– 4.
8. Fukuda S, Saracino G, Matsumura Y, Daimon M, Tran H, Greenberg NL, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation:a real-time, 3-dimensional echocardiographic study. Circulation 2006;114:I492– 8.
9. Tei C, Pilgrim JP, Shah PM,Ormiston JA, Wong M. The tricuspid valve annulus: study of size and motion in normal subjects and in patients with tricuspid regurgitation.Circulation 1982;66:665– 71.
10. Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation:which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127– 32.
11. McCarthy PM, Bhudia SK,Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674– 85.
12. Tang, GH, David TE, Singh SK,Maganti MD, Armstrong S, Borger MA. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes.Circulation 2006;114:I577– 81.
13. Navia JL, Nowicki ER, Blackstone EH, Brozzi NA, Nento DE, Atik FA,et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or lea flet procedure? J Thorac Cardiovasc Surg 2010;139(6):1473– 82.
14. Huffman LC, Nelson JS, Lehman AN, Krajacic MC, Bolling SF.Identical tricuspid ring sizing in simultaneous functional tricuspid and mitral valve repair: a simple and effective strategy. J Thorac Cardiovasc Surg 2014;147:611– 4.
15. Mukherjee D, Nader S, Olano A,Garcia MJ, Grif fin BP. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation.J Am Soc Echocardiogr2000;13:650–4.
16. Desai RR, Vargas Abello LM, Klein AL, Marwick TH, Krasuski RA,Ye Y, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasac Surg 2013;146:1126–32.
17. Bertrand PB, Koppers G, Verbrugge FH, Mullens W, Vandervoort P,Dion R, et al. Tricuspid annuloplasty concomitant with mitral valve surgery: effects on right ventricular remodeling. J Thorac Cardiovasc Surg 2014;147:1256–64.
18. Gammie JS, O’ Brien SM, Grif fith BP, Ferguson TB, Peterson ED.In fluence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation.Circulation 2007;115:881– 7.
19. Castedo E, Canas A, Cabo RA,Burgos R, Ugarte J. Edge-to-edge tricuspid repair for redeveloped valve incompetence after DeVega’ s annuloplasty. Ann Thorac Surg 2003;75:605– 6.
20. Castedo E, Monguio E, Cabo RA,Ugarte J. Edge-to-edge technique for correction of tricuspid valve regurgitation due to complex lesions. Eur J Cardiothorac Surg 2005;27:933– 4.
21. Lapenna E, De Bonis M, Verzini A,La Canna G, Ferrara D, Calabrese MC, et al. The clover technique for the treatment of complex tricuspid valve insuf ficiency: midterm clinical and echocardiographic results in 66 patients. Eur J Cardiothorac Surg 2010;37:1297– 303.
22. Badhwar V, Rankin JS, He M,Jacobs JP, Furnary AP, Fazzalari FL,et al. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality.Ann Thorac Surg 2017;103:587– 94.
23. Cala fiore AM, Gallina S, Iacò AL,Contini M, Bivona A, Gagliardi M,et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? A propensity score analysis. Ann Thorac Surg 2009;87(3):698–703.
24. Nishimura RA, Otto CM, Bonow RO,Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;129:e57– 185.
25. Vahanian A, Al fieri O, Andreotti F,Antunes MJ, Baron-Esquivias G,Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology(ESC) and the European Association for Cardio-Thoracic Surgery(EACTS). Eur J Cardiothorac Surg 2012;42:S1– 44.
26. David TE, David CM, Fan CP,Manlhiot C. Tricuspid regurgitation is uncommon after mitral valve repair for degenerative diseases. J Thorac Cardiovasc Surg 2017;154:110– 22.el.
27. Chikwe J, Itagaki S, Anyanwu A,Adams DH. Impact of concomitant tricuspid annuloplasty on tricuspid regurgitation, right ventricular function and pulmonary artery pressure after repair of mitral valve prolapse J Am Coll Cardiol 2015;65,1931– 8.
28. Ward S, Baker M, Bolling SF. Long term effect of concomitant tricuspid repair. In: American Association for Thoracic Surgery Centennial Meeting; 2017 April 29– May 3;Boston, MA. Beverly, MA: AATS;2017. Abstract nr 11.
29. Lee TC, Desai B, Glower DD.Results of 141 consecutive minimally invasive tricuspid valve operations: an 11-year experience. Ann Thorac Surg 2009;88:1845– 50.
 Cardiovascular Innovations and Applications2018年1期
Cardiovascular Innovations and Applications2018年1期
- Cardiovascular Innovations and Applications的其它文章
- Mitral Stenosis: A Review
- Misdiagnosed Aortic Intramural Hematoma and the Role of Intravascular Ultrasound Imaging in Detection of Acute Aortic Syndrome: A Case Report
- Management of Mitral Regurgitation in a Patient Contemplating Pregnancy
- An Asymptomatic Patient with Severe Mitral Regurgitation
- Clinical Evaluation of a Patient with Asymptomatic Severe Aortic Stenosis
- Low-Gradient, Low Ejection Fraction SevereAortic Stenosis: Still a Management Conundrum
