基于席夫碱配体的Gdバ/Dyバ配合物的结构和磁性
靳平宁 闫瑞芳 胡 鹏 吴燕妮 高媛媛黄玲珠 朱怡璇 苏 妍 汪应灵
(1新乡医学院基础医学院分析测试实验室,新乡 453003)
(2肇庆学院环境与化学工程学院,肇庆 526061)
(3南开大学先进能源材料化学教育部重点实验室,天津 300071)
(4内蒙古工业大学化工学院,呼和浩特 010051)
(5新乡医学院基础医学院,新乡 453003)
0 Introduction
Since the discovery of Single-molecule magnets(SMMs)during the 1990s,the study of SMMs has a booming development for its potential application in high-density information storage and quantum computing devices[1-4].To achieve these applications,carefully designed structures of SMMs are required.Among all the central ions,owning to their significant magnetic anisotropy which arising from the large,unquenched orbital angular momentum,lanthanide-ions are widely used to construct SMMs[5-15].For example,Zheng′s group reported a monometallic Dy complex of magnetization reached an effective energy barrier to magnetic relaxation of Ueff=1 815(1)K[16].Tong′s group reported another Dy-based compound with a record anisotropy barrier up to 1 837 K in zero field and a record magnetic blocking temperature of 60 K[17].Notably,previous studies show that the magnetic interaction between centre metalsand the alteration ofcoordination geometry on them are two important factors to adjust the relaxation dynamics of lanthanide-based SMMs[18-19].For example,Tang′s group reported an asymmetric dinuclear Dyバcomplex which shows the high axiality and Ising exchange interaction that can efficiently suppress quantum tunneling of magnetization and the exchange interaction between the Dy sites contribution leading to an increase of the relaxation time by 3 orders of magnitude[20].In addition,the dinuclear SMMs are ideal configurations to study the single-ion effective anisotropic barriers versus the energy barriers which arising from the two interaction metal centers[21-22].To date,various researches have been performed to study how the exchange coupling affects magnetic properties of lanthanide-based complexes and some innovative results have been reported[23-25].
To construct dinuclear SMMs,the choice of ligands is very important.As ligands can influence the construction of coordination polymers and moreover,ligand field is a key factor to control the magnetic anisotropy of SMMs.Therefore,Schiff-base ligands with rich O,N-based multichelating sites have been regarded as excellent candidates to construct lanthanide-based multinuclear coordination compounds.In addition,by modifying the terminal group of acylhydrazine derivatives,various ligand systems will be built in which ligand field will also affect the SMM properties of Lnバcoordination compounds[26-28].
Herein,we choose 2-hydroxy-3-methoxy-5-bromobenzaldehyde oxime(HL)as ligand in combination with methane acid and acetic acid to build new Lnbased coordination polymers.Two new complexes,namely,[Gd2(L)4(HCOO)2(CH3OH)2](1)and[Dy2(L)4(CH3COO)2(CH3OH)2](2)have been constructed successfully.Their syntheses,crystal structures and magnetic properties are reported.
Scheme 1 Molecular structure of HL
1 Experimental
1.1 Materials and measurements
All reagents and solvents were purchased from Aladdin and used without further purification.The ligand HL was synthesized according to the literature[29].Elemental analyses for C,H and N were performed on a Perkin-Elmer elemental analyzer model 240.IR spectra were recorded on a Nicolet IS10.IR spectrometer using KBr pellets in the range of 4 000~500 cm-1.Variable temperature magnetic susceptibilities were measured on SQUID MPMSXL-7 magnetometer in the temperature range of 2~300 K.
1.2 Synthesis of complex 1
Ligand HL (0.1 mmol)was dissolved in mixed solvent of 10 mL CH3OH and 5 mL CH3CN followed by addition of GdCl3·6H2O (0.1 mmol)and sodium methoxide (0.2 mmol),which gave a clear solution after stirring for 2.5 h.Diethyl ether was diffused slowly into the solution at room temperature,and light-yellow single crystals were obtained in 5 days.Yield:42.5%based on rare-earth.Elemental analysis calculated for C40H54Br4Gd2N4O22(%):C 30.47;H 3.45;N 3.55.Found(%):C 30.61;H 3.52;N 3.61.FT-IR(KBr,cm-1):1 689(w),1 612(m),1 568(m),1 473(s),1 312(w),1 174(w),1 072(m),1 010(w),952(w),842(s),753(m),587(m).
1.3 Synthesis of complex 2
Ligand HL (0.1 mmol)was dissolved in mixed solvent of 10 mL CH3OH and 5 mL CH3CN followed by the addition of Dy(CH3COO)3·4H2O(0.1 mmol)and triethylamine(0.2 mmol).A clear solution was obtained after stirring for 3 h.Diethyl ether was used to diffuse slowly into this solution at room temperature,and light-yellow single crystals were obtained in 5 days.Yield:44.5%based on rare-earth.Elemental analysis calculated for C40H50Br4Dy2N4O20(%):C 30.97;H 3.25;N 3.61.Found(%):C 30.81;H 3.21;N 3.51.FTIR(KBr,cm-1):1 860(w),1 601(m),1 478(m),1 380(s),1 221(m),1 110(s),1 005(w),941(w),840(m),734(s),558(w).
1.4 X-ray crystallographic study
The suitable single crystals of complexes 1 and 2,with crystal size of 0.34 mm×0.34 mm×0.29 mm and 0.32 mm×0.31 mm×0.28 mm respectively,were used for X-ray diffraction data collection with a BRUKER SMART 1000 CCD,all using graphitemonochromated Mo Kα radiation (λ=0.071 073 nm).The structures were solved primarily by direct methods and refined by the full-matrix least squares method.The computations were performed with the SHELX-97 program[30].All non-hydrogen atoms were refined with anisotropic thermalparameters.The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.The crystal data and refinement parameters of complexes 1 and 2 are summarized in Table 1.
CCDC:1570377,1;1570378,2.
2 Results and discussion
2.1 Crystal structures of complexes 1 and 2
The crystal structure of complex 1 is depicted inFig.1.Complex 1 crystallizes in the space group of P1 in triclinic structure.Two symmetric nine-coordinated Gdバions are bridged by two phenoxide groups from two Schiff base ligands and two molecules of formate ion with Gd-Ophenoxidebond length of 0.241 7(5)and 0.237 4(3)nm,respectively.Then,each Gdバion is further coordinated with two N ions,one Ohydroxylion and one Omethoxylion.The intramolecular Gd…Gd distance is 0.376 6(2)nm,as well as Gd-O-Gd angle of 103.65(3)°.

Table 1 Crystal data and structure refinement for complexes 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2

Fig.1 Crystal structure of complex 1 with thermal ellipsoids drawn at 30%probability
Complex 2 crystallize in monoclinic with space group of P21/c(Fig.2).The crystal structure of complex 2 is similar to complex 1 except that Dyバions are bridged by two acetate ions instead of formate ions.The Dy-O and Dy-N distances are in the range of 0.228 9(4)~0.265 0(4)nm and 0.257 7(3)~0.262 9(2)nm,respectively.The intramolecular Dy…Dy distance is 0.375 7(1)nm with Dy-O-Dy angle of 103.30(5)°.Selected bond lengths and angles for two complexes are shown in Table 2.

Fig.2 Crystal structure of complex 2 with thermal ellipsoids drawn at 30%probability
2.2 Magnetic properties of complex 1
Variable-temperature magnetic susceptibilities of complexes 1 and 2 were measured from 300 to 2.0 K in an applied field of 1 kOe.As showed in Fig.3,the χMT value for complex 1 at 300 K is 15.67 cm3·K·mol-1,which is in agreement with the expected value of 15.76 cm3·K·mol-1for two uncoupled Gdバ ions(8S7/2,S=7/2).The χMT value is almost constant until the temperature cooling to 30 K,after that,this value decreases rapidly,reaching a minimum value of 11.04 cm3·K·mol-1at 2.0 K.This phenomenon indicates the presence ofa weak antiferromagnetic interaction between the Gdバions.
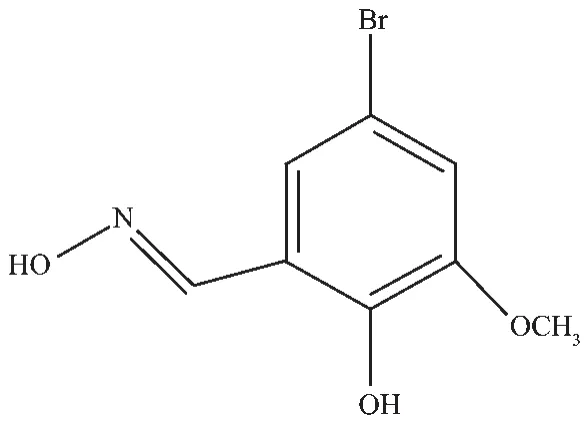
To evaluate the coupling parameters,the magnetic data were analyzed by a theoretical expression[31].The best fit from equation below gives g=1.99,J=-0.1348 which confirms the weak antiferromagnetic interaction between the Gd ions.

Fig.3 Temperature dependence of χMT for complex 1
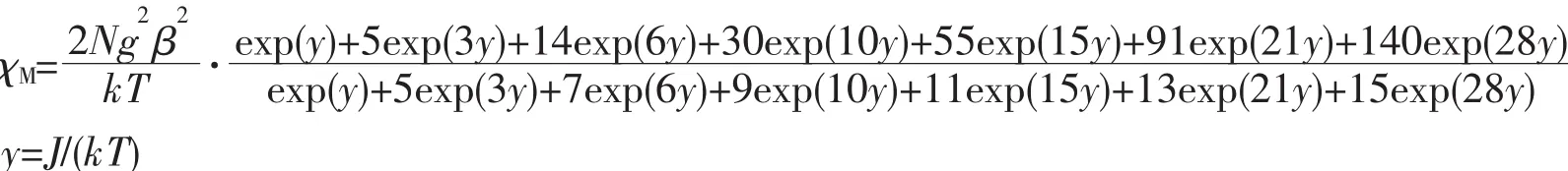
2.3 Magnetic properties of complex 2
For complex 2 (Fig.4),at room temperature,the χMT value(28.61 cm3·K·mol-1)is close to the theoretical value of 28.34 cm3·K·mol-1for two non-interacting Dyバ ions (6H15/2,g=4/3).Upon cooling,the χMT value decreases gradually in the temperature range of 300~50 K,then drops quickly and reaches 6.8 cm3·K·mol-1at 2 K.This observed decrease of χMT value under high temperatures results from the depopulation of the excited Stark sublevels.However,the decrease behavior at low temperature mainly results from intramolecular antiferromagnetic interaction between Dyバions[32].
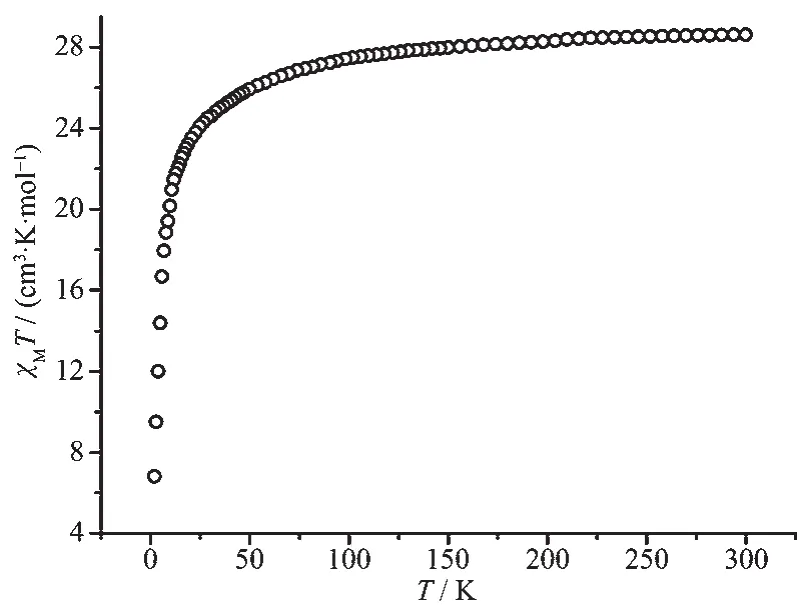
Fig.4 Temperature dependence of χMT for complex 2
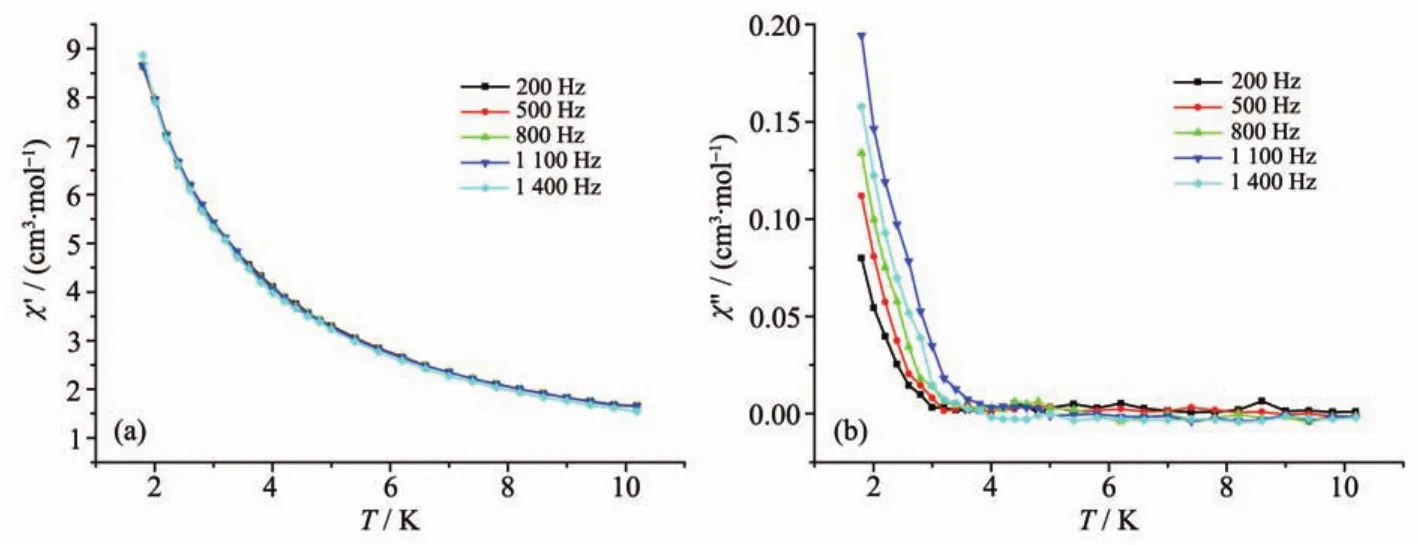
Fig.5 In-phase(a)and out-of-phase susceptibilities(b)of complex 2 at different frequencies under zero applied field
To investigate the dynamic magnetic properties of complex 2,the temperature frequency dependencies of magnetic susceptibility measurements under alternating current were measured in the range of 200~1 400 Hz.As shown in Fig.5,no maximum in-phase signal(χ′)and out-of-phase signal(χ″)values are observed until 2 K which might arise from the quantum tunneling of magnetization(QTM).However,the values of inphase signal(χ′)and out-of-phase signal( χ″)exhibit a frequency dependent character,indicating the slow relaxation of magnetization.
3 Conclusions
Two novel dinuclear Lnバbased complexes have been successfully synthesized.These two complexes have similar structures,in which two symmetric ninecoordinated Lnバions are bridged by two phenoxide groups from two Schiff base ligands,two molecules of formate ion for 1 and acetate ion for 2.The magnetic studies show that weak antiferromagnetic interaction between the Gd ions existing in complex 1,while complex 2 shows slow relaxation of magnetization.
[1]Vincent R,Klyatskaya S,Ruben M,et al.Nature,2012,488:357-360
[2]Thiele S,Balestro F,Ballou R,et al.Science,2014,344:1135-1138
[3]Bar A K,Pichon C,Gogoi N.Chem.Commun.,2015,51:3616-3619
[4]Cervetti C,Rettori A,Pini M G,et al.Nat.Mater.,2016,15:164-168
[5]LI Cheng-Hui(李承辉),GU Zhi-Guo(顾志国),ZUO Jing-Lin(左景林),et al.Chinese J.Inorg.Chem.(无机化学学报),2007,23(9):1583-1586
[6]Pointillart F,Gal Y L,Golhen S,et al.Dalton Trans.,2013,42:1949-1960
[7]Hu P,Gao Y Y,Xiao F P,et al.Polyhedron,2017,130:40-46
[8]Han H,Li X,Zhu X,et al.Eur.J.Inorg.Chem.,2017:2088-2093
[9]Du F X,Hu P,Gao Y Y,et al.Inorg.Chem.Commun.,2014,48:166-170
[10]Escobar L B L,Guedes G P,Soriano S,et al.Inorg.Chem.,2014,53:7508-7517
[11]Li C,Sun J,Yang M,et al.Cryst.Growth Des.,2016,16:7155-7162
[12]Hu P,Guo H F,Li Y,et al.Inorg.Chem.Commun.,2015,59:91-94
[13]LIU Sui-Jun(刘遂军),CUI Yu(崔雨),SONG Wei-Chao(宋伟朝),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(9):1894-1902
[14]HU Peng(胡鹏),GAO Yuan-Yuan(高媛媛),XIAO Feng-Yi(肖凤仪),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(1):34-40
[15]Yang M,Li H D,Li L C.Inorg.Chem.Commun.,2017,76:59-61
[16]Ding Y S,Chilton N F,Winpenny R E P,et al.Angew.Chem.Int.Ed.,2016,55:1-5
[17]Guo F S,Day B M,Chen Y C,et al.Angew.Chem.Int.Ed.,2017,56:1-6
[18]Sorace L,Benelli C,Gatteschi D.Chem.Soc.Rev.,2011,40:3092-3104
[19]Guo Y N,Chen X H,Xue S,et al.Inorg.Chem.,2011,50:9705-9713
[20]Guo Y N,Xu G F,Wernsdorfer W,et al.J.Am.Chem.Soc.,2011,133:11948-11951
[21]Xue S F,Guo Y N,Ungur L,et al.Chem.Eur.J.,2015,12:14099-14106
[22]Zhang L,Jung J L,Zhang P,et al.Chem.Eur.J.,2016,22:1392-1398
[23]Zhang K,Yuan C,Guo F S,et al.Dalton Trans.,2017,46:186-192
[24]Li M,Wu H P,Zhang S,et al.Eur.J.Inorg.Chem.,2017:811-819
[25]Zhang K,Guo F S,Wang Y Y.Inorg.Chem.Commun.,2017,76:95-99
[26]Bag P,Rastogi C K,Biswas S,et al.Dalton Trans.,2015,44:4328-4340
[27]Biswas S,Das S,Hossain S,et al.Eur.J.Inorg.Chem.,2016:4683-4692
[28]Hutchings A J,Habib F,Holmberg R J,et al.Inorg.Chem.,2014,53:2102-2112
[29]XU Tao(许涛),LI Lian-Zhi(李连之),JI Hai-Wei(冀海伟).Chinese Journal of Synthetic Chemistry(合成化学),2004,12:22-24
[30]Sheldrick G M.SHELXS-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[31]Mukherjee S,Lu J J,Velmurugan G,et al.Inorg.Chem.,2016,55:11283-11298
[32]Kahn M L,Ballou R,Porcher P,et al.Chem.Eur.J.,2002,8:525-531

