lmmunogenicity and protective efficacy of DHBV DNA vaccines expressing envelope and capsid fusion proteins in ducks delivered by attenuated Salmonella typhimurium
LlU Si-yang, JlA Ren-yong, Ll Qing-qing, FENG Dai-shen, SHEN Hao-yue, YANG Cui, WANG Ming-shu, ZHU De-kang, CHEN Shun, LlU Ma-feng, ZHAO Xin-xin, YlN Zhong-qiong, JlNG Bo, CHENG An-chun
1 Avian Disease Research Center, Sichuan Agricultural University, Chengdu 611130, P.R.China
2 Institute of Preventive Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, P.R.China
3 Key Laboratory of Animal Disease and Human Health of Sichuan Province, Chengdu 611130, P.R.China
1. lntroduction
Duck hepatitis B virus (DHBV), which belongs to theHepadnaviridaefamily, is commonly regarded as a representative of theAvihepadnavirusgenus (Piaseckiet al.2012). And most of the breakthroughs in our understanding ofHepadnaviridaeviruses, such as replication and pathologic mechanisms, were achieved through studies of DHBV (Liuet al. 2015). DHBV shares many basic characteristics with Human hepatitis B virus (HBV), thus making it an indispensable natural model for HBV research(Guidotti 2014).
L and S proteins encoded by the PreS/S and S genes,respectively, are large and small envelope proteins,respectively, that form the viral surface antigen. This antigen functions as the locator when the virus bridges the endoplasmic reticulum membrane (Grecoet al. 2014).In addition, this antigen participates in the assembly and release of viral particles (Siegler and Bruss 2013) and plays an important role in maintaining the stability of covalently closed circular DNA (cccDNA) in hepatocytes(Cuiet al. 2015), the numbers of which indicate whether the virus is actively replicating (Werle-Lapostolleet al.2004). The icosahedral nucleocapsid, which is made up of core (C) proteins, is enclosed by the envelope proteins and surrounds the DNA genome and viral polymerase.The nucleocapsid, which serves as the viral core antigen,functions in the maturation of the viral particles (Cuiet al.2013) and the release of nucleic acid during the assembly of viral progeny. It also transports the relaxed circular DNA to the cell nucleus, where it is then transformed into cccDNA,causing persistent DHBV infection in ducks (Nassal 2015).Current DHBV vaccines mainly target the L and C proteins(Khawajaet al. 2012a; Saadeet al. 2013a), and there have been few studies on the S protein, let alone fusion proteins.
Recently, some modified enteropathogenic bacteria,such asListeria monocytogenes,Salmonellaspp. andShigellaspp., have been employed as carriers for DNA vaccines (Frickmannet al. 2013; Pereiraet al. 2013;Handleyet al. 2016). With the help of attenuated live bacteria (Yuet al. 2012; Qianet al. 2015), antigen genes obtain easier access to mucosal surfaces than when they are introduced by intramuscular injections, and the process of capture by antigen-presenting cells (APCs) after translation is accelerated, motivating systemic responses against pathogens (Muhammadet al. 2012; Huet al. 2015).In addition, the price of DNA vaccines is greatly reduced compared with that of inactivated vaccines and protein vaccines. Notably, attenuatedSalmonella typhimuriumSL7207 has been widely applied in trials to deliver DNA vaccines (Liet al. 2012; Wanget al. 2012; Bergeret al.2013) but has not been used to deliver DHBV vaccines.
To determine whether attenuated live bacteria can increase the immune response against DHBV, we developed DNA vaccines expressing envelope and capsid fusion proteins and delivered them into ducks using attenuatedS.typhimuriumSL7207. Vaccine strains containing recombinant plasmids were obtained by electroporation,and their growth kinetics and genetic stabilitiesin vitrowere measured to ensure safety and efficacy. Animal experiments were carried out to analyze humoral immunity(IgY in pooled sera) and cellular immunity (CD4 and CD8 T cells in spleen). After that, the protective efficacy of our DNA vaccines was determined based on the cccDNA copy number, which indicates if the virus is active in hepatocytes(Reaiche-Milleret al. 2013). The results of this study have enriched our knowledge of the antigenicity of envelope and capsid fusion proteins in ducks and demonstrate the value of using attenuatedS.typhimurium(SL7207) to deliver DHBV vaccines.
2. Materials and methods
2.1. Bacterial strains, viruses, plasmids and ducks
AttenuatedS.typhimuriumSL7207 [2337-65 (WRAY)hisG46 ΔaroA407 (TCS aroA544:Tnl0)], was a kind gift from Prof. Kai Schulze at the Helmholtz Research Center for Infection (HZI, Germany). DHBV strain SCP01 (GenBank:KM676220.1) was isolated by our lab (Avian Disease Research Center, Sichuan Agricultural University, Sichuan,China).
The antigen genes (L, S, C) of DHBV were amplified and the fused genes (LC and SC) separated by a linker were generated by overlap PCR. The linker encoded a hydrophobic polypeptide, (Gly4Ser)3, that was 15 amino acids in length:5´-GGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGC GGTGGCGGCAGC-3´ (Xueet al. 2014). The genes were separately cloned into the pVAX1 expression vector to obtain recombinant plasmids. The constructed plasmids pVAX1-L, pVAX1-S, pVAX1-C, pVAX1-LC and pVAX1-SC were used for genetic immunization of ducks. The empty plasmid pVAX1 was used as a negative control. The plasmids pET-32a(+)-L, pET-32a(+)-S and pET-32a(+)-C were constructed to express truncated DHBV L, S and C proteins, respectively, for use in antibody (IgY) detection by enzyme-linked immunosorbent assay (ELISA) (described below). Additionally, pGEMT-cccDNA was constructed to set up a standard curve for cccDNA measurement.
One-day-old DHBV-negative and unvaccinated Pekin ducklings were purchased from a commercial duck farm(Ya’an, China) and fed in cages under standard conditions.Animal experiments were performed in accordance with animal care guidelines at the Sichuan Agricultural University(Chengdu, China).
2.2. Construction of recombinant attenuated S. typhimurium DHBV DNA vaccine
The constructed plasmids pVAX1-L, pVAX1-S, pVAX1-C,pVAX1-LC, and pVAX1-SC were transformed into attenuatedS.typhimuriumSL7207 by Electroporation (MicroPulserTM,Bio-Rad, USA). The positive clones were selected based on kanamycin resistance and confirmed by PCR using specific primers.
2.3. Measurement of the growth kinetics and genetic stabilities in vitro
The growth kinetics of the recombinantS.typhimuriumstrains were determined by measuring optical density (OD)at 600 nm with a spectrophotometer (Biorad, US) at hours 2,4, 6, 8, 10, 12 and 14. To determine the genetic stabilities of the transformants, transformed cells were passaged daily for 10 days (approximately 200 generations) in the absence of antibiotic (Gahanet al. 2007). On days 2, 4, 6,8 and 10, 100 μL of each culture was diluted 106, 107, and 108and 100 μL of bacteria from each dilution were plated on 3 kanamycin-free LB plates and 3 LB plates containing kanamycin. After 20 hours of cultivation, the colony forming units (CFU) were counted, and the plasmid loss rate was calculated as follows: Plasmid loss rate (%)=1–(The average number of colonies perkanamycin LB plate/Average number of colonies on kanamycin-free LB plates)×100
2.4. Safety and oral doses
To determine the immunization dose, 81 7-day-old ducks were randomly divided into 3 groups (SL7207-pVAX1-L,SL7207-pVAX1-S and SL7207-pVAX1-C). Each vaccine group was administrated orally with a dosage of 109,1010, or 1011CFU diluted in 2-mL PBS. The safety of the vaccines was tested by evaluating the spirit, appetite and activity of the objects. The appropriate vaccine dosage was determined by measuring the relative transcriptional level of the L, S and C genes in liver tissues 2, 4 and 6 weeks after inoculation.
2.5. Oral grouping and experimental protocol
A total of 180 1-day-old Pekin ducklings were randomly divided into 9 groups (Fig. 1-A). Before and after inoculation,ducks were deprived of water and food for 2 hours. Treated groups received 1011CFU of a vaccine strain diluted in 2-mL PBS taken orally. In parallel, the blank control group was orally administered the same volume of PBS. The experimental protocol is outlined in Fig. 1-B. Three ducks from each group were sacrificed, and pooled sera, liver and spleen were collected and stored at –80°C before measurement.
2.6. Determination of S, C and L gene transcription levels by real-time PCR
Fourteen days after immunization, the experimental and control groups were sacrificed, and the relative transcription levels of the S, C and L genes were determined. Total RNA was extracted from fresh liver and then reverse-transcribed to cDNA using HiScript®IIQ Select RT Supermix (Vazyme,USA), which served as the template for real-time PCR. The relative transcription levels of the S, C and L genes were measured using the Bio-Rad CFX96 Real-Time Detection System (Bio-Rad). Each 10 μL reaction contained 5 μL iQTMSYBR®Green Supermix (Bio-Rad), 0.2 μL of each primer(10 μmol L–1), 0.2 μL cDNA and 4.4 μL ultrapure water.The primers used in real-time PCR are shown in Table 1.The optimized real-time PCR program was 95°C for 3 min,followed by 40 cycles of 10 s at 95°C for denaturation,30 s at 64.1°C (M), or 64.1°C (S), or 59.5°C (C) for annealing. Melting curve analysis was performed following completion of the real-time PCR program. Target gene expression was normalized to duck GAPDH (GenBank:AY436595.1). Standard curves were generated to obtain the amplification efficiency.
2.7. Measurement of the relative transcription levels of the duck CD4 and CD8 genes by real-time PCR
Total RNA from fresh spleen was isolated and then reversetranscribed to cDNA, which served as the template for realtime PCR. The primers used in real-time PCR are shown in Table 1. The extension temperature was 59°C for the CD4 gene (GenBank: AF378701.1) and 55.7°C for the CD8 gene (GenBank: AF378373.1). Duck GAPDH (GenBank:AY436595.1) served as the internal control. Standard curves were generated to obtain the amplification efficiency.
2.8. Measurement of antibody levels by indirect ELlSA
Pooled sera were isolated from peripheral blood to analyze the DHBV-specific IgY responses induced by DNA vaccines.After that, 96-well polystyrene microtiter plates were coated with purified truncated L, S or C proteins. Goat Anti-Bird(Abcam, Cambridge, UK) IgY conjugated with horseradish peroxidase (HRP) at a 1:5 000 dilution was added as the secondary IgY. The substrate 3,3´,5,5´-tetramethylbenzidine(TMB) was used for staining. The reaction was terminated by the addition 50 μL of 2 mol L–1H2SO4, and the OD value at 450 nm was recorded using a Bio-Rad Model 680 Microplate Reader (Bio-Rad).
2.9. Protection studies
To assess the efficiency of DNA vaccines against DHBV in immunized ducks, 8 ducks from each group were intravenously challenged with a viremic serum pool (2×1010viral genome equivalents per duck) (Khawajaet al. 2012)on day 50, and a second challenge was carried out on day 64 (Fig. 1-B). To quantify the level of DHBV cccDNA in hepatocytes of each group, real-time PCR was performed 7 days after every challenge. Briefly, viral DNA wasextracted from duck livers using TIANamp Genomic DNA Kit(Tiangen, CHN). Linear DNA was digested by mung bean nuclease (New England Biolabs, UK) and primers (Table 1)were designed to span the gaps of the DHBV genome(Pollicinoet al. 2004). These 2 steps guaranteed the amplification of cccDNA rather than linear DNA. The number of DHBV particles was calculated based on an estimation of 6.667 pg hg–1DNA per cell (Zhonget al. 2011).
图4~7为完整试样和预制裂纹试样在三点弯曲试验中,试样变形破裂过程微震和电荷感应信号的监测结果。图中的(a,b,c,d)分别为试样变形破裂过程的时间-力曲线、位移-力曲线、时间-微震和时间-电荷感应信号曲线,(c)图中上、中、下三图分别为电荷传感器1、2、3接收到的电荷感应信号,(d)图中上、中、下三图分别为垂向振动速度传感器和两水平向振动速度传感器接收到的微震信号。

Table 1 List of primers used in this study and their sequences
2.10. Statistical analysis
Data are presented as the mean and standard deviation,which were calculated using GraphPad Prism 5. Statistical analysis of differences in means between groups was performed using an independent Tukey’s test. AP-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Construction of recombinant attenuated SL7207-DHBV DNA vaccines and determination of safety and immunization dose
The DHBV DNA vaccine plasmids were constructed and transformed into SL7207 to obtain the following recombinant attenuatedSalmonella-DHBV DNA vaccine strains: SL7207-pVAX1, SL7207-pVAX1-L, SL7207-pVAX1-S, SL7207-pVAX1-C, SL7207-pVAX1-LC and SL7207-pVAX1-SC. All plasmids were genetically stable as confirmed by PCR (Fig. 2) and screening for antibiotic resistance in subcultured bacteria. No dramatic symptoms were observed, and ducks receiving the highest dosage had the highest relative transcriptional levels of the S,C and L genes in their livers at 2, 4 and 6 weeks postadministration (Table 2). Thus 1011CFU was considered as the best dose for oral immunization.
3.2. The growth kinetics of mutant strains and plasmid stabilities in vitro
There was no significant difference in the growth of the 5 vaccine candidate strains (SL7207-pVAX1-L, SL7207-pVAX1-S, SL7207-pVAX1-C, SL7207-pVAX1-LC and SL7207-pVAX1-SC) (Fig. 3) in comparison with the negative control group (SL7207-pVAX1) and parent strain SL7207.Furthermore, among all groups, the recombinant plasmid loss rate after growth in LB without antibiotics for 10 days(200 generations) was less than 30% (Fig. 4), which indicates that the recombinant plasmids are sufficiently stable in the absence of antibiotics for at least 10 days.
3.3. The breadth of immunization in fusion groups was enhanced but the expression of antigen gene fusions was suppressed
Livers were collected 14 days after each immunization to analyze the relative transcription levels of the S, C and L genes delivered by SL7207. For all groups the transcript levels of the L, S and C genes increased gradually from days 21 to 49 (Fig. 5). The relative transcription levels ofland S in the livers of ducks receiving the single gene vaccines were slightly higher (about 1-fold) than in ducks receiving the gene fusion vaccines; however, the opposite trend was observed for the C gene (Fig. 5). The transcript levels of the L gene were almost 3-fold higher than the S and C genes and showed a more rapid rise, reaching the highest level on day 49. The transcript levels of the C gene delivered as a fusion protein were higher than when C was delivered simultaneously with the L or S genes located on separate plasmids. Thus, our gene fusion vaccines enhanced the breadth of immunization, although transcription of the L, S and C gene fusions was slightly suppressed.
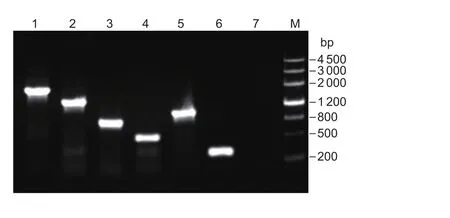
Fig. 2 PCR testing of the target plasmids. Lane M, Marker III;lane 1, plasmid pVAX1-SC (1 358 bp); lane 2, plasmid pVAX1-LC (1 908 bp); lane 3, plasmid pVAX1-C (789 bp); lane 4,plasmid pVAX1-S (437 bp); lane 5, plasmid pVAX1-L (987 bp);lane 6, plasmid pVAX1 (271 bp); lane 7, negative control.
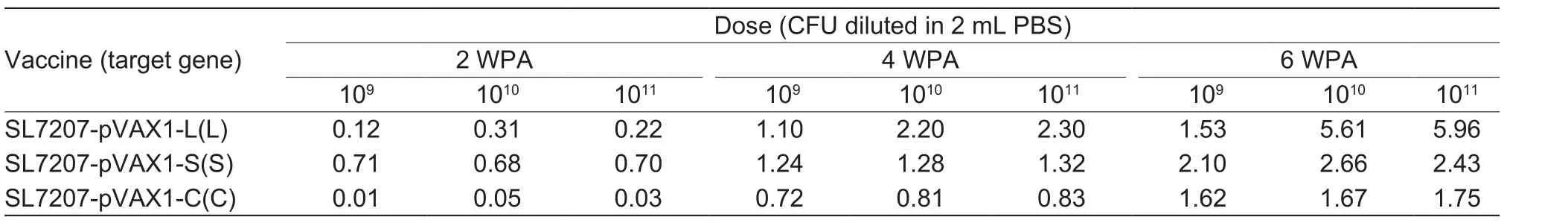
Table 2 The relative transcript levels of immune-related genes in the liver of DNA vaccine-treated duck1)
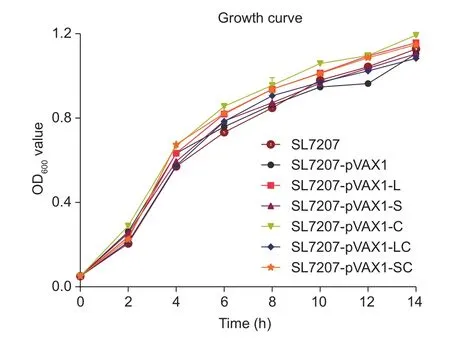
Fig. 3 Growth curves for recombinant bacteria in LB liquid medium containing kanamycin over a 14-hour period. Warmed LB was inoculated with overnight bacterial liquid cultures.The starting OD600 value was 0.05. At 2-hour intervals, 1 mL samples were taken and OD600 was measured. The experiment was repeated 3 times. There was no significant differences compared with the SL7207 groups using Tukey’s tests. Bars are shown in standard deviation.
3.4. Enhancement of humoral antibody responses to DHBV proteins by gene fusion DNA vaccines
To examine the potential of the gene fusion DNA vaccine to enhance humoral immune responses against DHBV, pooled sera were obtained 14 days after every administration;7 days after each DHBV-positive serum challenge. As shown in Fig. 5, the 2 control duck groups did not develop a detectable anti-antigen response. In contrast, starting 14 days after the first DNA immunization with SL7207-pVAX1-LC or SL7207-pVAX1-SC, humoral responses targeting the L, S and C proteins were significantly higher (P<0.001) than the control groups. This response was further enhanced after the last 2 DNA vaccine administrations, and the IgY levels reached the highest titers on day 57 and were 8-fold higher than that in the control groups. There was also a significant difference in antibody titer between groups receiving vaccines targeting the C protein. For the SL7207-pVAX1-LC group in particular, the levels of antibodies targeting the C protein were much higher than for the SL7207-pVAX1-C and SL7207-pVAX1-L+SL7207-pVAX1-C groups at every time point (Fig. 6-C). Slightly lower antibody titers were observed for all experimental groups after the second challenge, which might be the consequence of the reaction with neutralizing antibodies. Taken together, these results suggest that expressing a fusion protein enhances the magnitude and breadth of IgY responses to a DNA vaccine.
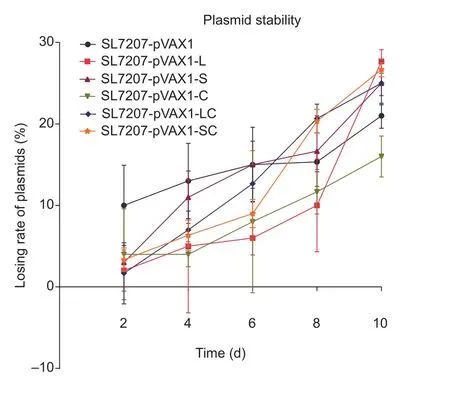
Fig. 4 Stability of plasmids in SL7207 grown on kanamycin-free LB plates over a 10-day period. Every day, 1 mL bacterial liquid culture was inoculated into fresh culture medium. In addition,200 μL bacterial culture was removed and gradient dilution was performed to find the appropriate concentration for colony counts. Plasmid loss rate (%)=1–(The average number of colonies per kanamycin LB plate)/Average number of colonies per kanamycin-free LB plate)×100. There was no significant differences compared with the SL7207-pVAX1 groups using Tukey’s tests. Bars are shown in standard deviation.
3.5. Enhancement of cellular immunity responses by fusion DNA vaccines
To estimate the numbers of CD4 and CD8 cells, we determined the transcript levels of the CD4 and CD8 genes after oral immunization. As shown in Fig. 7, levels of CD4 and CD8 were dramatically higher when compared to the control groups after the last 2 inoculations, with the greatest difference observed on day 49 (P<0.05). The level of CD4 transcription was approximately double the level of CD8 transcription at every time point for all treated groups.The level of CD4 expression in the negative control group(SL7207-pVAX1) also increased gradually, indicating that the live attenuatedS.typhimuriumcould induce cellular immunity. The capacity of the S antigen to induce cellular immunity was relatively lower than the L and C antigens.These data provide evidence that the fusion vaccines,especially SL7207-pVAX1-LC, effectively induce the cellular immune response in ducks.
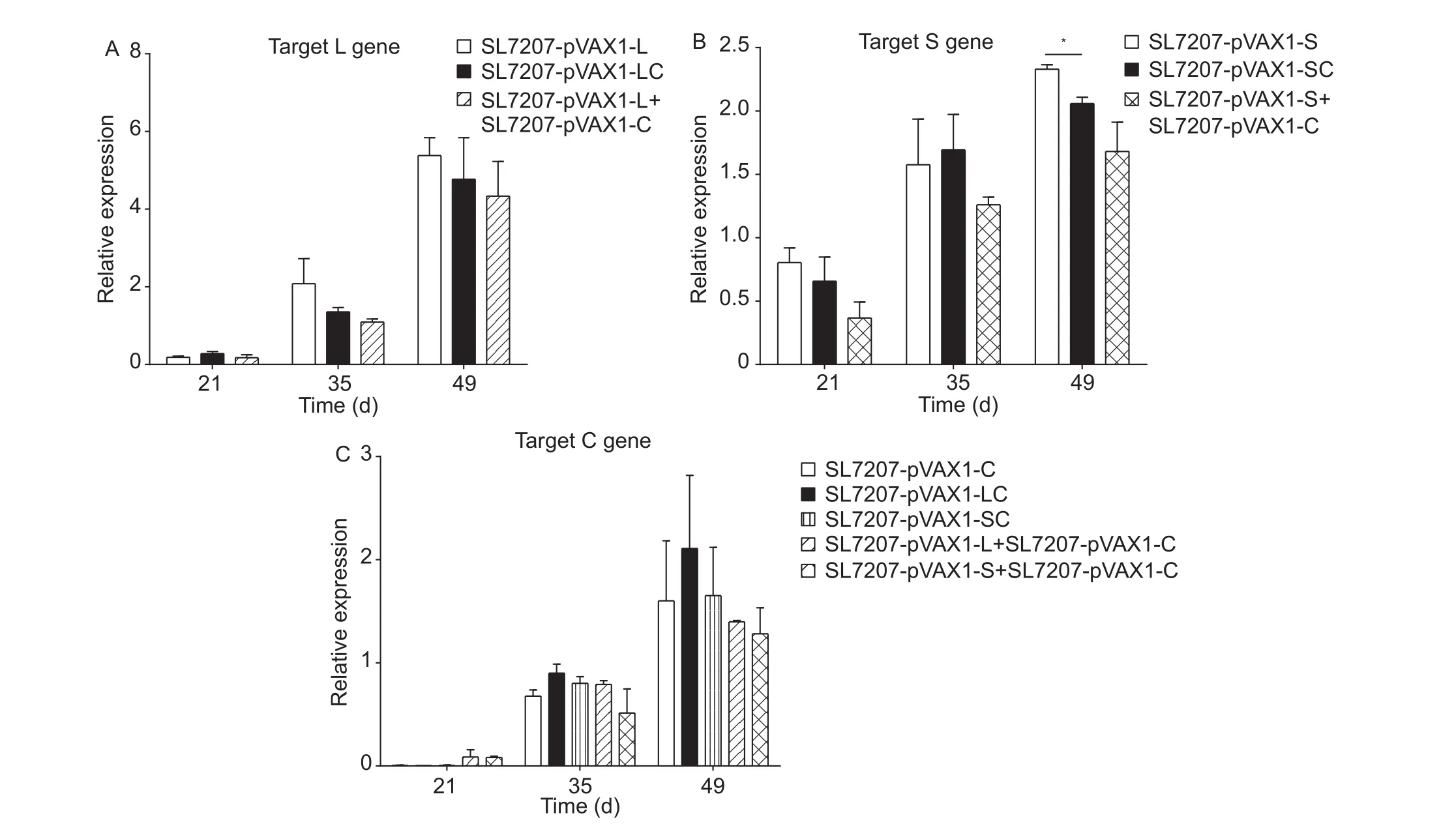
Fig. 5 The relative transcript levels of immune-related genes in the liver of DNA vaccine-treated duck. Liver was obtained from individual ducks on days 21, 35 and 49. The mRNA level of GAPDH was used as an internal control. Specific primers were used to detect the relative transcript levels targeting genes L (A), S (B) and C (C). The SL7207-pVAX1 group was used as the negative control and the PBS group as the blank control. Analyses of differences compared with the SL7207-pVAX1 groups were done using Tukey’s tests (*, P<0.05). Bars are shown in standard deviation.
3.6. Enhanced resistance to cccDNA in hepatocytes
As shown in Fig. 8, the level of cccDNA in the PBS control group dropped slightly during the 2 challenges, providing evidence that as ducks grow older, their immune system becomes much stronger. In addition, the cccDNA copy number of experimental groups decreased significantly compared with the PBS group, indicating that inoculated ducks could resist DHBV challenge at least 2 times.Furthermore, the fusion vaccine groups (SL7207-pVAX1-LC and SL7207-pVAX1-SC) had the lowest level of cccDNA after every challenge. The cccDNA copy number in the SL7207-pVAX1-LC group was 81.17% of the number in the SL7207-pVAX1 group on day 57, and then went up to 85.58% on day 71 (Table 3). The cccDNA copy number in the SL7207-pVAX1-SC group was 70.50 and 77.19% of the number in the SL7207-pVAX1 group at days 57 and 71, respectively. Additionally, only 3 ducks in the SL7207-pVAX1-LC group were infected after every challenge. The decrease in both infection rates and cccDNA copy number indicate that the delivery of fusion proteins, especially the L and C fusion proteins, enhance duck resistance against DHBV infection.
4. Discussion
There is an evidence that live attenuated bacterial vectors are superior (Linet al. 2015) to other methods of antigen presentation, such as intramuscular injection, electroporation and so on. Bacterial vaccines are convenient, efficient and can be produced inexpensively at a high-volume. Bacteria can also serve as adjuvants, providing immunostimulatory molecules such as lipopolysaccharide. Multiple antigens can be delivered orally, relieving animals from the pain of intravenous or intramuscular administration. In addition,antigens delivered by live bacteria are easily absorbed and processed. In this study, we have provided further evidence that administrationvialive attenuated bacteria is a potent delivery method that enhances the magnitude of immune response to a DNA vaccine.
Previous studies have shown that DNA vaccines have therapeutic efficiency for preventing DHBV infection, but we had insufficient proof that gene fusion DNA vaccines prevent infection with high-efficiency (Saadeet al. 2013;Isorceet al. 2015). In this study, to upgrade the immune efficacy in ducks, in addition to employing attenuatedS.typhimuriumSL7207 as a vector to deliver the eukaryotic expression plasmid, we adopted a wider range of immunity targeting both humoral immunity and cellular immunity. We used electroporation to obtain attenuatedS.typhimuriumharboring DHBV antigen genes and detected the transcript levels of these antigen genes in duck liver. Our results suggested that using attenuatedS.typhimuriumSL7207 as the oral delivery vector for the DHBV DNA vaccine did help the vaccines reach the hepatic cells where they were then transcribed. As we know, generating a strong immune response that lasts for a long period of time is one of the most important vaccine evaluation criteria (Duthieet al.2012), and the level of immune response is closely related to the growth kinetics of recombinant bacteria and the stability of antigen genes during bacterial delivery (Gahanet al. 2007). By comparing the growth kinetics with the parent strain SL7207 during the 14 hours after inoculation,no significant change was observed, which suggests that transformation had no influence on the proliferation of bacteria used for vaccine delivery. After 10 days cultivationin vitroin the absence of kanamycin, the number of positive bacteria was reduced by approximately 30%. In a safety study, neither deaths nor abnormal reactions were observed after inoculation with different dosages (109, 1010, and 1011CFU) of the recombinant attenuated SL7207 DNA vaccine.DHBV is hepatotropic (Locarnini and Roggendorf 2013);therefore, the use of salmonella for cloning in the liver could introduce plasmids directly into hepatocytes and strengthen immunoreaction. Our results were also consistent with previous studies where strong immune responses were obtained using attenuatedS.typhimuriumas a DNA vaccine carrier to prevent infection by viruses, such as transmissible gastroenteritis virus (TGEV) and duck plague virus (DPV)(Zhonget al. 2011; Yuet al. 2012). Taken together, our results indicate that attenuatedS.typhimuriumis an ideal candidate for DNA vaccine delivery.
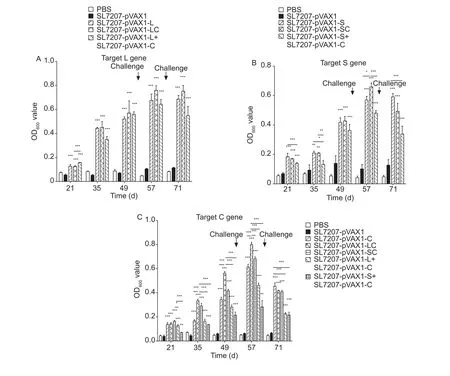
Fig. 6 Humoral immune responses to duck hepatitis B virus (DHBV)-specified L, S and C proteins measured by indirect enzymelinked immunosorbent assay (ELISA). Serum was obtained from individual ducks on days 21, 35, 49, 57 and 71. Serum antibody responses to L (A), S (B) and C (C) protein were measured. The SL7207-pVAX1 group was used as the negative control, and the PBS group was used as the blank control. Data are expressed as the means and standard deviation of IgY levels in each group.Analyses of differences compared with the SL7207-pVAX1 groups were done using Tukey’s tests (*, P<0.05; **, P<0.01; ***, P<0.001).
The expression of antigen genes could be detected in the liver where SL7207 colonizes. Herein, we only detected antigen gene expression in liver tissue because DHBV is hepatotropic, and the data in this organ are more representative. We found that the relative expression levels of antigen genes increased gradually after every inoculation time point. Interestingly, when we tried to express 2 different antigen genes, the expression of each gene was inhibited slightly compared with the expression of single genes. We speculated that this was due to the competition for nutrients in hosts, which inhibited the expression of exogenous antigen genes. In addition, the relative expression levels of genes encoding fusion proteins were higher than the genes located on separate plasmids, indicating that expressing a fusion gene was more efficient than expressing 2 separate genes.
The humoral immune response was previously found to neutralize circulating viral particles and prevent viral spread throughout a multicellular host (Khawajaet al. 2012b). In this study, the levels of specific serum antibodies (IgY)were evaluated 14 days after the administration of every oral vaccine and every challenge, and we found significant differences (P<0.001) between the experimental groups and the negative control group (SL7207-pVAX1) at the same time points. The levels of IgY increased gradually from day 21,peaking at day 57 and then dropping at a slow rate after the second challenge, indicating that our novel vaccines could markedly induce serum IgY. But the levels of IgY could also have decreased as a consequence of immune tolerance after the DHBV challenge, especially the second challenge.
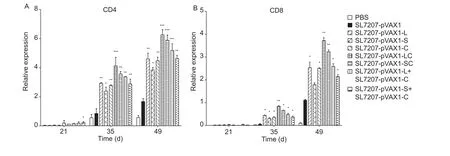
Fig. 7 The relative transcription levels of the CD4 and CD8 genes in the spleen of DNA vaccine-treated duck. Liver was obtained from individual ducks on days 21, 35, 49, 57 and 71. The mRNA level of GAPDH was used as an internal control. The SL7207-pVAX1 group was used as the negative control, and the PBS group was used as the blank control. Analyses of differences compared with the SL7207-pVAX1 groups were done using Tukey’s tests (*, P<0.05; **, P<0.01; ***, P<0.001). Bars are shown in standard deviation.

Fig. 8 The number of copies of covalently closed circular DNA(cccDNA) in hepatocytes after challenges. Immunized ducks were intravenously challenged with a viremic serum pool (2×1010 viral genome equivalents per duck) on days 50 and 64. Liver was obtained from individual ducks on days 57 and 71. Total DNA was extracted and then digested by Mung Bean Nuclease to degrade linear DNA. The number of copies of cccDNA were determined by PCR using specific primers. Analyses of differences compared with the SL7207-pVAX1 groups were done using Tukey’s tests (**, P<0.01; ***, P<0.001). Bars are shown in standard deviation.
CD4 and CD8 T cells play the powerful roles in preventing virus infection. Specifically, CD4 T cells regulate immunoreaction, not only by up-regulating costimulatory molecules on antigen presenting cells (APCs) (Cederbomet al. 2000) and improving their capacity to process and present antigens, but also by assisting the expression of the CD8 gene (Suvaset al. 2003). Therefore, the magnitude of the CD4 T cell response is a significant criterion when judging a potential vaccine. In our tests, the numbers of CD4 and CD8 T cells surged after the second and third DNA vaccine immunizations. At the last 2 time points, the number of CD4 T cells almost doubled compared with negative control groups, and the fusion groups displayed better effects than others. These results indicated that antigen fusion proteins may enhance mutual promotion effects and induce dramatic cellular immunoreaction.
We found that DNA vaccines stimulated both humoral and cellular immune responses, providing evidence for hepatitis B prevention. Moreover, challenge studies provided the first direct evidence of the ability of vaccinated ducks to resist DHBV infection. These findings suggested that vaccines,especially those expressing a fusion protein ofland C,show a protection efficacy that is superior to vaccines expressing 2 separate genes, and may stimulate a wider range of immune responses against different parts of the virus. The lower cccDNA copy number in the PBS groups after the second challenge demonstrated that the ability to fight against DHBV was strengthened among all ducks.But the infection might be cleared by the natural immune response even in the absence of vaccination. Ducks of that age (50- and 64-days old) are likely to experience a transient DHBV infection. Therefore, other immunization data such as the level of IgY in the blood and number of CD4 and CD8 T cells in spleen were assessed to support DHBV transient infection.
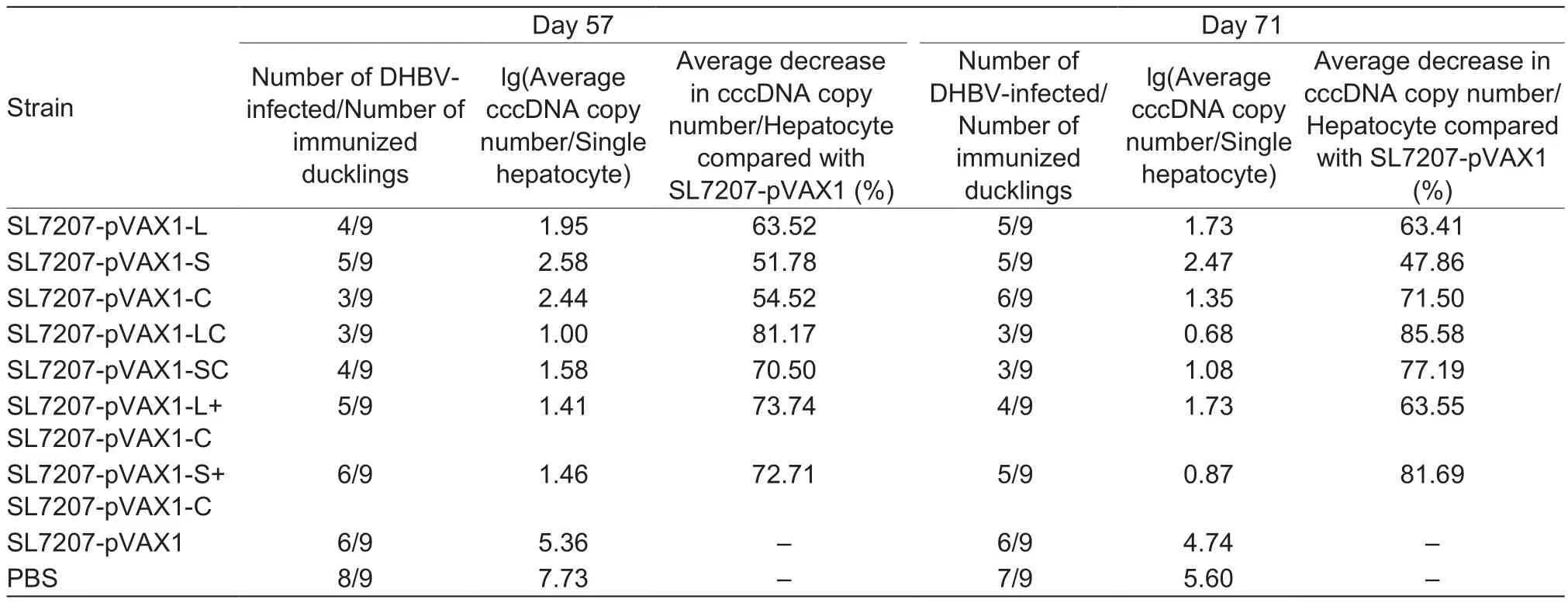
Table 3 Protective efficiency data
5. Conclusion
On the whole, our results were consistent with previous observation (Derricket al. 2004) that fused antigens, which contained a wider range of protein epitopes, reduced the likelihood of suffering from MHC-related restriction in heterologous populations. Moreover, in this study, we found that a recombinant molecule encouraged equivalent uptake of the antigen protein by APCs, reducing the possibility of immunodominant responses. In general, our novel fusion vaccine, SL7207-pVAX1-LC delivered by attenuatedS.typhimuriuminduced better systemic immune responses and reduced the infection of hepatocytes by DHBV more than vaccines consisting of single gene counterparts. Based on this finding, SL7207-pVAX1-LC may be a promising DHBV vaccine. In the future, it is worth further exploring DNA vaccines expressing multiple antigen genes delivered by attenuatedS.typhimurium, as well as the therapeutic benefits when applied to the duck model of DHBV infection.
Acknowledgements
This research was supported by the National Key Technology R&D Program of China (2015BAD12B05), the earmarked fund for China Agricultural Research System (CARS-43-8),the Integration and Demonstration of Key Technologies for Duck Industry in Sichuan Province, China (2014NZ0030),and the Sichuan Province Research Programs, China(2014-002).
Berger E, Soldati R, Huebener N, Hohn O, Stermann A,Durmus T, Lobitz S, Zenclussen A C, Christiansen H, Lode H N. 2013. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for neuroblastoma.Cancer Letters, 331, 167–173.
Cederbom L, Hall H, Ivars F. 2000. CD4+CD25+regulatory T cells down-regulate co-stimulatory molecules on antigenpresenting cells.European Journal of Immunology, 30,1538–1543.
Cui X, Luckenbaugh L, Bruss V, Hu J. 2015. Alteration of mature nucleocapsid and enhancement of covalently closed circular DNA formation by hepatitis B virus core mutants defective in complete-virion formation.Journal of Virology,89, 10064–10072.
Cui X, Ludgate L, Ning X, Hu J. 2013. Maturation-associated destabilization of hepatitis B virus nucleocapsid.Journal of Virology, 87, 11494–11503.
Derrick S C, Yang A L, Morris S L. 2004. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection withMycobacterium tuberculosisand boosts BCG-induced protective immunity.Vaccine, 23, 780–788.
Duthie M S, Raman V S, Piazza F M, Reed S G. 2012. The development and clinical evaluation of second-generation leishmaniasis vaccines.Vaccine, 30, 134–141.
Frickmann H, Dekker D, Boahen K, Acquah S, Sarpong N,Adu-Sarkodie Y, Schwarz N G, May J, Marks F, Poppert S. 2013. Increased detection of invasive enteropathogenic bacteria in pre-incubated blood culture materials by realtime PCR in comparison with automated incubation in Sub-Saharan Africa.Scandinavian Journal of Infectious Diseases, 45, 616–622.
Gahan M E, Webster D E, Wesselingh S L, Strugnell R A.2007. Impact of plasmid stability on oral DNA delivery by Salmonella enterica serovarTyphimurium.Vaccine, 25,1476–1483.
Greco N, Hayes M H, Loeb D D. 2014. Snow goose hepatitis B virus (SGHBV) envelope and capsid proteins independently contribute to the ability of SGHBV to package capsids containing single-stranded DNA in virions.Journal of Virology, 88, 10705–10713.
Guidotti L G. 2014. Animal models of Hepatitis B and C. In:Encyclopedia of Medical Immunology. Springer, New York.pp. 44–49.
Handley S A, Desai C, Zhao G, Droit L, Monaco C L, Schroeder A C, Nkolola J P, Norman M E, Miller A D, Wang D.2016. SIV infection-mediated changes in gastrointestinal bacterial microbiome and virome are associated with immunodeficiency and prevented by vaccination.Cell Host& Microbe, 19, 323–335.
Hu Q, Wu M, Fang C, Cheng C, Zhao M, Fang W, Chu P K,Ping Y, Tang G. 2015. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy.Nano Letters, 15, 2732–2739.
Isorce N, Lucifora J, Zoulim F, Durantel D. 2015. Immunemodulators to combat hepatitis B virus infection: From IFN-α to novel investigational immunotherapeutic strategies.Antiviral Research, 122, 69–81.
Khawaja G, Buronfosse T, Jamard C, Abdul F, Guerret S, Zoulim F, Luxembourg A, Hannaman D, Evans C F, Hartmann D.2012a.In vivoelectroporation improves therapeutic potency of a DNA vaccine targeting hepadnaviral proteins.Virology,433, 192–202.
Khawaja G, Buronfosse T, Jamard C, Guerret S, Zoulim F,Luxembourg A, Hannaman D, Evans C, Hartmann D, Cova L. 2012b. Enhanced magnitude and breadth of neutralizing humoral response to a DNA vaccine targeting the DHBV envelope protein delivered byin vivoelectroporation.Virology, 425, 61–69.
Li B, He H, Zhang S, Zhao W, Li N, Shao R. 2012.Salmonella typhimuriumstrain SL7207 induces apoptosis and inhibits the growth of HepG2 hepatoma cellsin vitroandin vivo.Acta Pharmaceutica Sinica(B), 2, 562–568.
Lin I Y, Van T T H, Smooker P M. 2015. Live-attenuated bacterial vectors: Tools for vaccine and therapeutic agent delivery.Vaccines, 3, 940–972.
Liu Q, Huang J, Jia R, Wang M, Zhu D, Chen S, Liu M, Yin Z,Wang Y, Cheng A. 2015. The pregenome/C RNA of duck hepatitis B virus is not used for translation of core protein during the early phase of infectionin vitro.Virus Research,196, 13–19.
Locarnini S A, Roggendorf M. 2013. OtherHepadnaviridae(Avihepadnaviridae(DHBV) andOrthohepadnaviridae(WHV)). In:Viral Hepatitis. 4th ed. John Wiley & Sons, Ltd.,USA. pp. 96–106.
Muhammad A, Champeimont J, Mayr U B, Lubitz W, Kudela P. 2012. Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications.Expert Review of Vaccines, 11, 97–116.
Nassal M. 2015. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B.Gut, 64,1972–1984.
Pereira V B, Azevedo M S P, Saraiva T D L, Souza B M, Turk M Z, De Castro C P, Agresti P M, Lima F A, Azevedo V,Pontes D S. 2013. Use of bacteria in DNA vaccine delivery.Vaccine, 12, 15.
Piasecki T, Kurenbach B, Chrząstek K, Bednarek K, Kraberger S, Martin D P, Varsani A. 2012. Molecular characterisation of an avihepadnavirus isolated fromPsittacula krameri(ring-necked parrot).Archives of Virology, 157, 585–590.
Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G,Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. 2004.Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection.Gastroenterology, 126,102–110.
Qian F, Guo A, Li M, Liu W, Pan Z, Jiang L, Wu X, Xu H. 2015.Salmonella flagellin is a potent carrier-adjuvant for peptide conjugate to induce peptide-specific antibody response in mice.Vaccine, 33, 2038–2044.
Reaiche-Miller G Y, Thorpe M, Low H C, Qiao Q, Scougall C A, Mason W S, Litwin S, Jilbert A R. 2013. Duck hepatitis B virus covalently closed circular DNA appears to survive hepatocyte mitosis in the growing liver.Virology, 446,357–364.
Saade F, Buronfosse T, Guerret S, Pradat P, Chevallier M,Zoulim F, Jamard C, Cova L. 2013.In vivoinfectivity of liver extracts after resolution of hepadnaviral infection following therapy associating DNA vaccine and cytokine genes.Journal of Viral Hepatitis, 20, e56–e65.
Siegler V D, Bruss V. 2013. Role of transmembrane domains of hepatitis B virus small surface proteins in subviral-particle biogenesis.Journal of Virology, 87, 1491–1496.
Suvas S, Kumaraguru U, Pack C D, Lee S, Rouse B T. 2003.CD4+CD25+T cells regulate virus-specific primary and memory CD8+T cell responses.The Journal of Experimental Medicine, 198, 889–901.
Wang C, Kang Y, Jiang Z, Wang P. 2012. Construction and expression of recombinant Salmonella SL7207/pEGFP-N1-AQP9 and SL7207/pshRNA-AQP9.Chongqing Medicine,33, 001. (in Chinese)
Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K,Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z,Delaney W E. 2004. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy.Gastroenterology, 126, 1750–1758.
Xue Q J, Dai J, Li X Z, Zhu W, Si C P, Chen T. 2014.Construction of a recombinant - BCG containing the LMP2A and BZLF1 genes and its significance in the Epstein- Barr virus positive gastric carcinoma.Journal of Medical Virology, 86, 1780–1787.
Yu X, Jia R, Huang J, Shu B, Zhu D, Liu Q, Gao X, Lin M, Yin Z, Wang M. 2012. AttenuatedSalmonella typhimuriumdelivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge.Veterinary Research, 43, 1.
Zhong Y, Han J, Zou Z, Liu S, Tang B, Ren X, Li X, Zhao Y,Liu Y, Zhou D. 2011. Quantitation of HBV covalently closed circular DNA in micro formalin fixed paraffin-embedded liver tissue using rolling circle amplification in combination with real-time PCR.Clinica Chimica Acta, 412, 1905–1911.
 Journal of Integrative Agriculture2018年4期
Journal of Integrative Agriculture2018年4期
- Journal of Integrative Agriculture的其它文章
- Climate change and Chinese farmers: Perceptions and determinants of adaptive strategies
- Estimating the average treatment effect of adopting stress tolerant variety on rice yield in China
- Effects of conditioners (single-layer, double-layer and retentionconditioner) on the growth performance, meat quality and intestinal morphology of growing and finishing pigs
- Sub-lethal effects of Beauveria bassiana (Balsamo) on field populations of the potato tuberworm Phthorimaea operculella Zeller in China
- Regionalization of wheat powdery mildew oversummering in China based on digital elevation
- Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii
