Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii
Simi Jacob, Ramgopal Rao Sajjalaguddam,, Hari Kishan Sudini
1 International Crops Research Institute for the Semi-Arid Tropics, Patancheru 502324, India
2 Jawaharlal Nehru Technological University, Kukatpally, Hyderabad 500085, India
3 Biocon Academy, Biocon House, Bangalore 560100, India
1. Introduction
Stem rot also known as white mold or southern blight is caused bySclerotium rolfsiiSacc. This soilborne pathogen is necrotrophic with a wide host range (Aycock 1966)affecting productivity and causing large scale economic losses (Punja 1985; Shewet al. 1987; Wokocha 1990;Cillierset al. 2003; Singhet al. 2003). Among its many host genera, peanut sustains high yield losses (Aycock 1966).This is one of the major soilborne diseases affecting peanut productivity around the world (Backman and Brenneman 1997). According to Mayee and Datar (1988), typical yield losses due to this disease was reported to be over 25% and under severe conditions this may go to 80%. The pathogen survives in the soil as resistant structures called sclerotia that are found associated with plant debris or near the soil surface remaining viable for a long period in the absence of a susceptible host, serving as primary inoculum for disease(Aycock 1966; Backman and Brenneman 1997). Moist conditions, dense planting, high temperature and frequent irrigations are considered favourable conditions for disease development (Aycock 1966; Punja 1985). Initial symptoms include browning and wilting of leaves and branches (Koike 2004). Further, pods are infected and covered by white mycelia. Sclerotia in the soil germinate in the presence of volatiles and alcohols from decomposing plant material present on the surface of the soil (Clark and Moyer 1988;Backman and Brenneman 1997). Punja (1985) reported that increase in temperatures and damp conditions enhance sclerotial germination. Deep ploughing is an important cultural practice that can keep the amount of viable inoculum in check.
Currently application of fungicides are in use to reduce stem rot incidence to a large extent as most of the peanut varieties in cultivation are either susceptible or have low levels of resistance. But their continuous use may lead to the development of resistant strains apart from posing risk to the environment. Moreover fungicide application is not a feasible option for the resource poor smallholder farmers of the Asian and African countries due to its high cost.Hence there is a need for sustainable and environmentally safe way of disease control. Alternative strategies involve the use of natural resources and products (Copping and Menn 2000; Rimando and Duke 2006; Baleet al. 2008).Biological control is a reliable and sustainable alternative to better manage soilborne pathogens and to increase productivity and soil health. Biocontrol can be broadly defined as the reduction in amount of pathogen inoculum or disease producing activity of a pathogen accomplished by or through one or more organisms other than man (Cook and Baker 1983). This property relies on the ability of a microbial species to antagonize another organism through parasitism, competition and antibiosis (Alabouvetteet al.2006). Biological control ofS.rolfsiiin different crop species have been earlier reported (Madiet al. 1997; Ozgonenet al.2010; Ikaet al. 2011; Rakhet al. 2011; Adhilakshmiet al.2014). However, most of the reported studies make use of culture broth or talc formulations of whole organisms for disease control. In the current study we investigate use of metabolites for disease control.
Actinobacteria are excellent choices as plant disease control agents due to their ability to produce fungicidally important compounds that antagonize many phytopathogens(Xiaoet al. 2002; Meschkeet al. 2012). These are a diverse group of free living saprobic mycelial bacteria present abundantly in the soil, maintaining the structure and integrity of soil. They also take part in the recycling of soil nutrients (Ameset al. 1984; Halderet al. 1991; Elliot and Lynch 1995). They are widely studied for the production of many economically important antimicrobial metabolites(Lazzariniet al. 2000; Bentleyet al. 2002; Saugaret al.2002; Basilioet al. 2003; Terkinaet al. 2006). Among the different actinobacterial genera,Streptomycesspp. have been commonly isolated and studied as biological control agents. They are one of the major sources of bioactive natural products (Berdy 2012). The objective of the present study is to evaluate the competence of a previously reported potential biocontrol agent and its metabolites to manage disease incidence and improve crop yield under field conditions.
2. Materials and methods
2.1. Source and selection of the antagonist
The strainStreptomycessp. RP1A-12 used in this study was isolated from peanut rhizosphere soil of the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) fields and selected for field evaluation based on its antagonistic abilities on the stem rot pathogenS.rolfsii. The strain was identified using 16S rRNA gene sequencing asStreptomycesflocculus(Simiet al. 2016).This gene sequence of RP1A-12 was deposited at GenBank(accession no. KR049226).
2.2. Isolation and source of pathogen
The stem rot pathogen,S.rolfsiiculture was obtained from the culture collection of the Peanut Pathology Lab at ICRISAT. The culture was originally isolated from diseased peanut plants with typical stem rot symptoms at ICRISAT,Patancheru fields and maintained on potato dextrose agar(PDA; HiMedia) plates incubated at 25°C. The pathogenicity of the fungal culture was confirmed earlier using Koch’s postulates. Sclerotia were harvested from media plates cultured for 14 days and stored at 4°C for further studies.
2.3. Preparation of bio-agent formulations
Biomass ofStreptomycessp. RP1A-12 was extracted by inoculating actively growing cells into glucose casamino yeast extract (GCY) medium and incubated for 3 days at 180 r min–1. Following incubation, cells were centrifuged at 9 000 r min–1for 20 min and pellets collected and stored at–20°C. As required, the pellets were diluted with distilled water till a final concentration of 108colony-forming units(CFU) mL–1was obtained using the formula:
CFU mL–1=Number of colonies×Dilution factor/Volume of culture plate
Talc formulations (TF) were prepared by mixing culture biomass with sterilized talc powder at the rate of 400 mL kg–1talc (Vidhyasekaran and Muthamilan 1995). Partially purified crude metabolites of the same bioagent were obtained from culture filtrates grown in GCY for 5 days and extracted using diaion HP-20 (SUPELCO Sigma Aldrich)resin (Sterner 2012). Adsorbed fractions were eluted with methanol and concentrated on rotary evaporator and this served as metabolic extract. The obtained metabolite extract was formulated with distilled water (Simiet al. 2016) and used as crude metabolite formulations (CMF). Peanut seeds used in this study were surface sterilized using 2.5% sodium hypochlorite solution for 2 min and washed several times with distilled water. Seed treatments (ST) were prepared by treating surface sterilized seeds with talc formulations at a rate of 10 g kg–1seed for 1 h for TFtreatments and soaked in crude metabolite formulation for 30 min for CMF treatments.
2.4. lnfluence of RP1A-12 and its metabolites on peanut seed germination and seedling vigor
The influence ofStreptomycessp. RP1A-12 and its metabolites on germinability and vigor of peanut seedlings was evaluated using the Ragdoll method (Chamblee and Green 1995) with slight modifications. JL-24 cultivar seeds were used for this purpose. Seeds were stored at normal conditions in paper bags until required for experimentation.Treated peanut seeds were placed on germination towels and incubated in growth chambers at 28°C for 15 days.There were four replications in each treatment, with 10 seeds per replication. Seeds soaked in sterile distilled water were served as the control. Seven days after incubation,number of germinated seeds was recorded. At the end of 15 days, roots and shoot lengths of seedlings, lateral root growths were recorded and the seedling vigor index 1 (VI-1) and seedling vigor index 2 (VI-2) were calculated using the formulae:
VI-1=Percent of germination (%)×Seedling growth (shoot length+root length) (cm)
VI-2=Percent of germination (%)×Seedling dry weight (g)
For VI-2, the seedlings were air-dried and then placed in a hot air oven at 105°C for 2 h. After that they were weighed to determine mean seedling dry mass (g) in each replication.
2.5. Pot trials for growth promotion studies
The ability of the biocontrol agent to promote growth and enhance pod yield of peanut plants was evaluated. Treated seeds (as described above) were sown in 6-inch pots filled with sterile sand and soil mixed in a ratio of 1:2. Seven treatments were designed for this study,viz., 1) seed treatment (ST) with RP1A-12 talc formulations (ST TF); 2)soil application (SA) with RP1A-12 talc formulations (SA TF);3) seed treatment and soil application with RP1A-12 talc formulations ((ST+SA) TF); 4) seed treatment with RP1A-12 metabolites (ST CMF); 5) soil application with RP1A-12 metabolites (SA CMF); 6) seed treatment and soil application with RP1A-12 metabolites ((ST+SA) CMF). Soil application was done by mixing pot mixture with TFat the rate of 10 g kg–1soil. CMFwas applied as a soil drench at the rate of 50 mL per pot after planting seeds. Un-treated seeds were used as the controls. Seven days after sowing, the number of germinated seeds was counted. The experiment was carried out in a completely randomized design (CRD) with each treatment replicated thrice. The pots were maintained in a controlled environment (16-h day/8-h night cycle, at a constant temperature of 28°C and a relative humidity of 90%) and watered regularly. Observations for effective (pink coloured) nodule count and root length were recorded by harvesting the plants at 45 days after sowing (DAS) and for parameters such as plant height, plant biomass, vigor index and pod yield at 120 DAS (Deyet al. 2004). This study was conducted twice and the mean data were analyzed and presented.
2.6. Field evaluations for stem rot disease control
The biocontrol agentStreptomycessp. RP1A-12 was evaluated against the stem rot pathogen,S.rolfsii, for its disease suppressing and yield enhancing abilities under field conditions consecutively for 2 years. Stem rot susceptible peanut variety JL-24 was used in the study. Both the field experiments were conducted at ICRISAT research farm during the rainy seasons of 2014 and 2015 (June 23rd sowing in 2014 and June 26th sowing in 2015). The same piece of land was used for the second year evaluations.No other crop was grown in between. Type of soil was red sandy loam and the plot area used for conducting the investigation was 30 m×6.3 m with three blocks as three replications. Each block was divided into beds with each bed representing one treatment. Each bed having dimensions of 2 m×1.5 m (length×breadth) was divided into four rows and a total of 80 peanut seeds sown in each bed. Inter (distance between rows) and intra (distance between plants within a row) spacing of 30 and 10 cm respectively was maintained.The experiment was carried out in a randomized complete block design (RCBD). During field preparation, single super phosphate (SSP) 372 kg ha–1was applied as basal dose. The bioagent RP1A-12 was used both as crude metabolites and whole organism based formulations. The treatments include: 1) seed treatment and soil application with RP1A-12 talc formulations (TF); 2) seed treatment and soil application with RP1A-12 metabolites (CMF); 3) chemical control (azoxystrobin); 4) pathogen inoculated control;and 5) un-inoculated control. The fungicide azoxystrobin(250 g L–1(23.1%, w/w), Amistar®, Syngenta, Switzerland)solution diluted at a rate of 1 mL L–1of water was used for experimental purpose. Chemical was applied twice at 50 and 65 DAS respectively in both the seasons.
Pathogen inoculum preparation and inoculationThe pathogen,S.rolfsii, was mass multiplied on autoclaved sorghum grains for inoculating the field plots. Overnight soaked sorghum grains sterilized in glass flasks and polythene bags were inoculated with fresh mycelial disks and incubated for 10–15 days at (28±2)°C. The field soil was infested artificially with the prepared pathogen inoculum, by opening a furrow on both sides of rows in each bed, with an inoculum rate of 1 kg inoculum per 9 m2area. Inoculum was added around 40 DAS. The plots were irrigated on the same day and repeated as and when required.
Antagonist inoculum preparation and inoculationTalc formulations of the bioagent were prepared according to Vidhyasekaran and Muthamilan (1995). Culture biomass extracted as mentioned above was mixed with sterilized talc at a rate of 400 mL kg–1. Talc powder used was mixed with calcium carbonate (CaCO3) and carboxy methyl cellulose prior to sterilization to maintain its pH and stickiness. Partially purified metabolites of the same bioagent were extracted from culture filtrates using diaion HP-20 (SUPELCO Sigma Aldrich) resin with methanol (Sterner 2012). The obtained metabolites were mixed with water at the rate of 1:100 for field use. For use as soil application, talc formulations were mixed with farmyard manure (FYM) at the rate of 1 kg formulated talc in 90 kg of FYM and applied at a rate of 4.8 kg of formulated FYM per 9 m2area. For seed treatments,surface sterilized seeds were treated with the formulated talc powder at a rate of 10 g kg–1seed. A total of 400 mL of the partially purified metabolite formulation was applied as soil drench on alternate rows of the specific treatments.Application of whole organism based formulation and crude metabolites based formulation were done 15 days after pathogen inoculation (Shokeset al. 1996).
Data collectionDisease infection parameters such as number of infected plants (incidence) and number of dead plants (mortality) were recorded 15 days after pathogen inoculation. Subsequent observations were taken after every 2 weeks interval. Crop yields were measured upon harvest stage (approximately 120 DAS). Percent disease control by treatment over inoculated control and production losses were also calculated. Percent disease incidence(PDI) (Kokalis-Burelleet al. 1992) and percent disease control (PDC) (Rakhet al. 2011) were measured using the below formulae:
PDI (%)=Number of infected plants/Total number of plants×100
PDC (%)=(Percent of disease in inoculated control–Percent of disease inoculated control)/Percent of disease in inoculated control×100
2.7. Statistical analysis
All statistical analyses were performed using GenStat 14.0 statistical package (2013, Lawes Agricultural Trust,Rothamsted Experimental Station). Data were analyzed by analysis of variance (ANOVA). Differences between the treatment means were compared by Duncan’s multiple range test at a 5% level of significance. Field experiment data were arc-sine transformed in order to stabilize the variance.
3. Results
3.1. In vitro seed germination and seedling vigor studies
Results of thein vitrostudies indicate seed germination and seedling vigor was enhanced significantly over the control when treated with TFof the candidate bioagentStreptomycessp. RP1A-12 and was on par with control when treated with CMF(Table 1). VI-1 was significantly (P=0.05) increased in TFtreatment (4 220) which was above control treatments(3 424) followed by CMFtreated seeds (3 350) (Table 1).Root length and shoot length of TFtreated seeds were alsoincreased by 17 and 10% compared to control, respectively(Table 1).
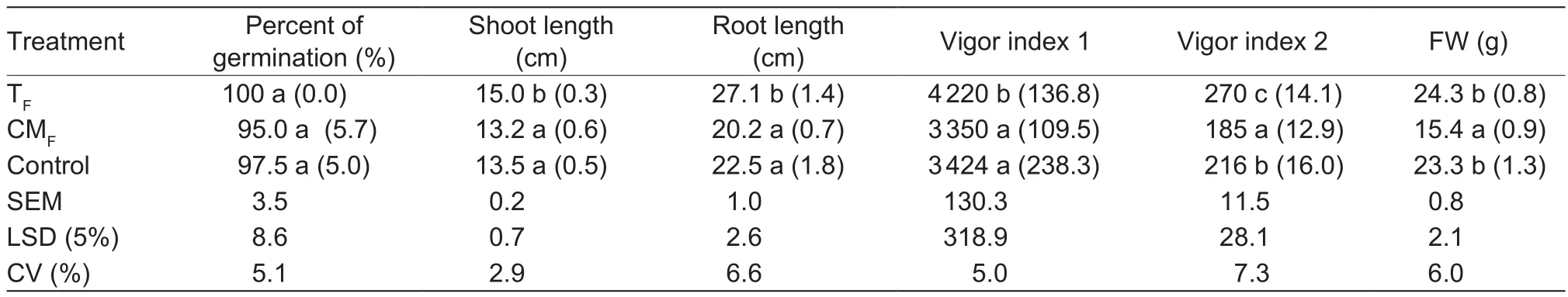
Table 1 In vitro study on the influence of Streptomyces sp. RP1A-12 and its metabolites on peanut seed germination and seedling vigor1)
3.2. Pot trials
Results of the pot trials conducted for growth promotion studies are summarized in Table 2. In general, significant(P<0.05) improvement was seen in nodule count, plant height, plant fresh weight and yield when treated with TFStreptomycessp. of RP1A-12 (Table 2). Pod yields of treated plants were increased by 31, 42 and 30% when treated with ST TF, SA TFand (ST+SA) TF, respectively.Significant improvement (50%) in the nodule number of bioagent treated seeds was observed. Treatment with TFhad a superior effect on peanut growth compared to CMFtreatments which were on par with the control treatments(Table 2).
3.3. Field evaluations
Disease incidence was low during 2014 rainy season compared to 2015 rainy season. Average disease incidence during 2014 was noted as 24% where as it was 90% in 2015. Statistically significant difference in disease occurrence was seen among the treatments amended with bioagents compared with control treatments without any bio-treatments during both the seasons (Table 3). In the first season, stem rot incidence in both bioagent talc formulation (7.34%) and crude metabolites (8.34%) treated plants was lower compared to inoculated control (23.84%).The results were on par with uninoculated healthy control(8.34%) and chemical control (10.63%; Table 3). During the second season, the least percent disease incidence among the treatments was recorded in the metabolite treatment(45.82%) which was significantly lower than chemical control(64.52%), un-inoculated control (68.93%) and inoculated control (90.19%; Table 3). In 2014, both RP1A-12 crude metabolites and talk formulation exhibited similar amounts of disease control which was on par with the un-inoculated control. However during 2015, RP1A-12 metabolites treated plants displayed a superior disease control compared to all the other treatments tested (Table 3). Application of bioagent RP1A-12 as TFand CMFlowered the severity index which was on par with the un-inoculated control(Fig. 1). Overall in the combined data, lower incidence was recorded in the crude metabolite treatment (27%) which was significantly lower than the inoculated control (57%),un-inoculated control (38%) and chemical control (37%).Disease incidence with bioagent’s TF(38%) treatment was also significantly less compared to the inoculated control(Table 3). Pod yields of different treatments were recorded during the two seasons and their means were pooled as the trend remained the same across the seasons. Pod yields of treated plants were higher, which was statistically superior over inoculated control treatment (Table 3).
4. Discussion
During the past decade actinomycetes have been given considerable importance due to their ecological role in nutrient cycling and as plant growth promoters (Jianget al.2006; Pathom-areeet al. 2006; Franco-Correaet al. 2010).The beneficial effects of actinomycetes on plants are brought about by plant growth promotion and disease suppression activities. In our earlier study, we assessed the prospects ofStreptomycessp. RP1A-12 in managing groundnut stem rot disease caused byS.rolfsiiunder the greenhouse conditions(Simiet al. 2016). In this study,Streptomycessp. RP1A-12 was evaluated for growth promotion of peanut plants and stem rot disease control under field conditions. Biocontrolagents isolated from the rhizosphere of a particular crop adapt and provide better disease control when applied to the same crop (Cook 1993). Several reports are available on plant growth promotion activities of actinomycetes (Gopalakrishnanet al. 2011; Adhilakshmiet al. 2014; Simi and Sudini 2016) and the use of biological agents for the control of stem rot (Karthikeyanet al. 2006; Ganesanet al. 2007; Rakhet al. 2011; Bashaet al. 2012). In our study, the strain was found to improve vigor index 1 and 2 which is a measure of the influence on seed germination and seedling growth on treating with talc formulation of biomass(Table 1). Similar results were achieved in the studies conducted by Dochhilet al. (2013) and Tančićet al. (2013) where the seedling vigor, root and shoot lengths were increased by the beneficial microbes. Apart from this, production of growth regulators like auxins and gibberellin-like compounds were reported to be involved in growth promotion (Manuliset al. 1994; Bloemberg and Lugtenberg 2001; Doumbouet al. 2001).Streptomycessp. RP1A-12 was reported to produce indole-3-acetic acid (IAA) (Simiet al. 2016). Increase in root length and shoot length of peanut seedlings may be attributed to the production of such growth regulators. Studies conducted by Garridoet al. (2002) and Lopezet al. (2004)showed an increase in lateral roots and root elongation due to IAA production.Results of pot experiments conducted to assess growth promotion were in agreement within vitrostudies to enhance seed germination and seedling vigour(Table 2) and also with other reports using microbes as plant growth promoters(Patten and Glick 2002; Gopalakrishnanet al. 2011). Furthermore significant increase in nodule count, biomass accumulation and yield parameters were also noticed (Table 2). In bothin vitroand greenhouse studies, crude metabolites used did not improve seed germination and seedling vigor significantly but the results were comparable to control plants.
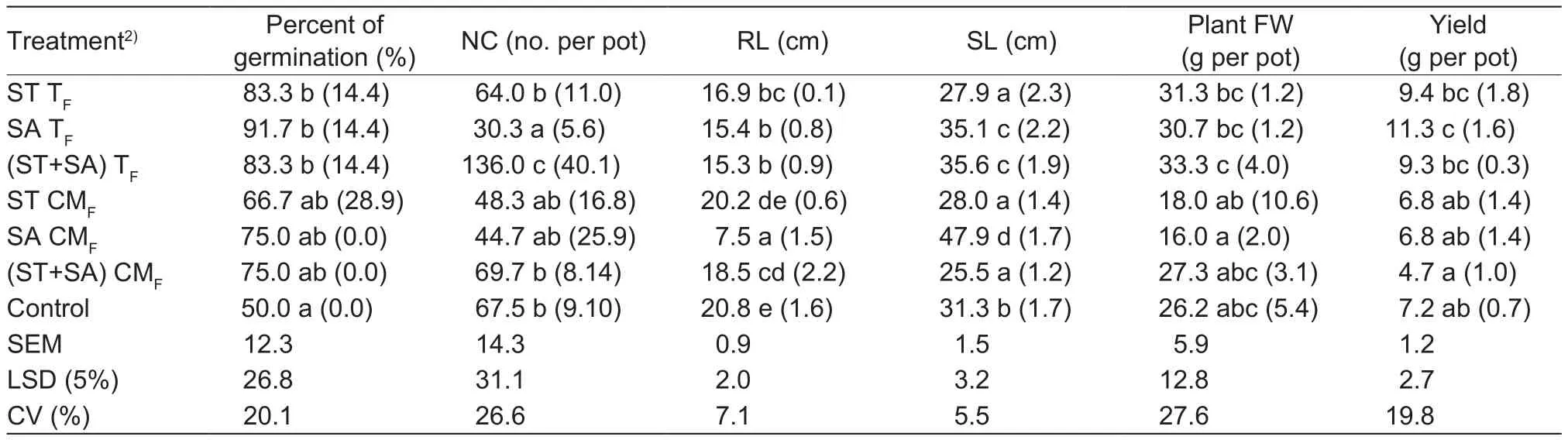
Table 2 Peanut growth promotion by Streptomyces sp. RP1A-12 in greenhouse studies1)
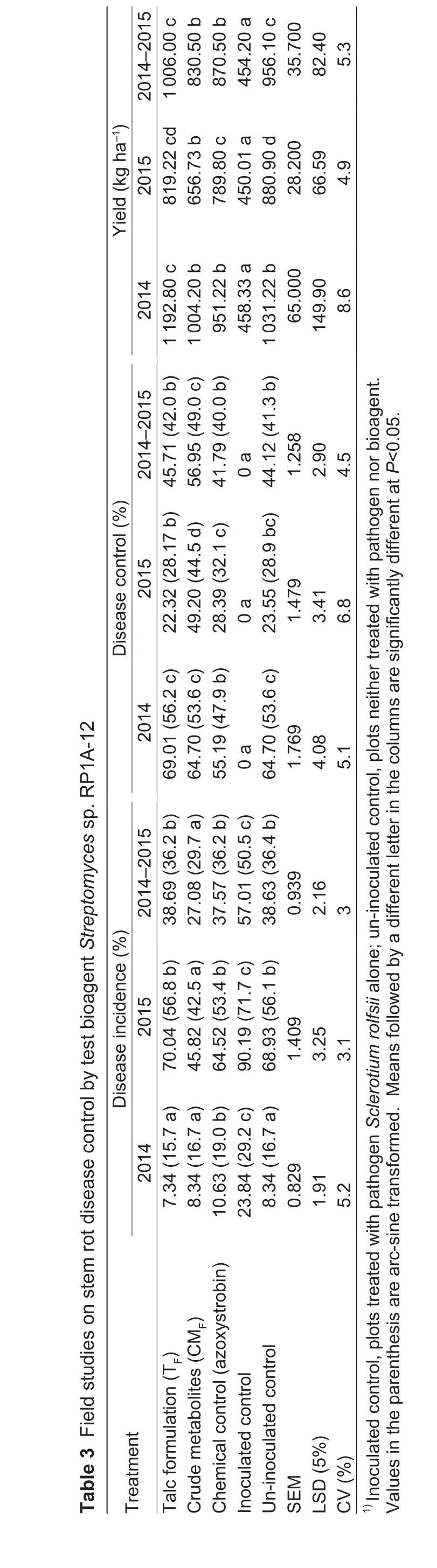
?
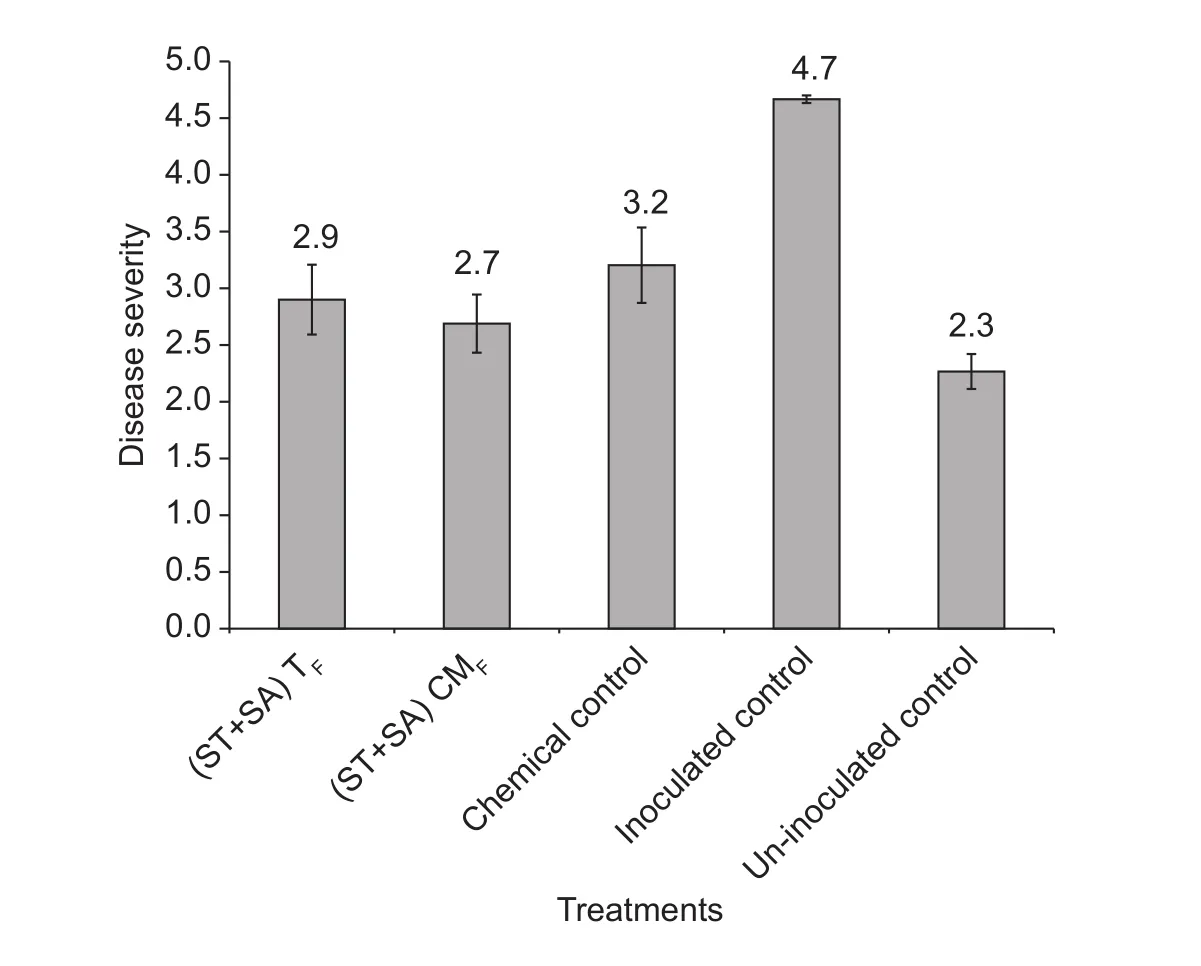
Fig. 1 Effect of Streptomyces sp. RP1A-12 formulations on stem rot disease severity in field studies. ST+SA, seed treatment and soil application; TF, talc formulations;CMF, crude metabolite formulation; inoculated control, plots treated with pathogen Sclerotium rolfsii alone; un-inoculated control, plots neither treated with pathogen nor bioagent. Data are means±SE.
Effective decrease in disease incidence was seen with the use of talc formulations (45%) and also by the application of crude metabolite formulations(56%). However, when the disease pressure is high with 90%incidence in 2015, percent disease control brought by CMF(49%) is significantly high compared to TF(22%). This can be due to the antifungal activity of the secondary metabolites present in the partially purified extract. Heavy disease incidence and incessant rains in September (173 mm)and October (63.6 mm) of 2015 as against to 47.6 and 47.4 mm total rainfall in September and October of 2014 might be the reason for lower performance of bioagents applied as TF. It is a well-known fact that actinomycetes produce a wide variety of chemically diverse and biologically active substances that are involved in the antagonism against microbial pests (Ensign 1992; Behal 2000). Also inhibition of oxalic acid production by crude metabolites established in our earlier study (Simiet al. 2016) might have played a crucial role in reducing stem rot incidence.Oxalic acid is reported as one of the important pathogenicity factor of stem rot pathogenS.rolfsii(Marcianoet al. 1983).Streptomyces isolates known to produce siderophores and hydrogen cyanide (HCN) (Haaset al. 1991; Weiet al. 1991)play a direct role in disease suppression was established in earlier studies. The isolateStreptomycessp. RP1A-12 was also shown to produce siderophores which are known to chelate iron and make it readily available for plant nutritional requirements. Results indicate that soil drench with crude metabolite-based formulation had better efficiency in controlling disease severity (Fig. 1). This can be attributed to the presence of one or more bioactive metabolites in the crude fractions that can antagonise the pathogen. Earlier reports have shown the isolation of specific metabolites from actinobacteria for the control of several plant diseases (Kimet al. 2000; Ismetet al. 2004; Taechowisanet al. 2005).Data from the study showing an increase in peanut pod yield can be related to increased growth promotion (Tables 1 and 2) as well as decreased disease incidence and severity(Table 3; Fig. 1). This is in agreement with previous studies as reviewed by Doumbouet al. (2001). We have also observed reasonably high disease incidence (68.93%) in 2015 in un-inoculated control plot. It could be due to the partial movement of primary inoculum while preparing the field in the second season.
5. Conclusion
Results from the present study point out that the strainStreptomycessp. RP1A-12 is effective in controlling peanut stem rot while acting as growth promoter under the present situation. Incidence of stem rot was significantly reduced during both the seasons under study. Use of preliminaryin vitrostudies as a mode of selection of biological control agents for field evaluation continues to be a key factor(Campbell 1986; Swadling and Jeffries 1996). Most importantly for a biological control agent to be effective,it must showcase multiple mechanisms of pathogen population control which was evident from the production of HCN, siderophores and also the role of the antifungal metabolites.
Acknowledgements
The authors are thankful to Biotechnology Industry Research Assistance Council (BIRAC), a government of India enterprise, for providing financial support under Biotechnology Industry Partnership Programme (BIPP)(BT/BIPP0429/11/10). The authors are also thankful to Sri Biotech Laboratories India Pvt. Ltd., Hyderabad, India for their collaboration on this project and to Dr. K. Vijay Krishna Kumar (Acharya NG Ranga Agricultural University, Andhra Pradesh, India) for his critical inputs while preparing the manuscript.
Adhilakshmi M, Latha P, Paranidharan V, Balachandar D,Ganesamurthy K, Velazhahan R. 2014. Biological control of stem rot of groundnut (ArachishypogaeaL.) caused bySclerotium rolfsiiSacc. with actinomycetes.Archives of Phytopathology and Plant Protection, 47, 298–311.
Alabouvette C, Olivain C, Steinberg C. 2006. Biological control of plant diseases: The European situation.European Journal of Plant Pathology, 114, 329–341.
Ames R N, Reid C P, Ingham E R. 1984. Rhizosphere bacterial population responses to root colonization by a vesiculararbuscular mycorrhizal fungus.New Phytologist, 96,555–563.
Aycock R. 1966. Stem rot and other diseases caused bySclerotium rolfsii.Technical Bulletin, North Carolina Agricultural Experiment Station, 174, 202.
Backman P A, Brenneman T B. 1997. Stem rot. In: Kokalis-Burelle N, Porter D M, Rodriguez-Kabana R, Smith D H,Subrahmanyam P, eds.,Compendium of Peanut Diseases.2nd edition. APS Press, St. Paul, Minn, USA. pp. 36–37.
Bale J S, van Lenteren J C, Bigler F. 2008. Biological control and sustainable food production.Philosophical Transactions of the Royal Society(B), 363, 761–776.
Basha T S, Radhaiah A, Nagalakshmi D M, Eswara R N P.2012. Biocontrol potential of indigenousPseudomonasspp.againstSclerotiumrolfsiicausing stem rot of groundnut.International Journal of Food Agriculture and Veterinary Science, 2, 134–141.
Basilio A, Gonzalez I, Vicente M F, Gorrochategui J,Cabello A, González A, Genilloud O. 2003. Patterns of antimicrobial activities from soil actinomycetes isolated under different conditions of pH and salinity.Journal of Applied Microbiology, 95, 814–823.
Behal V. 2000. Bioactive products fromStreptomyces.Advances in Applied Microbiology, 47, 113–157.
Bentley S D, Chater K F, Cerdeño-Tárraga A M, Challis G L,Thomson N R, James K D, Harris D E, Quail M A, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen C W,Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T,et al. 2002. Complete genome sequence of the model actinomyceteStreptomycescoelicolorA3 (2).Nature, 417,141–147.
Berdy J. 2012. Thoughts and facts about antibiotics: where we are now and where we are heading.Journal of Antibiotics,65, 385–395.
Bloemberg G V, Lugtenberg B J J. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria.Current Opinion in Plant Biology, 4, 343–352.
Campbell R. 1986. The search for biological control agents against plant pathogens: A pragmatic approach.Biological Agriculture and Horticulture, 3, 317–327.
Chamblee D S, Green J T. 1995. Ragdoll test for seed germination. Appendix F. In:Production and Utilization of Pastures and Forages in North Carolina. North Carolina Agricultural Research Service Technical Bulletin No. 305.p. 153.
Cilliers A J, Pretorius Z A, van Wyk P S. 2003. Integrated control ofSclerotium rolfsiion groundnut in South Africa.Journal of Phytopathology, 151, 249–258.
Clark C A, Moyer J W. 1988.Compendium of Sweet Potato Diseases. American Phytopathological Society, Minnesota,USA.
Cook R, Baker K F. 1983.The Nature and Practice of Biological Control of Plant Pathogens. American Phytopathological Society, St Paul, Minnesota. p. 539.
Cook R J. 1993. Making greater use of introduced microorganisms for biological control of plant pathogens.Annual Review of Phytopathology, 31, 53–80.
Copping L G, Menn J J. 2000. Biopesticides: A review of their action, applications and efficacy.Pesticide Management Science, 56, 651–676.
Dey R, Pal K K, Bhatt D M, Chauhan S M. 2004. Growth promotion and yield enhancement of peanut (Arachis hypogaeaL.) by application of plant growth-promoting rhizobacteria.Microbiological Research, 159, 371–394.
Dochhil H, Dkhar M S, Barman D. 2013. Seed germination enhancing activity of endophyticStreptomycesisolated from indigenous ethno-medicinal plantCentella Asiatica.International Journal of Pharma and BioSciences, 4,256–262.
Doumbou C L, Salove M K H, Crawford D L, Beaulieu C. 2001.Actinomycetes, promising tools to control plant diseases and to promote plant growth.Phytoprotection, 82, 85–102.
Elliot L F, Lynch J M. 1995. The international workshop on establishment of Microbial inocula in soils: Cooperative research project on biological resource management of the Organization for Economic Cooperation and Development(OECD).American Journal of Alternative Agriculture, 10,50–73.
Ensign J C. 1992. Introduction to the actinomycetes. In: Balows A, Trijper H G, Dworkin M, Harder W, Schleifer K H, eds.,The Prokaryotes. Springer-Verlag, New York. pp. 811–815.
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez M X, Barea J M. 2010. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities.Applied Soil Ecology, 45,209–217.
Ganesan S, Kuppusamy R G, Sekar R. 2007. Integrated management of stem rot disease (Sclerotiumrolfsii) of groundnut (ArachishypogaeaL.) using Rhizobium andTrichoderma harzianum(ITCC-4572).Turkish Journal of Agriculture and Forestry, 31, 103–108.
Garrido G, Guerrero J, Cano E, Acosta M, Sánchez-Bravo J.2002. Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings.Physiologia Plantarum,114, 303–312.
Gopalakrishnan S, Pande S, Sharma M, Humayun P, Kiran B K, Sandeep D, Vidya M S, Deepthi K, Rupela O M. 2011.Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea.Crop Protection, 30, 1–9.
Haas K, Keel C, Laville J, Maurhofer M, Oberhansli T F,Schnider U, Voisard C, Wuthrich B, Defago G. 1991.Secondary metabolites ofPseudomonasfluorescensstrain CHAO involved in the suppression of root diseases. In:Hennecks H, Verma D P S, eds.,Advances in Molecular Genetics of Plant-Microbe Interactions. Interlaken,Switzerland. pp. 450–456.
Halder A K, Mishra A K, Chakarbarthy P K. 1991. Solubilization of inorganic phosphates byBradyrhizobium.Indian Journal of Experimental Biology, 29, 28–31.
Ika R S, Syamsuddin D, Nasir S, Anton M. 2011. Control of damping off disease caused bySclerotiumrolfsiiSacc.using actinomycetes and VAM fungi on soybean in the dry land based on microorganism diversity of rhizosphere zone.AGRIVITA, 33, 140–146.
Ismet A, Vikineswary S, Paramaswari S, Wong W H, Ward A, Seki T, Fiedler H P, Goodfellow M. 2004. Production and chemical characterization of antifungal metabolites fromMicromonosporasp. M39 isolated from mangrove rhizosphere soil.World Journal of Microbiology and Biotechnology, 20, 523–528.
Jiang Y, Li W J, Xu P, Tang S K, Xu L H. 2006. Study on Actinomycetes diversity under salt and alkaline environments.Acta Microbiologica Sinica, 46, 191–195.(in Chinese)
Karthikeyan V, Sankaralingam A, Nakkeeran S. 2006. Biological control of groundnut stem rot caused bySclerotiumrolfsii(Sacc.).Archives of Phytopathology and Plant Protection,39, 239–246.
Kim B S, Moon S S, Hwang B K. 2000. Structure elucidation and antifungal activity of an anthracycline antibiotic,daunomycin, isolated fromActinomadura roseola. Journal of Agricultural and Food Chemistry, 48, 1875–1881.
Koike S T. 2004. Southern blight of Jerusalem artichoke caused bySclerotiumrolfsiiinCalifornia. Plant Disease, 88, 769.
Kokalis-Burelle N, Backman P A, Rodriguez-Kabana R,Ploper L D. 1992. Potential for biological control of early leafspot of peanut usingBacillus cereusand chitin as foliar amendments.Biological Control, 2, 321–328.
Lazzarini A, Cavaletti L, Toppo G, Marinelli F. 2000. Rare genera of actinomycetes as potential producers of new antibiotics.Antonie van Leewenhoek, 78, 399–405.
López J, Acosta M, Sánchez-Bravo J. 2004. Role of basipetal auxin transport and lateral auxin movement in rooting and growth of etiolated lupin hypocotyl.PhysiologiaPlantarum,121, 294–304.
Madi L, Katan T, Katan J, Henis Y. 1997. Biological control ofSclerotiumrolfsiiandVerticilliumdahliaebyTalaromyces flavusis mediated by different mechanisms.Phytopathology,87, 1054–1060.
Manulis S, Shafir H, Epstein E, Lichter A, Barash I. 1994.Biosynthesis of indole-3-actetic acidviathe indole-3-acetamide pathway inStreptomycessp.Microbiology,140, 1045–1050.
Marciano P, Lenna P D, Magro P. 1983. Oxalic acid, cell wall-degrading enzymes and pH in pathogenesis and their significance in the virulence of twoSclerotiniasclerotiorumisolates on sunflower.PhysiologicalPlantPathology, 22,339–345.
Mayee C D, Datar V V. 1988. Diseases of groundnut in the tropics.Review of Tropical Plant Pathology, 5, 85–118.
Meschke H, Walter S, Schrempf H. 2012. Characterization and localization of prodiginines fromStreptomyces lividanssuppressingVerticillium dahliaein the absence or presence ofArabidopsis thaliana.EnvironmentalMicrobiology, 14,940–592.
Ozgonen H, Akgul D S, Erkilic A. 2010. The effects of arbuscular mycorrhizal fungi on yield and stem rot caused bySclerotiumrolfsiiSacc. in peanut.African Journal of Agricultural Research, 5, 128–132.
Pathom-aree W, Stach James E M, Ward A, Horikoshi K, Bull A T, Goodfellow M. 2006. Diversity of actinomycetes isolated from challenger deep sediment (10,898 m) from the mariana trench.Extremophiles, 10, 181–189.
Patten C, Glick B R. 2002. Role ofPseudomonasputidain indole acetic acid in development of the host plant root system.Applied and Environmental Microbiology, 68,3795–3801.
Punja Z K. 1985. The biology, ecology, and control ofSclerotium rolfsii.AnnualReviewofPhytopathology, 23, 97–127.
Rakh R R, Raut L S, Dalvi S M, Manwar A V. 2011. Biological control ofSclerotiumrolfsii, causing stem rot of groundnut byPseudomonascf.monteilii9.Recent Research in Science and Technology, 3, 26–34.
Rimando A M, Duke S O. 2006.Natural Products for Pest Management. ACS Symposium Series No. 927. American Chemical Society. p. 319.
Saugar I, Sanz E, Rubio M A, Espinosa J C, Jimenez A. 2002.Identification of a set of genes involved in the biosynthesis of the aminonucleoside moiety of antibiotic A201A fromStreptomycescapreolus.European Journal of Biochemistry,269, 5527–5535.
Shew B B, Wynne J C, Beute M K. 1987. Field, microplot, and greenhouse evaluations of resistance toSclerotium rolfsiiin peanut.Plant Disease, 71, 188–191.
Shokes F M, Rozalski K, Gorbet D W, Brenneman T B,Berger D A. 1996. Techniques for inoculation of peanut withSclerotiumrolfsiiin the greenhouse and field.Peanut Science23, 124–128.
Simi J, Sajjalaguddam R R, Kumar K V K, Varshney R, Sudini H K. 2016. Assessing the prospects ofStreptomycessp. RP1A-12 in managing groundnut stem rot disease caused bySclerotiumrolfsiisacc.Journal of General Plant Pathology, 82, 96–104.
Simi J, Sudini H K. 2016. Indirect plant growth promotion in grain legumes: Role of actinobacteria. In: Subramaniam G,Sathya A, Vijayabharathi R, eds.,Plant Growth Promoting Actinobacteria. Springer, Singapore. pp. 17–32.
Singh A, Mehta S, Singh H B, Nautiyal C S. 2003. Biocontrol of collar rot disease of betelvine (PiperbetleL.) caused bySclerotiumrolfsiiby using rhizosphere-competentPseudomonasfluorescensNBRI-N6 andP.fluorescensNBRI-N.Current Microbiology, 47, 153–158.
Sterner O. 2012. Isolation of microbial natural products. In:Satyajit S D, Lutfun N, eds.,Natural Products Isolation.Springer, Humana Press, New York. pp. 393–413.
Swadling I, Jeffries P. 1996. Isolation of microbial antagonists for biocontrol of grey mould diseases of strawberries.Biocontrol Science and Technology, 6, 125–136.
Taechowisan T, Lu C, Shen Y, Lumyong S. 2005. Secondary metabolites from endophyticStreptomycesaureofaciensCMUAc130 and their antifungal activity.Microbiology, 151,1691–1695.
Tančić S, Skrobonja J, Lalošević M, Jevtić R, Vidić M. 2013.Impact ofTrichodermaspp. on soybean seed germination and potential antagonistic effect onSclerotinia sclerotiorum.Journal of Pesticides and Phytomedicine(Belgrade), 28,181–185.
Terkina I A, Parfenova V V, Ahn T S. 2006. Antagonistic activity of actinomycetes of Lake Baikal.Applied Biochemistry and Microbiology, 42, 173–176.
Vidhyasekaran P, Muthamilan M. 1995. Development of formulations ofPseudomonas fluorescensfor control of chickpea wilt.PlantDisease, 79, 782–786.
Wei G, Kloepper J W, Sadik T. 1991. Induction of systemic resistance of cucumber toColletotrichumorbiculareby select strains of plant growth-promoting rhizobacteriaPhytopathology, 81, 1508–1512.
Wokocha C R. 1990. Integrated control ofSclerotiumrolfsiiinfection of tomato in the Nigerian Savanna: Effect ofTrichodermavirideand some fungicides.CropProtection,9, 231–234.
Xiao K, Kinkel L L, Samac D A. 2002. Biological control of phytophthora root rots on alfalfa and soybean withStreptomyces.BiologicalControl, 23, 285–295.
 Journal of Integrative Agriculture2018年4期
Journal of Integrative Agriculture2018年4期
- Journal of Integrative Agriculture的其它文章
- Climate change and Chinese farmers: Perceptions and determinants of adaptive strategies
- Estimating the average treatment effect of adopting stress tolerant variety on rice yield in China
- lmmunogenicity and protective efficacy of DHBV DNA vaccines expressing envelope and capsid fusion proteins in ducks delivered by attenuated Salmonella typhimurium
- Effects of conditioners (single-layer, double-layer and retentionconditioner) on the growth performance, meat quality and intestinal morphology of growing and finishing pigs
- Sub-lethal effects of Beauveria bassiana (Balsamo) on field populations of the potato tuberworm Phthorimaea operculella Zeller in China
- Regionalization of wheat powdery mildew oversummering in China based on digital elevation
