Sub-lethal effects of Beauveria bassiana (Balsamo) on field populations of the potato tuberworm Phthorimaea operculella Zeller in China
YUAN Hui-guo, WU Sheng-yong, LEl Zhong-ren, Silvia l. Rondon, GAO Yu-lin
1 State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, P.R.China
2 Department of Crop and Soil Sciences, Hermiston Agricultural Research and Extension Center, Oregon State University,Hermiston, OR 97838, USA
1. lntroduction
The potato tuberworm,Phthorimaea operculellaZeller(Lepidoptera: Gelechiidae), is considered to be one of the most important potato (Solanum tuberosumL.) pests worldwide including China. In recent years, China has been boosting potato production to make potato the fourth major crop produced in the country following rice, wheat and maize (Zhanget al. 2017).Phthorimaea operculellahas a close evolutionary relationship withSolanaceouscrops that is enhanced by the insect high adaptability to daily and seasonal changes, high reproductive potential,and high survival rate even under extreme temperatures(Dogramaciet al. 2008). The pest can thrive under field and storage conditions (Bacon 1960; Foot 1974b; Haines 1977;Shelton and Wyman 1979a, b; Briese 1986; Hermanet al.2005). In China,P.operculellawas first reported in Guangxi in 1937 (Li and Zhang 2005); at present,P.operculellais widely distributed in China and mainly occurs in Yunnan,Guizhou and Sichuan (Xu 1985). All these regions are key potato production areas. The pest is difficult to control, and over the years insecticides have been used extensively(Foot 1974a; Trivedi and Rajagopal 1992), however, no resistance to pesticides has been reported yet, but basic line information to determine pesticide susceptibility has already been collected (Dobie 2010).
P.operculellaadults deposit eggs in potato foliage or close to tubers, while the larvae mine leaves, stems,and petioles and excavate tunnels through potato tubers(Rondonet al. 2007; Yuanet al. 2017). This wider niche ability makes this insect difficult to control. In the U.S. Pacific Northwest, there are many pesticides registered to controlP.operculella(Rondonet al. 2007), while in China, there are several products that effectively control the pest (Duet al.2006); however, El-kady (2011) reported that continuous use of pesticides promoted pesticide resistance inP.operculellaand better management program are needed.
Under low pesticide input conditions,P.operculellacan be controlled effectively by the use of natural enemies(Kroschel and Koch 1996; Collet al. 2000).Beauveria bassiana(Bals.-Criv) Vuill has proved to be an effective biological control agent ofP.operculellain the laboratory(Li and Zhang 2005). The advantages of using natural products such asB.bassianaare numerous, including being safe to the environment when compared to chemical insecticides, relatively easy to handle and safer to handlers.However,B.bassianadepends on favorable environmental conditions during application and they are heavily humidity dependable (Shah and Pell 2003). Microbial control is not yet developed for massive commercial production and more information is still needed to prove the effectiveness of this method (Kroschel and Koch 1996; Sporlederet al. 2001).In high pesticide input systems, the effect of pesticides on natural enemies ofP.operculellais unknown (Koss 2003).Most researches of pesticides have focused on their lethal effects onP.operculelladevelopmental stages (Desneuxet al. 2007); however, data concerning sub-lethal effects are limited. In general, besides the direct mortality induced by any given pesticide, the sub-lethal effect must be considered for a completely assessment of pesticide impact and effectiveness (Desneuxet al. 2007).
Entomopathogens are a good alternative to pesticides since they contribute to the natural regulation of arthropod populations (Evans 2008). Seyed-Talebiet al. (2012)studied the sub-lethal effect ofB.bassianaon the life table parameters of two-spotted spider miteTetranychus urticaeKoch. They found that the duration of the immature stage was significantly longer on female when compared to male longevity, while oviposition period and fecundity were significantly lower on fungus-treated mites. Hafezet al.(1997) indicated thatB.bassianainfluence the longevity ofP.operculellalarvae and adults, however, sub-lethal effects were not thoroughly studied. In potatoes, early studies by Arthurset al. (2008) determined the effects of granulovirus andB.thuringensisfor season-long control ofP.operculellawith mixed results. Quesada-Moragaet al. (2004), Latifianet al. (2010), and Seyed-Talebiet al. (2012) suggested that entomopathogenic fungi should be evaluated further to determine its influence in offsprings’ life-history, including growth, development and reproduction. Thus, this study was designed to evaluate the sub-lethal effects ofB.bassianaon different biological parameters ofP.operculella. Information will provide valuable insight regarding the effects of this fungus inP.operculellathat could potentially be used in pest management programs.
2. Materials and methods
2.1. lnsect colony
Following modifications of Gui and Li (2003) and Rondonet al. (2009) protocols, a colony ofP.operculellawas established by collecting over 500 adults in the Yunnan Province, China (103.79´E, 25.51´N). Potato tuberworm adults were reared on potato tubers placed in cylindrical food containers (14 cm diameter×3.5 cm depth) which were covered with a fine cheesecloth adjusted with a rubber band. Adults were fed with a 5% sugar suspension which was applied using a small brush applied in a small cotton wick. Also, a round filter paper (5 cm diameter) was placed on top of the cheese cloth to be used as oviposition substrate. Eggs were collected daily, and filter paper with eggs was transferred to an empty container until hatching.After hatching, a (6±1) cm-diameter-hemispherical tuber was added as a feeding substrate; tubers were changed every other day. Also, a layer of fine sand was added at the bottom of the container to be used as a pupation substrate.Colony was kept in an environmental chamber (MLR-351H,SANYO Electric Co., Ltd., Moriguchi City, Osaka, Japan) at(27±2)°C, 12 h L:12 h D, and 80–90% (RH). About every two months,P.operculellaadults were added to the colony to prevent introgression and to increase genetic diversity in the confined population. Insects from the colony were used in all bioassays.
2.2. Fungal strain
A strain ofB.bassianaJLGZL-14, derived fromOstrinia furnacalis(Guenee), was collected in Gongzhuling, Jilin Province, China in 2011. The strain was maintained, and conidia were produced on Sabouraud dextrose agar (SDA)at (26±1)°C under continuous darkness. Conidia were then harvested from one- to three-week-old cultures. Conidial concentrations were determined with a haemocytometer and adjusted with 0.05% Tween-80 in sterile water. Suspension was diluted to 1×107conidia mL–1. Subsample of the conidial suspension was placed in a shaking plate for culture at(26±1)°C, 195 r min–1for 16 h. A haemocytometer was used to count the total number of conidia and conidial germination under the microscope (Li 2015). Germination rate of fungal conidia was>90%.
2.3. Bioassay
In bioassays, two treatments were used: (1) fungus treated(FT), where first instarP.operculellalarvae were dipped for 5 s in a conidial suspension (1×107conidia mL–1), which is the concentration based on the median lethal concentration(LC50) against 3rd instar larvae ofP.operculellain our preliminary test; (2) control treatment (CT), where larvae were dipped in 0.05% Tween-80.
To determine the effect ofB.bassianaonP.operculellasurvival, larvae were collected from filter papers with a small brush and transferred to a Petri dish (7 cm diameter) containing 10 mL of conidia suspension for immersion.P.operculellalarvae were allowed to dry on a separate filter paper for 5 s and then carefully transferred to a separate Petri dish which was placed over an arena containing a layer of fine sand and two cuboid potato tubers (2 cm×2 cm×2 cm) which served as a food source. Larvae of 200 and 50 were used in FT and CT, respectively. Both, fungal and control treatments,were replicated four times. Each arena was placed in an environmental chamber ((27±2)°C, RH (80–90)%, 12 h L:12 h D). Once theP.operculellareached the pupae stage,pupae were harvested and used in the life table study.Survival was estimated by counting the number of larvae that reached the pupal stage. Pupae that developed from larvae exposed toB.bassianaare referred to as FT pupae,and those that were treated with sterile water containing Tween-80 are referred to as CT pupae.
2.4. Life table study
A sub-sample of FT (n=20) and CT (n=20) pupae were sexed and placed in a cylindrical food container (14 cm diameter×3.5 cm depth). For each treatment, about 20 h after adult emergence, egg laying started. A circular filter paper served as oviposition substrate. A sub-sample of forty eggs was separately collected from FT and CT and then placed in containers (3.5 cm in diameter×8 cm depth)until hatching. After hatching, a single newly hatched first instar larva was placed in an individual container (3.5 cm in diameter×8 cm depth) containing a potato tuber cube(2 cm×2 cm×2 cm) that served as a food source. The lid of the container was pricked to allow ventilation and the potato tuber cube was changed every three days. The developmental stage and survival of eachP.operculellalife stage were recorded daily for each treatment. Each immature stage was determined based on measuring the cephalic capsule. Once the larvae reached the pupal stage;pupae were again separated by sex and transferred to a 6 cm wide×8 cm depth container tighten with a fine cloth adjusted with a rubber band. After emergence, one male and one female were placed together and oviposition was measured by counting daily the number of eggs per filter paper. Filter paper had to be replaced on a daily basis.Adults were fed with sugar suspension. Sixteen couples were separately chosen from fungal and control treatments to assess oviposition. All measurements were made at(27±2)°C and a photoperiod of 12 h L:12 h D in a growth chamber.
2.5. Life table analysis
Number of days from egg to adulthood, survival, and female daily fecundity were analyzed according to the age-stagetwo-sex life table described by Chi and Liu (1985) and Chi(1988). Data were evaluated using the computer program TWOSEX-MSChart (Chi 2012). Following Chi and Liu(1985) protocol, the age-stage-specific survival rate (sxj)was determined. In the formula below,xandywere age and stage, respectively.lxwas the age-specific survival rate(lx);fxjwas the age-stage-specific fecundity;mxwas the agespecific fecundity (mx). Also, the intrinsic rate of increase(r), gross reproductive rate (GRR), and net reproductive rate(R0) were calculated. The intrinsic rate of increase (r) was calculated by using the interactive bisection method and the Euler-Lotka equation:

The net reproductive rate (R0), finite rate of increase (k),the mean generation time (T) and gross reproductive rate(GRR) were calculated as follows:

The bootstrap technique (Efron and Tibshirani 1993)was used to estimate the means and standard errors of population parameters (Zhanget al. 2015). The differences in the development times, fecundities and the population parameters between the FT and CT were compared byt-test atP<0.05. According to the age-stage, two-sex life table (Chi and Liu 1985), the program TIMING-MSChart was used to estimate project population growth (Chi 2009).Because bootstrap technique uses random resampling, a small number of replications will generate variable means and standard errors.
3. Results
Bioassay tests showed thatP.operculellalarvae treated withB.bassianapresented a high rate, with mortality of(90.3±2.1)%, while mortality rate in the control treatment was(13.8±0.7)%. Moreover,B.bassianasignificantly affected the growth and development ofP.operculellaoffspring,including survival.
Table 1 shows the mean number of days for eachP.operculellalife stages in FT and CT, respectively. The egg, pupal, and teneral stages were significantly different between the FT and CT. The immature stage was significantly higher in FT compared to the CT treatment(P<0.05). No differences in longevity were observed between males and females in both treatments (both forP>0.05). However, there were significant differences in the mean number eggs per female where untreated females laid a higher number of eggs as compared to treated ones(P<0.05).
Thesxjdepicts the probability that an individual will survive to agexand stagej(Fig. 1). The survival rate of 4th instar larvae ofP.operculellaat FT decreased rapidly and was lower than that of CT larvae. The probability from the 4th instar to pupal of FT (0.61) was numerically lower than the CT (0.95). The numbers of adult in the FT treatment (0.4)were lower than that in CT (0.85). The curve of thelxand themxis shown in Fig. 2. Also, the daily mean number of offspring produced by females of agexand stagejper day is shown with thefxj(Fig. 2). Thelxof FT individuals rapidly declined and the curve decline of the FT is quicker than CT. Themxof FT individuals is similar than the CT one. In addition,lxmxof FT was lower than CT. Moreover, thefxof FT treatment had a peak of 23.88 at 23 d, which is lower than the peak of CT that was 46 at 24 d.
The age-stage-specific life expectancy (Fig. 3), which is the expected total time of an individual of agexand stagey,was shorter for FT individuals compared to untreated ones.The age-stage-specific reproductive values (vxj) described the contribution of an individual of agexand stagejto the future population. The reproductive value increased at age 21 d and reached a peak of 69.59 eggs, which is loewer than the peak of CT (102.50) at later time (24 d) (Fig. 4).
Errors of ther,λ,R0andTwere calculated in the different treatments by using the bootstrap method (Table 2).Software analysis results showed thatR0andTwere lower in FT (17 per day at 25 d) compared to the CT (66 per day at 27 d).
4. Discussion
The damage ofP.operculellato potato plants mainly occurred during larvae period (Rondon 2010). Most researches have been designed to evaluate the effect ofB.bassianaonP.operculellaby choosing 2nd to 4th instar larvae (Li and Zhang 2005; Kauret al. 2011). The virulence ofB.bassianastrain JLGZL-14 was tested against 3rd instars in our preliminary test and showed that LC50value was 1×107conidia mL–1, which was used to evaluate the effect on 1st instar larvae ofP.operculellain this study.The bioassays showed that higher susceptibility of 1st as compared to 3rd instars.
Although most of 1st instar larvae ofP.operculelladied from fungal infection in our study, there was still approximately 8% that successfully pupated. This result is consistent with the results reported by Li and Zhang (2005).Because of the high reproductive potential of adults (Hermanet al. 2005; Rondon 2010), theB.bassiana-treated larvae that pupated and eclosed as adults would establish the new population and continue to damage potato plants. In addition, the economic damage on potato plants was mainly caused by larval feeding, it is critical to evaluate sub-lethal effects ofB.bassianaonP.operculellalarva.

Table 1 Life history table of Phthorimaea operculella Zeller F1 generation untreated (control) or treated with Beauveria bassiana
The age-stage, two-sex life table can accurately and precisely described the survival rate and stage structure,thereby evaluating the sub-lethal effects ofB.bassianaonP.operculella. The life table study showed that the survival rate ofP.operculellaimmatures, especially in 4th instar and pupa decreased dramatically in fungal treatment.The overlap of the stage-specific survival curves in the female and male is due to the difference of growth rate between individuals (Chi and Yang 2003). In addition,the developmental periods from 1st and 3rd instar larvae were not affected byB.bassiana. Similar results were obtained by Seyed-Talebiet al. (2012) who studied the development of all stages of the two-spotted spider mite,Tetranychus urticae(Acari: Tetranychidae), and found that instars were not affected by fungal infection. However,immature stages (egg+larvae+pupa) of FT individuals in our study were shorter than those in CT. It is hypothesized that larvae infected by fungus may have acquired and stored lesser nutrient resources than that of control larvae, which might have affected the development of an insect (Kauret al. 2011). The smaller size in the fungus-treated larvae observed during the test supported the hypothesis.
Sub-lethal effects such as reduction in fecundity indicates that the fungus is invading the host (Mulock and Chandler 2001). Fewer conclusive studies are available concerning sub-lethal effects of fungal pathogen on reproductive capacity of insects. Our study showed that fungal treatment resulted into significant reduction in reproductive potential. Likewise Seyed-Talebiet al. (2012) reported that the fecundity was significantly lower on fungus-treated mites. Wanget al.(2014) also found theR0for treated whiteflies’ offspring is lower than that of control, which is consistent with our results.It can also be hypothesized that nutritional deficiency caused by fungal infection can drastically affect the reproduction of females which have high energetic demands.

Fig. 1 Age-stage-specific survival rate (sxj) ofPhtorimaea operculella Zeller untreated or treated with Beauveria bassiana.FT, fungus treated; CT, control treatment; L1–L4 indicate first-,second-, third- and fourth-instar-stage larvae, respectively.

Fig. 2 Age-specific survival rate (lx), female age-specific fecundity (fxj), age-specific fecundity of total population (mx), and age-specific maternity (lxmx) of Phtorimaea operculellaZeller F1 generation untreated or treated with Beauveria bassiana.FT, fungus treated; CT, control treatment; L1–L4 indicate first-,second-, third- and fourth-instar-stage larvae, respectively.
We showed thatB. bassiananot only has high pathogenicity toP.operculellalarvae, but also causes sublethal effects, which include shortening the development period of one generation, reducing the fecundity of female of offspring, and affecting their population parameters.In addition, malformations in offspring wings were observed after fungal treatment. This study provides the basic information to help us understand the effects of entomopathogenic fungi onP.operculellalarvae and demonstrated that the strain ofB.bassiana, JLGZL-14, has potential for control ofP.operculellawith a concentration of 1×107conidia mL–1. Field studies that spray withB.bassianasuspension are necessary to determine their outcome in the suppression of the target pest,P.operculella.

Fig. 3 Age-stage-specific life expectancies (exj) of Phtorimaea operculella Zeller untreated or treated with Beauveria bassiana.FT, fungus treated; CT, control treatment; L1–L4 indicate first-,second-, third- and fourth-instar-stage larvae, respectively.

Fig. 4 Age-stage-reproductive value (vxj) of Phtorimaea operculella Zeller untreated or treated with Beauveria bassiana.FT, fungus treated; CT, control treatment; L1–L4 indicate first-,second-, third- and fourth-instar-stage larvae, respectively.
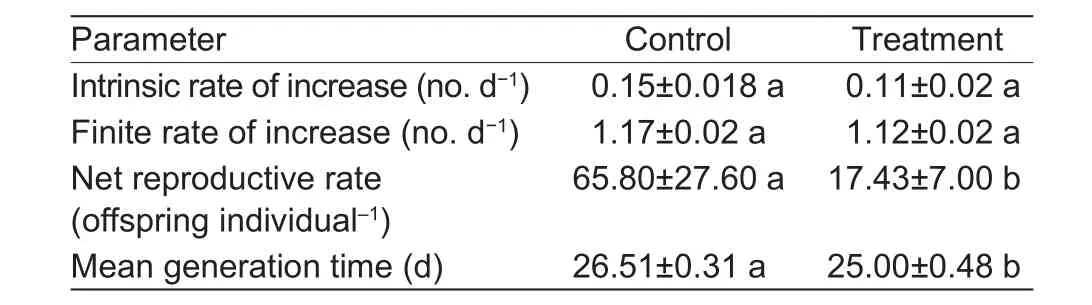
Table 2 Estimated population parameters (mean±SE) for Phthorimaea operculellaZeller F1 population untreated (control)or treated with Beauveria bassiana
5. Conclusion
This study provides the basic information to help us understand the effects of entomopathogenic fungi onP.operculellalarvae and demonstrated that the strain ofBeauveria bassiana, JLGZL-14, has potential for control ofP.operculellawith a concentration of 1×107conidia mL–1.
Acknowledgements
The research project was supported by the External Cooperation Program of Yunnan Province, China(2014IB002).
Arthurs S P, Lacey L A, Pruneda J N, Rondon S I. 2008. Semifield evaluation of a granulovirus andBacillus thuringensisssp. Kurstaki for season-long control of the potato tuber moth,Phthorimaea operculella.Entomologia Experimentalis et Applicata, 129, 276–285.
Bacon O G. 1960. Systemic insecticides applied to cut seed pieces and to soil at planting time to control potato insects.Journal of Economic Entomology, 53, 835–839.
Briese D T. 1986. Geographic variability in demographic performance of the potato moth,Phthorimaea operculellaZell. in Australia.Bulletin of Entomological Research, 76,719–726.
Chi H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals.Environmental Entomology, 17, 26–34.
Chi H. 2009. TIMING-MSChart: A computer program for the timing of pest management. [2016-01-11].http://140.120.197.173/Ecology/
Chi H. 2012. TWOSEX-MS chart: Computer program for age stage, two-sex life table analysis. [2016-01-03]http://140.120.197.173/ecology/
Chi H, Liu H. 1985. Two new methods for the study of insect population ecology.Academia Sinica, 24, 225–240.
Chi H, Yang T C. 2003. Two-sex life table and predation rate ofPropylaea japonica Thunberg(Coleoptera: Coccinellidae)fed onMyzus persicae(Sulzer) (Homoptera: Aphididae).Environmental Entomology, 32, 327–333.
Coll M, Gavish S, Dori I. 2000. Population biology of the potato tubermoth,Phthorimaea opercuella(Lepidoptera:Gelechiidae) in two potato cropping systems in Israel.Bulletin of Entomological Research, 90, 309–315.
Desneux N, Decourtye A, Delpuech J M. 2007. The subletahl effects of pesticides on beneficial arthopods.Annual Review of Entomology, 52, 81–106.
Dobie C H. 2010. Pesticide susceptibility of potato tuberworm in the Pacific Northwest. MSc thesis, School of Earth and Environmental Sciences, Washington State University,USA. p. 52.
Dŏgramaci M, Rondon S I, DeBanoand S J. 2008. The effect of soil depth and exposure to winter conditions on survival of the potato tuberwormPhthorimaea operculella(Lepidoptera: Gelechiidae).Entomologia Experimentalis et Applicata, 129, 332–339.
Du L T, Li Z Y, Zhou L M. 2006. Comparative test on the control effects of three kinds of pesticides on potato tuberwormPhthorimaea opercuella(Zeller).Chinese Potato Journal,20, 92–93. (in Chinese)
Efron B, Tibshirani R J. 1993.An Introduction to the Bootstrap.Chapman & Hall, New York.
El-kady H. 2011. Insecticide resistance in potato tuber mothPhthorimaea operculellaZeller in Egypt.American Journal of Science, 7, 263–266.
Evans J. 2008. Biopesticides: From cult to mainstream. Agrow,October 2008, 11–14. [2016-12-16]. www.agrow.com
Foot M A. 1974a. Cultural practices in relation to infestation of potato crops by the potato tuber moth (Phthorimaea operculella). I. Effect of irrigation and ridge width.New Zealand Journal of Experimental Agriculture, 2, 447–450.
Foot M A. 1974b. Field assessment of several insecticides against the potato tuber mothPhthorimaea operculella(Zell.) at Pukukohe.New Zealand Journal of Experimental Agriculture, 2, 191–197.
Gui F R, Li Z Y. 2003. A method for rearing the potato tuber mothPhthorimaea operculellaon potato.Entomological Knowledge, 40, 187–189.
Haines C P. 1977. The potato tuber moth,Phthorimaea operculella(Zeller): A bibliography of recent literature and a review of its biology and control on potatoes in the field and in store. Tropical Products Institute, London.
Hafez M, Zaki F N, Moursy A. 1997. Biological effects of the entomopathogenic fungus,Beauveria bassianaon the potato tuber mothPhthorimaea operculella(Seller).Journal of Pest Science, 70, 158–159.
Herman T J B, Clearwater J R, Triggs C M. 2005. Impact of pheromone trap design, placement and pheromone blend on catch of potato tuber moth.New Zealand Plant Protection, 58, 219–223.
Kaur S, Kaur H P, Kaur K, Kaur A. 2011. Effect of different concentrations ofBeauveria bassianaon development and reproductive potential ofSpodoptera litura(Fabricius).Journal of Biopesticides, 4, 161–168.
Koss A. 2003. Integrating chemical and biological control in Washington State potato fields. MSc thesis, Washington State University, USA.
Kroschel J, Koch W. 1996. Studies on the use of chemicals,botanicals andBacillus thuringiensisin the management of the potato tuber moth in potato stores.Crop Protection,15, 197–203.
Latifian M, Soleimannejadian E, Ghazavi M, Mosadegh M S, Hayati J. 2010. Effects of sublethal concentrations of fungusBeauveria bassianaon the reproductive potentials of sawtoothed beetleOryzaephilus surinamensison commercial date cultivars.Plant Protection Journal, 2,279–292.
Li J. 2015. Research on thermotolerance ofBeauveria Bassianaagainst western flower thrips and development of wettable powder formulation. MSc thesis, Chinese Academy of Agricultural Sciences. (in Chinese)
Li Z Y, Zhang Q W. 2005. Relative virulence of seven isolates ofBeauveria bassianato the potato tuber moth,Phthorimaea operculella(Zeller) and their biological compatibility with ten insecticides.Plant Protection, 31, 57–62. (in Chinese)
Mulock B S, Chandler L D. 2001. Effect ofBeauveria bassianaon the fecundity of western corn rootworm,Diabrotica virgifera(Coleoptera: Chrysomelidae).Biological Control,22, 16–21.
Quesada-Moraga E, Santos-Quirós R, Valverde-Garcîa,Santiago-Alvarez C. 2004. Virulence, horizontal transmission, and sublethal reproductive effects ofMetarhizium anisopliae(Anamorphic fungi) on the German cockroach (Blattodea: Blattellidae).Journal of Invertebrate Pathology, 87, 51–58.
Rondon S I. 2010. The potato tuberworm: A literature review of its biology, ecology, and control.American Journal of Potato Research, 87, 149–166.
Rondon S I, Debano S J, Clough G H, Dogramaci M,Schreiber A, Jensen A. 2007.Biology and Management of the Potato Tuberworm In the Pacific Northwest. Biology and management of the potato tuberworm in the Pacific Northwest. A Pacific Northwest. Extension Publication.Oregon State University.
Rondon S I, Hane D C, Brow C R, Dogramaci M. 2009. Resistance of potato germplasm to the potato tuberworm (Lepidoptere:Gelechiidae).Journal of Economic Entomology, 102,1649–1653.
Seyed-Talebi F S, Kheradmand K, Talaei-Hassanloui, Talebi-Jahromi K. 2012. Sublethal effects ofBeauveria bassianaon life table parameters of two-spotted spider mite,Tetranychus urticae(Acari:Tetranychidae).Biocontrol Science and Technology, 22, 293–303.
Shah P A, Pell J K. 2003. Entomopathogenic fungi as biological control agents.Applied Microbiology and Biotechnology,61, 413–423.
Shelton A M, Wyman J A. 1979a. Time of tuber infestation and relationships between catches of adult moths, foliar larval populations, and tuber damage by 435 potato tuber worm.Journal of Economic Entomology, 72, 599–601.
Shelton A M, Wyman J A. 1979b. Potato tuberworm damage to potato grown under different irrigation and cultural practices.Journal of Economic Entomology, 72, 261–264.
Sporleder M, Zegarra O, Kroschel K, Huber J, Lagnaoui A.2001. Assessment of the inactivation time of phthorimaea operculella granulovirus 441 (PoGV) at different intensities of natural irradiation.International Journal of Radiation Oncologybiologyphysics, 57, 123–128.
Trivedi T P, Rajagopal D. 1992. Distribution, biology, ecology and management of potato tuber moth,Phthorimaea operculella(Zeller) (Lepidoptera: Gelechiidae): A review.Tropical Pest Management, 38, 279–285.
USDA. 2015. China potatoes and potato products annual. USDA Foreign Agricultural Service. Global Agricultural Information Network. [2015-09-25]. https://gain.fas.usda.gov/Recent%20GAIN%20Publications/Potatoes%20and%20 Potato%20Products%20Annual_Beijing_China%20-%20 Peoples%20Republic%20of_9-25-2015.pdf
Wang D J, Zang L S, Zhang Y. 2014. Sublethal effects ofBeauveria bassianaBalsamo on life table parameters of subsequent generations ofBemisia tabaci Gennadius.Scientia Agricultura Sinica, 47, 3588–3595. (in Chinese)
Xu G J. 1985. Potato tuberwormPhthorimaea operculella(Zeller).MSc thesis, Chinese Academy of Agricultural Sciences. (in Chinese)
Yuan H, Lei Z, Rondon S I, Gao Y. 2017. Potential of a strain ofBeauveria bassiana(Hypocreales: Cordycipitaceae) for the control of the potato tuberworm,Phthorimaea operculella(Zeller).International Journal of Pest Management, 63,352–354.
Zhang M, Luo Q Y, Gao M J, Liu Y, Yang Y D. 2017. Research progress and prospect of potato market.Chinese Potato,2,113–118. (in Chinese)
Zhang T, Reitz S R, Wang H. 2015. Sublethal effects ofBeauveria bassiana(Ascomycota: Hypocreales) on life table parameters ofFrankliniella occidentalis(Thysanoptera:Thripidae).Journal of Economic Entomology, 108, 975–986.
 Journal of Integrative Agriculture2018年4期
Journal of Integrative Agriculture2018年4期
- Journal of Integrative Agriculture的其它文章
- Climate change and Chinese farmers: Perceptions and determinants of adaptive strategies
- Estimating the average treatment effect of adopting stress tolerant variety on rice yield in China
- lmmunogenicity and protective efficacy of DHBV DNA vaccines expressing envelope and capsid fusion proteins in ducks delivered by attenuated Salmonella typhimurium
- Effects of conditioners (single-layer, double-layer and retentionconditioner) on the growth performance, meat quality and intestinal morphology of growing and finishing pigs
- Regionalization of wheat powdery mildew oversummering in China based on digital elevation
- Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii
