NR2A、NR2B在慢性胰腺炎大鼠大脑前扣带回的表达变化及意义
周文 高峻 李桂香 李兆申
·论著·
NR2A、NR2B在慢性胰腺炎大鼠大脑前扣带回的表达变化及意义
周文 高峻 李桂香 李兆申
目的检测慢性胰腺炎(CP)大鼠大脑前扣带回(ACC)的N-甲基-D-天冬氨酸受体亚基NR2A和NR2B基因表达水平,探讨其在CP疼痛形成中的作用。方法成年雄性SD大鼠按数字表法随机分为CP组、对照组和正常组。通过尾静脉一次性注射二丁基二氯化物8 mg/kg体重的方法制备CP模型,对照组尾静脉注射等容积乙醇和甘油混合液。4周后依次用不同粗细(3.85、5.50、7.05、10.4、17.8 g)的von frey纤维丝刺激大鼠腹部,记录疼痛阳性反应次数的百分率。然后处死大鼠,取胰腺组织行病理学检查,采用实时PCR和蛋白质印迹法检测大脑ACC区NR2A、NR2B mRNA及蛋白表达量。结果CP模型成功率为60%。用Von frey纤维丝以3.85 g至17.8 g递增刺激大鼠腹部,CP组大鼠阳性反应百分率从(38.33±7.53)%递增到(73.33±8.17%),对照组大鼠从(7.80±6.70)%递增到(34.44±5.27)%,正常组大鼠从(6.57±5.48)%递增到(33.33±5.00)%,均随着刺激强度增强而增加。每个刺激强度CP组大鼠的阳性反应百分率均显著高于对照组及正常组,差异有统计学意义(P值均<0.05),而对照组与正常组间的差异均无统计学意义。CP组、对照组、正常组大鼠ACC区NR2A蛋白表达量分别为1.25±0.78、0.95±0.14、0.91±0.09,NR2B蛋白表达量为1.44±0.12、0.93±0.08、0.94±0.04,CP组显著高于对照组及正常组,差异均有统计学意义(P值均<0.01);NR2A mRNA表达量为1.43±0.20、0.80±0.10、1.01±0.13,NR2B mRNA表达量为1.40±0.09、0.98±0.14、0.94±0.05,CP组显著高于对照组及正常组,差异均有统计学意义(P值均<0.01)。结论CP大鼠大脑ACC区谷氨酸受体NR2A、NR2B的表达上调,可能参与CP疼痛的形成。
胰腺炎,慢性; 疼痛; 大脑皮质; N-甲基-D-天冬氨酸
慢性胰腺炎(CP)是各种病因引起的胰腺组织和功能持续性损害,疼痛为其主要临床表现,85%~90%的患者可发生腹痛,严重影响生活质量[1]。现有理论认为CP患者的腹痛可能与胰管压力增高、神经重塑、氧化应激、神经炎症等相关,但均不能完全阐述疼痛的原因。近年来开始探讨CP中枢神经系统的内脏痛觉传导通路和关键中枢皮质区域的功能是否发生变化[2]。大脑前扣带回(anterior cingulate cortex,ACC)是疼痛感知的关键皮层区域[3-4]。谷氨酸是中枢神经系统中感觉传递和感知的主要兴奋性递质,N-甲基-D-天冬氨酸(N-methyl-D-aspartate recepter, NMDA)是其离子型受体之一。研究发现,大脑ACC区NMDA受体表达变化与痛厌恶情绪的调节相关,且在慢性内脏痛动物模型中表达水平明显升高[5-6]。本研究观察CP大鼠大脑ACC区NMDA受体亚基NR2A、NR2B基因表达的变化,探讨其与CP疼痛的相关性。
材料和方法
一、实验动物及主要试剂
健康雄性SD大鼠20只,由第二军医大学实验动物中心提供,体重180~220 g,饲养在室温26℃左右、相对湿度60%~70%、昼夜周期12 h/12 h的环境中,水和食物供给充足。DBTC购自Sigma公司,逆转录试剂盒、PCR试剂盒购自TAKARA公司。兔抗鼠NR2A多抗购自Millipore公司、兔抗鼠NR2B多抗购自Abcam公司,辣根过氧化物标记的山羊抗兔二抗购自美国Millipore公司,内参Tubulin购自上海碧云天生物技术有限公司。
二、动物模型建立及分组
20只SD大鼠按随机数字表法分为CP组(10只),对照组(5只),正常组(5只)。CP模型采用尾静脉一次注射DBTC 8 mg/kg体重的方法制备[7-8]。对照组注射等容积乙醇和甘油混合液,正常组未予处理。实验前禁食24 h,不禁水。
三、机械痛测定
建模4周后大鼠腹部备皮,在大致相同的位置做标记。将大鼠置于悬挂的带罩网状架子上适应环境30 min。依次用不同粗细(3.85、5.50、7.05、10.4、17.8 g)的von frey纤维丝从下方刺激大鼠腹部标记处。每个规格使用10次,每次1~2 s,间隔15 s,以纤毛弯曲90°为准。大鼠舔舐腹部、收腹或身体收缩为阳性反应[9-10]。记录阳性反应次数的百分率。
四、胰腺组织病理学检查
4周后处死大鼠,取胰腺组织,4%甲醛溶液固定,常规行病理学检查。
五、蛋白质印迹法检测NR2A、NR2B蛋白表达
4周后腹腔注射7%的水合氯醛麻醉大鼠,断头后迅速将头置于冰上取出ACC区脑组织,置-80℃冰箱保存。用裂解液提取蛋白,定量后行蛋白质印迹法检测NR2A、NR2B蛋白表达。抗NR2A抗体1∶500稀释,抗NR2B抗体1∶1 000稀释、抗Tubulin抗体1∶2 000稀释。山羊抗兔二抗1∶5 000稀释。最后通过凝胶成像系统采集图像,ImageJ软件扫描,以目的条带与内参条带灰度值比表示蛋白相对表达量。
六、实时RT-PCR法检测NR2A、NR2B mRNA表达
使用Trazol提取大鼠大脑ACC区组织的总RNA,先反转录成cDNA,再行实时PCR反应扩增产物。根据GeneBank目的基因的全长序列,应用Primer3 Plus软件设计引物。NR2A 上游引物 5′-GCCAGTTACACAGCCAACCT-3′,下游引物 5′-CAAATCGGAAAGGTGGAGAA-3′;NR2B 上游引物 5′-CGGATGTGTCCTGAGACTGA-3′,下游引物 5′-ATTCCTCATGCAGGTTCCAC-3′;内参GAPDH 上游引物 5′-CCCCCAATGTATCCGTTGTG-3′,下游引物 5′-TAGCCCAGGATGCCCTTTAGT-3′。引物合成由上海生工生物工程有限公司完成。反转录反应条件:37℃ 15 min,80℃ 30 s。PCR反应条件:95℃ 10 min,95℃ 15 s、60℃ 1 min,48个循环。通过仪器自带软件获取Ct值,采用公式2-△△Ct计算mRNA的相对表达量。实验重复3次,取均值。
七、统计学方法
结 果
一、大鼠胰腺组织病理变化
建模4周后,CP组大鼠胰腺的腺泡萎缩,出现空泡状细胞,小叶结构破坏,胶原纤维散在分布于血管和小叶间,炎性细胞浸润(图1A)。CP制备成功率为60%。对照组与正常组大鼠胰腺组织未见明显病理改变(图1B、1C)。

图1 CP组(1A)、对照组(1B)、正常组(1C)大鼠胰腺组织病理改变(HE ×100)
二、大鼠机械痛反应百分率
用von frey纤维丝以3.85 g至17.8 g递增刺激大鼠腹部,CP组大鼠阳性反应次数百分率从(38.33±7.53)%递增到(73.33±8.17)%,对照组大鼠从(7.80±6.70)%递增到(34.44±5.27)%,正常组大鼠从(6.57±5.48)%递增到(33.33±5.0)%,均随着刺激强度增强而增加。每个刺激强度CP组大鼠的阳性反应次数百分率均显著高于对照组及正常组,差异有统计学意义(P值均<0.05),而对照组与正常组间的差异均无统计学意义。
三、大鼠大脑ACC区NR2A、NR2B蛋白表达量的变化
CP组、对照组、正常组大鼠大脑ACC区NR2A蛋白表达量分别为1.25±0.78、0.95±0.14、0.91±0.09;NR2B蛋白表达量为1.44±0.12、0.93±0.08、0.94±0.04。CP组显著高于对照组及正常组,差异均有统计学意义(P值均<0.01),而对照组与正常组的差异无统计学意义(P>0.05,图2)。
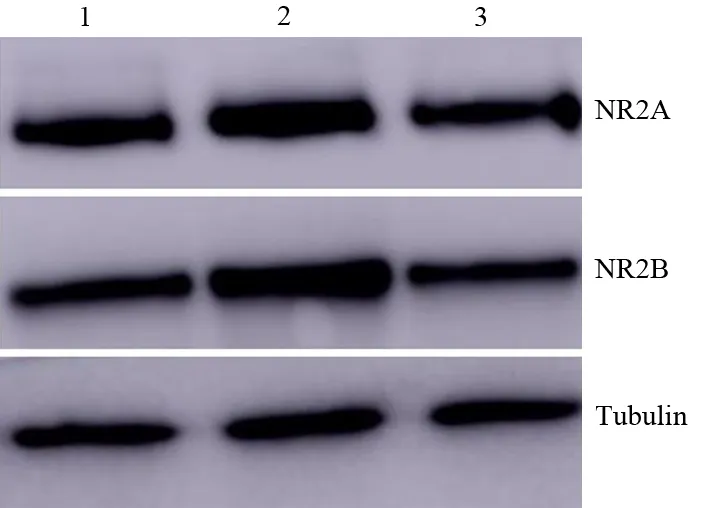
图2 正常组(1)、CP组(2)、对照组(3)大鼠大脑ACC区NR2A和NR2B蛋白表达
四、大鼠大脑ACC区NR2A、NR2B mRNA表达量的变化
CP组、对照组、正常组大鼠大脑ACC区NR2A mRNA表达量分别为1.43±0.20、0.80±0.10、1.01±0.13;NR2B mRNA表达量为1.40±0.09、0.98±0.14、0.94±0.05。CP组显著高于对照组及正常组,差异均有统计学意义(P值均<0.01),而对照组与正常组的差异无统计学意义(P>0.05)。
讨 论
CP为临床常见胰腺疾病,疼痛作为其主要临床表现之一,是部分患者就诊的首发症状并反复就医的原因,但目前公认的CP疼痛机制尚未明确。近年来的研究发现,CP患者大脑杏仁核和ACC等处的血流增加,参与疼痛加工的大脑皮质厚度显著变薄,且皮质厚度与疼痛评分呈正相关[11-12]。
ACC区是疼痛感知的关键皮层区域,其本身可参与疼痛传递和调节过程,还可通过调节多种疼痛相关受体参与痛觉行为的反应过程。NMDA受体在ACC区分布广泛,可兴奋突触后传递,并在神经可塑性中起重要作用,与记忆形成、慢性疼痛等多种中枢功能有关,其中NR2A、NR2B在多种慢性疼痛中表达明显增高[13-15]。有研究表明,通过慢病毒感染沉默ACC区NR2B表达可减弱ACC神经元的兴奋性,显著缓解疼痛反应[16]。NMDA受体依赖长时程增强效应(long-termpotentiation, LTP)是ACC长期可塑性的主要形式,与感觉传递和感知相关的中枢区域的突触传递一起被认为是慢性疼痛的关键细胞机制。在外周刺激长时间作用下,突触后NMDA受体触发ACC的锥体神经元中的LTP,通过激活谷氨酸受体,增加钙离子通透性。突触后神经元内钙离子浓度升高,激活钙依赖的信号通路,正反馈引起谷氨酸及谷氨酸受体的表达上调,增强兴奋性突触传递,形成LTP,导致慢性内脏痛的形成与增强[17-18]。
本研究结果显示,CP大鼠中枢ACC区域NR2A、NR2B表达上调,可增强ACC神经元的兴奋性,促进伤害性信号传递,参与CP疼痛的形成。至于NMDA受体依赖LTP的具体机制有待进一步研究。
[1] Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis[J]. Lancet, 2011, 377(9772): 1184-1197. DOI:10.1016/S0140-6736(10)61852-1.
[2] Pasricha PJ. Unraveling the mystery of pain in chronic pancreatitis[J]. Nat Rev Gastroenterol Hepatol, 2012, 9(3): 140-151. DOI:10.1038/nrgastro.2011.274.
[3] Li XY, Wang N, Zuo ZX, et al. Long-Term temporal imprecision of information coding in the anterior cingulate cortex of mice with peripheral inflammation or nerve Injury[J]. J Neurosci, 2014, 34(32): 10675-10687. DOI:10.1523/JNEUROSCI.5166-13.2014.
[4] Chen T, Koga K, Descalzi G, et al. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury[J]. Mol Pain, 2014, 10: 33. DOI:10.1186/s13041-014-0076-8.
[5] Liu SB, Zhang MM, Cheng LF, et al. Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain[J]. Mol Brain, 2015, 8(1): 76. DOI:10.1186/s13041-015-0169-z.
[6] Lei LG, Sun S, Gao YJ, et al. NMDA receptors in the anterior cingulate cortex mediate pain-related aversion[J]. Exp Neurol, 2004, 189(2): 413-421. DOI:10.1016/j.expneurol.2004.06.012.
[7] Sparmann G, Merkord J, Jaschke A, et al. Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride[J]. Gastroenterology, 1997, 112(5): 1664-1672.
[8] Zhao HF, Ito T, Gibo J, et al. Anti-monocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats[J]. Gut, 2005, 54(12): 1759-1767.DOI:10.1136/gut.2004.049403.
[9] Miyauchi M, Suda K, Kuwayama C, et al. Role of fibrosis-related genes and pancreatic duct obstruction in rat pancreatitis models: implications for chronic pancreatitis[J]. Histol Histopathol, 2007, 22(10): 1119-1127.DOI:10.14670/HH-22.1119.
[10] Qian NS, Liao YH, Feng QX, et al. Spinal toll like receptor 3 is involved in chronic pancreatitis-induced mechanical allodynia of rat[J]. Mol Pain, 2011, 7: 15. DOI:10.1186/1744-8069-7-15.
[13] Yang JX, Hua L, Li YQ, et al. Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit[J]. J Neurosci, 2015, 35(1): 36-52. DOI:10.1523/JNEUROSCI.1161-14.2015.
[14] Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain[J]. Philos Trans R Soc Lond B Biol Sci, 2014, 369(1633): 20130146. DOI:10.1098/rstb.2013.0146.
[15] Li TT, Ren WH, Xiao X, et al. NMDA NR2A and NR2B receptors in the rostral anterior cingulate cortex contribute to pain-related aversion in male rats[J]. Pain, 2009, 146(1-2): 183-193. DOI:10.1016/j.pain.2009.07.027.
[16] Guo SG, Lv XH, Guan SH, et al. Silencing the NR2B gene in rat ACC neurons by lentivirus-delivered shRNA alleviates pain-related aversion[J]. Int J Clin Exp Med, 2015, 8(5): 6725-6734.
[17] Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide[J]. Molecular Brain, 2013, 6: 5. DOI:10.1186/1756-6606-6-5.
[18] Yan X, Jiang E, Gao M, et al. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain[J]. The Journal of Physiology, 2013, 591: 2001. DOI:10.1113/jphysiol.2012.250522.
NR2AandNR2Bexpressionchangesinanteriorcingulatecortexofchronicpancreatitisratsanditssignificance
ZhouWen,GaoJun,LiGuixiang,LiZhaoshen.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LiZhaoshen,Email:zhsli@81890.net
ObjectiveTo observe the changes of expression levels of NMDA receptor NR2A and NR2B in anterior cingulate cortex (ACC) of chronic pancreatitis(CP) rats and explore its roles in the pathogenesis of pain in CP.MethodsMale adult SD rats were randomly divided into CP group, control group and normal group using random number method. CP rat model was established by injecting 8mg/kg dibutyltin dichloride (DBTC) through tail vein. Control group was injected with a equal volume mixture of ethanol and glycerol via tail vein. After 4 weeks, von Frey hair of different sizes (3.85, 5.50, 7.05, 10.4, 17.8 g) were used to stimulate the abdomen of the rats and the percentage of the positive pain response was recorded. Then the rats were sacrificed. The pancreas was collected for pathological examination. NR2A and NR2B subunit mRNA and protein expression in ACC was detected by realtime-PCR and Western Blotting, respectively.ResultsThe success rate of CP model establishment was 60%. As Van Frey hair used to stimulate the ratabdomen increased from 3.85 g to 17.8 g, the percentage of positive pain response increased from (38.33±7.53)% to(73.33±8.17)% in CP group,from (7.80±6.70)% to (34.44±5.27)% in control group,from(6.57±5.48)% to(33.33±5.00)% in normal group, which was increased with the increase of the stimulation intensity. For each stimulation intensity, the percentage of positive pain response in CP group was all obviously higher than those in control and normal group, and the differences were all statistically significant (allP<0.01), but there was no statistical difference between control group and normal group. The protein expression of NR2A of ACC in CP group, control group and normal group was 1.25±0.78, 0.95±0.14 and 0.91±0.09, respectively. The protein of NR2B in three groups were 1.44±0.12, 0.93±0.08 and 0.94±0.04, respectively.NR2A and NR2B protein expressions in the CP group were both significantly higher than those in control group and normal group, and the difference was statistically significant (P<0.01). The mRNA expression of NR2A in three groups were 1.43±0.20, 0.80±0.10 and 1.01±0.13, respectively. The mRNA expression of NR2B in three groups were 1.40±0.09, 0.98±0.14 and 0.94±0.05, respectively. The mRNA expressions of NR2A and NR2B in CP group were significantly higher than those in normal and control group, and the difference was statistically significant (P<0.01).ConclusionsThe expression of glutamate receptor NR2A and NR2B in ACC were upregulated in CP rats and may be involved in the development of the pain.
Pancreatitis, chronic; Pain; Cerebral cortex; Receptors, N-methyl-D-aspartate
FundprogramNational Natural Science Foundation of China(81270540)
10.3760/cma.j.issn.1674-1935.2017.06.003
200433 上海,第二军医大学长海医院消化内科
李兆申,Email:zhsli@81890.net
国家自然科学基金(81270540)
2017-04-26)
吕芳萍)

