血脂及炎症指标在非酒精性脂肪肝合并颈动脉粥样硬化患者中的改变
赵 波, 胡来明, 李小莉
湖北医药学院附属襄阳市第一人民医院老年病科,湖北 襄阳 441000
血脂及炎症指标在非酒精性脂肪肝合并颈动脉粥样硬化患者中的改变
赵 波, 胡来明, 李小莉
湖北医药学院附属襄阳市第一人民医院老年病科,湖北 襄阳 441000
目的检测并明确血脂及炎症指标在非酒精性脂肪肝合并颈动脉粥样硬化患者中的改变,以期为临床诊疗提供参考。方法分析2015年1月至2017年1月在湖北医药学院附属襄阳市第一人民医院接受诊疗的非酒精性脂肪肝患者的临床资料。依据患者是否合并颈动脉粥样硬化分为观察组(合并)及对照组(不合并)。共纳入观察组46例,对照组50例。检测两组血脂及血液炎症指标水平,并分析与颈动脉内膜中层厚度(IMT)的相关性。结果观察组患者HDL-C显著低于对照组(P<0.05),而LDL-C显著高于对照组(P<0.05)。两组患者TG及TC水平比较差异无统计学意义(P>0.05)。 观察组患者外周血IL-6水平均显著高于对照组(P<0.05)。两组患者外周血IL-10及TGF-β水平比较差异无统计学意义(P>0.05)。观察组患者IMT平均值为(1.39±0.25)mm。观察组患者IMT与LDL-C(r=0.631,P<0.05)及IL-6(r=0.518,P<0.05)水平均呈显著正相关,而与HDL-C(r=-0.690,P<0.05)水平呈显著负相关。结论伴有动脉粥样硬化的非酒精性脂肪肝患者脂质代谢紊乱及系统性慢性炎症反应程度更大,且与患者颈动脉粥样硬化程度相关。
非酒精性脂肪肝;颈动脉粥样硬化;血脂;炎症;脂质代谢紊乱
非酒精性脂肪肝(non-alcoholic fatty liver disease, NAFLD)是我国最为常见的脂肪性肝病,虽然其发病机制并不十分明确,但脂质代谢障碍、肥胖及遗传易感等被认为是高危因素[1-2]。而系统性慢性炎症则是疾病进展过程中的重要推手[3]。动脉粥样硬化是另一种与脂质代谢障碍密切相关的临床常见病[4],是引发心脑血管意外的基础疾病,具有一定致残及致死性。部分脂肪肝患者常在健康体检中意外发现颈动脉粥样硬化[5]。这一方面提示此两种疾病可能具有相似的发病机制,另一方面也给我们造成困惑:合并颈动脉粥样硬化的NAFLD患者与不合并颈动脉粥样硬化的患者在血脂、系统性炎症指标上是否有差异?回答这一问题将对临床诊疗提供参考。因而我们设计此研究检测并分析血脂及炎症指标在NAFLD合并颈动脉粥样硬化患者中的改变及临床意义。
1 资料与方法
1.1研究对象分析2015年1月至2017年1月在我院接受诊疗的NAFLD患者的临床资料。所有患者均经临床及影像学检查明确诊断为NAFLD,并同时排除以下情况的患者:有饮酒史;合并肝脏其他器质性病变;合并严重肾功能障碍;合并活动性感染。依据患者是否合并颈动脉粥样硬化分为观察组(合并)及对照组(不合并)。共纳入观察组46例,对照组50例。两组一般临床比较见表1,各项指标两组比较差异无统计学意义(P>0.05)。

表1 两组一般临床资料比较Tab 1 Comparison of general clinical data between two groups
1.2检测及观察指标对比两组患者如下血液学检测:(1)血脂:甘油三脂(Triglyceride, TG)、高密度脂蛋白(high-density lipoprotein, HDL-C)、低密度脂蛋白(low-density lipoprotein, LDL-C)及总胆固醇(total cholesterol, TC);(2)炎症指标:转化生长因子-β(transforming growth factor-β, TGF-β)、白介素-10(Interleukin-10, IL-10)及白介素-6(Interleukin-6, IL-6)。于清晨空腹状态下采集患者静脉血10 ml,血脂的检测采用全自动生化仪,炎症指标的检测采用酶联免疫吸附法,试剂盒购自Santa Cruz公司(USA)。对比两组患者颈动脉内膜中层厚度(intimal medial thickness, IMT),检查及评估采用彩色多普勒超声。分析观察组患者IMT与血脂及炎症指标的相关性。
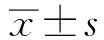
2 结果
2.1两组血脂水平比较观察组患者HDL-C显著低于对照组(P<0.05),而LDL-C显著高于对照组(P<0.05)。两组患者TG及TC水平比较差异无统计学意义(P>0.05)(见表2)。

表2 两组患者血脂水平的比较
2.2两组患者炎症指标的比较观察组患者外周血IL-6水平均显著高于对照组(P<0.05)。两组患者外周血IL-10及TGF-β水平比较差异无统计学意义(P>0.05)(见表3)。

表3 两组患者炎症指标的比较Tab 3 Comparison of inflammation between two groups
2.3观察组患者IMT与血脂及炎症指标的相关性分析观察组患者IMT平均值为(1.39±0.25)mm。如图1所示,观察组患者IMT与LDL-C(r=0.631,P<0.05)及IL-6(r=0.518,P<0.05)水平均呈显著正相关,而与HDL-C水平呈显著负相关(r=-0.690,P<0.05)。

图1 观察组患者IMT与血脂及炎症指标的相关性分析Fig 1 Correlation of IMT with blood lipid and inflammation indexes of patients in observation group
3 讨论
随着人口老龄化、西方化的生活饮食方式及食品安全等因素的影响,我国脂质代谢紊乱相关性疾病的发病率不断攀升。其中脂肪肝在人群中已然成为一种多发病、常见病,单纯的轻、中度脂肪肝尚不至于威胁患者生命等,但这提示患者可能存在脂质代谢紊乱、代谢综合征、系统性慢性炎症及肥胖等[6-7]。另外,临床上NAFLD患者在体检中常意外发现动脉粥样硬化[8-9],这提示两种疾病之间的密切联系。虽然具有相近的发病基础,但动脉粥样硬化具有更大的危害性[10]。本研究尝试分析合并或不合并动脉粥样硬化的NAFLD患者血脂及血液炎症指标是否有差异,及其水平与颈动脉粥样硬化程度的相关性,以期为临床诊疗提供参考。
本研究显示,观察组患者HDL-C显著低于对照组,而LDL-C显著高于对照组。两组患者TG及TC水平比较未见显著差异。此外,观察组患者外周血IL-6水平均显著高于对照组。LDL-C是运输内源性胆固醇的主要载体,其在高脂饮食及肥胖等病理生理过程中均可升高,且其水平与代谢综合征密切相关。HDL-C可将血管壁或细胞内的脂质转运至外周组织或器官中,以减少细胞或血管壁内的脂质沉积,这是HDL-C抗动脉粥样硬化及脂肪肝功能的机制之一[11-13]。IL-6是一种非特异性促炎介质,在慢性炎症状态的维持中发挥关键作用,可通过多条信号通路激活单核巨噬细胞系统而维持炎症状态。HDL-C在脂肪肝患者中可能因为消耗而降低,而LDL-C因脂质代谢障碍而升高。同时IL-6作为一种促炎介质同时参与脂质代谢障碍及动脉粥样硬化的病理生理过程,但其在伴有动脉粥样硬化的患者中水平更高,提示疾病的严重程度[14-15]。动脉粥样硬化存在慢性炎症状态,而炎症介质可干扰胆固醇的代谢,因而血脂水平可能降低[16-17]。我们的研究虽然没有发现观察组患者TG及TC水平低于对照组,两者水平差异并无统计学意义,这可能是由于样本量差异以及纳入患者标准差异所致。同时我们的数据反映,患者颈动脉粥样硬化程度与血脂及炎症水平相关,进一步提示两种疾病之间的内在联系。我们认为动脉粥样硬化和NAFLD虽然作为两种独立的疾病,但两者可能具有相似的发病机制和诱因。慢性炎症作为推动疾病发生、发展的重要因素贯穿于整个疾病病程中,炎症与脂质代谢障碍互为因果从而促进疾病进展,其中的复杂机制尚需进一步探索。
综上,伴有动脉粥样硬化的NAFLD患者脂质代谢紊乱及系统性慢性炎症反应程度更大,且与患者颈动脉粥样硬化程度相关。
[1] GLASSNER K, MALATY H M, ABRAHAM B P. Epidemiology and risk factors of nonalcoholic fatty liver disease among patients with inflammatory bowel disease [J]. Inflamm Bowel Dis, 2017, 23(6): 998-1003.
[2] AJMERA V, LOOMBA R. Editorial: non-alcoholic fatty liver disease-a pandemic in need of novel treatments and endpoints [J]. Aliment Pharmacol Ther, 2017, 46(1): 65-66.
[3] CARR R M, PATEL A, BOWNIK H, et al. Intestinal inflammation does not predict nonalcoholic fatty liver disease severity in inflammatory bowel disease patients [J]. Dig Dis Sci, 2017, 62(5): 1354-1361.
[4] GILL C, VATVHEVA K P, PAN J J, et al. Frequency of nonalcoholic fatty liver disease and subclinical atherosclerosis among young mexican americans [J]. Am J Cardiol, 2017, 119(11): 1717-1722.
[5] PATIL R, SOOD G K. Non-alcoholic fatty liver disease and cardiovascular risk [J]. World J Gastrointest Pathophysiol, 2017, 8(2): 51-58.
[6] PANDEY A, RAJ P, GORU S K, et al. Esculetin ameliorates hepatic fibrosis in high fat diet induced non-alcoholic fatty liver disease by regulation of FoxO1 mediated pathway [J]. Pharmacol Rep, 2017, 69(4): 666-672.
[8] CASERTA C A, MELE A, SURACE P, et al. Association of non-alcoholic fatty liver disease and cardiometabolic risk factors with early atherosclerosis in an adult population in Southern Italy [J]. Ann Ist Super Sanita, 2017, 53(1): 77-81.
[9] ARONER S A, MUKAMAL K J, ST-JULES D E, et al. Fetuin-A and risk of diabetes independent of liver fat content: the multi-ethnic study of atherosclerosis [J]. Am J Epidemiol, 2017, 185(1): 54-64.
[10] LONARDO A, BALLESTRI S, GUARALDI G, et al. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease-Evidence from three different disease models: NAFLD, HCV and HIV [J]. World J Gastroenterol, 2016, 22(44): 9674-9693.
[11] LIU Y, LIU C, SHI X, et al. Correlations of non-alcoholic fatty liver disease and serum uric acid with subclinical atherosclerosis in obese Chinese adults [J]. J Diabetes, 2017, 9(6): 586-595.
[12] PRIVITERA G, SPADARO L, ALAGONA C, et al. Hepatic insulin resistance in NAFLD: relationship with markers of atherosclerosis and metabolic syndrome components [J]. Acta Diabetol, 2016, 53(3): 449-459.
[13] 王志勇, 邓国孙. 脂肪肝患者腹部手术风险及一级亲属癌症的发病率研究[J]. 胃肠病学和肝病学杂志, 2016, 25(3): 318-319.
WANG Z Y, DENG G S. Research on the risk of abdominal operation in patients with fatty liver and the prevalence of cancer in first-degree relatives [J]. Chin J Gastroenterol Hepatol, 2016, 25(3): 318-319.
[14] WANG X, AI L, XU Q, et al. A20 Attenuates Liver Fibrosis in NAFLD and Inhibits Inflammation Responses [J]. Inflammation, 2017, 40(3): 840-848.
[15] TARIQ H, NAYUDU S, AKELLA S, et al. Non-alcoholic fatty pancreatic disease: a review of literature [J]. Gastroenterol Res, 2016, 9(6): 87-91.
[16] HADIZADEH F, FAGHIHIMANI E, ADIBI P. Nonalcoholic fatty liver disease: diagnostic biomarkers [J]. World J Gastrointest Pathophysiol, 2017, 8(2): 11-26.
[17] TISON G H, BLAHA M J, NASIR K, et al. Relation of anthropometric obesity and computed tomography measured nonalcoholic fatty liver disease (from the multiethnic study of atherosclerosis) [J]. Am J Cardiol, 2015, 116(4): 541-546.
Thechangesofserumlipidandinflammationmarkersinnon-alcoholicfattyliverdiseasepatientscombinedwithatherosclerosis
ZHAO Bo, HU Laiming, LI Xiaoli
Department of Geriatrics, Xiangyang First People’s Hospital Affiliated to Hubei University of Medicine, Xiangyang 441000, China
ObjectiveTo analyze the changes of serum lipid and inflammation markers in non-alcoholic fatty liver disease (NAFLD) patients combined with atherosclerosis.MethodsClinical data of NAFLD patients
treatment in hospital from 2015 to 2017 were analyzed. Patients were divided into two groups by whether sufferred from atherosclerosis.ResultsA total of 96 patients were collected, including group A (combined with atherosclerosis,n=46) and group B (not combined with atherosclerosis,n=50). Levels of IL-6 and LDL-C were higher and the level of HDL-C was lower in group A compared with group B, the difference was statistically significant (P<0.05). There was no significant difference in IL-10, TG, TC and TGF-β between two groups (P>0.05). Correlation analysis showed that the level of intimal medial thickness in group A had significant positive correlation with LDL-C (r=0.631,P<0.05), IL-6 (r=0.518,P<0.05) and negative correlation with HDL-C (r=-0.690,P<0.05).ConclusionNAFLD patients combined with atherosclerosis have more severe lipid metabolism disorders and systemic inflammatory response, they are associated with degree of atherosclerosis.
Non-alcoholic fatty liver disease; Atherosclerosis; Serum lipid; Inflammation; Lipid metabolism disorders
10.3969/j.issn.1006-5709.2017.12.020
R575
A
1006-5709(2017)12-1406-03
2017-07-01
王全楚)

