Improvement of The Performance of Planar Heterojunction Perovskite Solar Cells by Using Pyridine as Additive
-, -, , -, -, -*, -, -*
(1. Key Laboratory of Display Materials and Photoelectric Devices, Education Ministry of China, Tianjin University of Technology, Tianjin 300384, China;2. Tianjin Key Laboratory for Photoelectric Materials and Devices, School of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China;3. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China; 4. China Electronics Technology Group Corporation No.18th Research Institute, Tianjin 300384, China)*Corresponding Authors, E-mail: liyingyang@tjut.edu.cn; jww315@126.com; sgyin@tjut.edu.cn
ImprovementofThePerformanceofPlanarHeterojunctionPerovskiteSolarCellsbyUsingPyridineasAdditive
LIZhi-cheng1,2,WANGYa-ling1,2,YANGYin1,2,CAOHuan-qi1,2,QINWen-jing1,2,YANGLi-ying1,2*,JIWei-wei3,4*,YINShou-gen1,2*
(1.KeyLaboratoryofDisplayMaterialsandPhotoelectricDevices,EducationMinistryofChina,TianjinUniversityofTechnology,Tianjin300384,China;2.TianjinKeyLaboratoryforPhotoelectricMaterialsandDevices,SchoolofMaterialsScienceandEngineering,TianjinUniversityofTechnology,Tianjin300384,China;3.SchoolofChemicalEngineeringandTechnology,TianjinUniversity,Tianjin300072,China;4.ChinaElectronicsTechnologyGroupCorporationNo.18thResearchInstitute,Tianjin300384,China)
*CorrespondingAuthors,E-mail:liyingyang@tjut.edu.cn;jww315@126.com;sgyin@tjut.edu.cn
An efficient one-step solution method was demonstrated to prepare CH3NH3PbI3perovskite solar cells by adding pyridine to the precursor solution. The film morphology, crystallinity, and optical properties of CH3NH3PbI3perovskite films were investigated by SEM, AFM, XRD, UV-Vis and PL. The results show that the perovskite film properties (coverage of perovskite films and surface morphology) can be manipulated by incorporating a small amount of pyridine. The adding of pyridine is helpful to obtain a smooth, continuous and dense morphology. With the optimized pyridine volume fraction of1%, the power conversion efficiency of7.33% withJscof14.64mA/cm2,Vocof0.82V, and FF of0.61is obtained for the planar device structure. By contrast, the power conversion efficiency of the device without pyridine is only1.01%. However, the further increase of the content of pyridine can result in the decomposition of perovskite, because the pyridine can easily form complexes with perovskite and induce the perovskite dissociation.
perovskite solar cell; additive; morphology; pyridine
1 Introduction
During the past year, perovskite solar cells have received great attention due to their long charge carrier diffusion length[1-2], broad and strong light absorption[3], longer carrier lifetimes[4-5], and bipolar transport properties[6-7]. The main interest in inorganic-organic hybrid perovskite materials stems from the huge success of their application in perovskite solar cells (PSCs), which has led to power conversion efficiency (PCE) of >20%[8-11]. To date, a certified power conversion efficiency of 22.1% has been reported[12]. CH3NH3PbI3perovskite is the most commonly used sensitizer with a fixed energy bandgap of 1.52 eV and the absorption range up to 800 nm[13-15]. The optimal morphology and device performance have been achieved by various processing approaches, such as substrate annealing, solvent annealing, or solvent as additive, in order to control crystal growth during spin coating[16-19]. Introduction of additives in solution deposition process is an effective approach to modulate the growth of perovskite films, and to improve the film coverage and crystallinity. Several additives, such as NH4Cl[20], CH3NH3Cl[21], 1,8-diiodooctane (DIO)[22]and 1-chloronaphthalene[23], have been used to deposit continuous and uniform films, which significantly enhance the device performance. Snaithetal. reported a mixed halide precursor solution that could effectively tune the nucleation and growth kinetics of the perovskite films[24]. Jen and coworkers found that bidentate halogenated additives can temporarily chelate with Pb2+, which encourages homogenous nucleation and modifies interfacial energy, ultimately altering the kinetics of crystal growth[22].
Here, we presented a method of controlling the morphology growth of the perovskite absorber by adding pyridine as additive into the precursor solution for preparing efficient perovskite solar cells. The effect of the additive on the morphology, structure, optical and electrical properties of perovskite film has been investigated by photoluminescence spectra, SEM, UV-Vis, XRD and AFM. The steady-state PL measurement suggested that the films with pyridine as additive contained less traps and defects. The planar heterojunction perovskite solar cells fabricated from perovskite precursor solution containing pyridine additive demonstrated enhancement in performance compared to the devices with pristine films. However, further increasing the contents of pyridine, decomposition of perovskite was observed due to the fact that pyridine can easily combine with perovskite to form complexes, leading to the dissociation of perovskite.
2 Experiments
2.1 Materials

2.2 Preparation of Perovskite Films
The device structure is shown in the Fig.1. PbAc2and CH3NH3I solid were dissolved in 0.5 mL DMF solution according to the molar ratio of 1∶3. Then, a certain amount of pyridine was added into the DMF precursor solution as the additive. The thickness of PEDOT∶PSS layer is about 40 nm which is obtained by spin coating at 3 000 r/min for 30 s on ITO coated glass substrates, followed by annealing at 120 ℃ for 30 min in air. The perovskite layer was spin-coated on top of the PEDOT∶PSS by spin coating at a speed of 2 000 r/min for 40 s, followed by annealing at 90 ℃ for 10 min in the glovebox. The PCBM (24 mg/mL, o-dichlorobenzene) is spin-coated at 1 000 r/min for 20 s onto the perovskite layer. Finally, 150 nm Al is thermally evaporated under a vacuum of a pressure of 1×10-4Pa. The active layer area of the device is 0.09 cm2defined by a shadow mask.

Fig.1 Structures of the solar cells
3 Results and Discussion
To study the effects of pyridine additive on the device performance, solar cells with the structure of ITO/PEDOT∶PSS/CH3NH3PbI3/PCBM/Al were fabricated. Fig.2(a) shows theJ-Vcharacteristics of the devices containing different volume fraction of pyridine. And the performance parameters are alsosummarized in Tab.1. The control device without adding pyridine gives the PCE of 1.01%, withJscof 3.49 mA/cm2,Vocof 0.6 V, and FF of 48.41%. The device containing pyridine additive shows improved performance and reaches the maximum when the pyridine with 1% volume fraction. The optimized device gives the PCE of 7.33%, withJscof 14.64 mA/cm2,Vocof 0.82 V, and FF of 61.11%, respectively. Further increasing the amount of pyridine, reduced performance was observed. This may be caused by the corrosion of pyridine on the film, as reported by previous reports[25]. Although the addition of pyridine reduces the roughness and improves the uniformity of the film, the devices still show slightly hysteresis. We speculate the reason for the current hysteresis is probably attributed to the interfacial contact between perovskite layer and electron transport layers. To improve the interfacial contact and eliminate the hysteresis, further work on interfacial modification is under way (Fig.2(b)) .

Fig.2 (a)J-Vcharacteristics of the devices with different amount of pyridine. (b)J-Vcharacteristics of the optimal perovskite device measured with different scan directions.
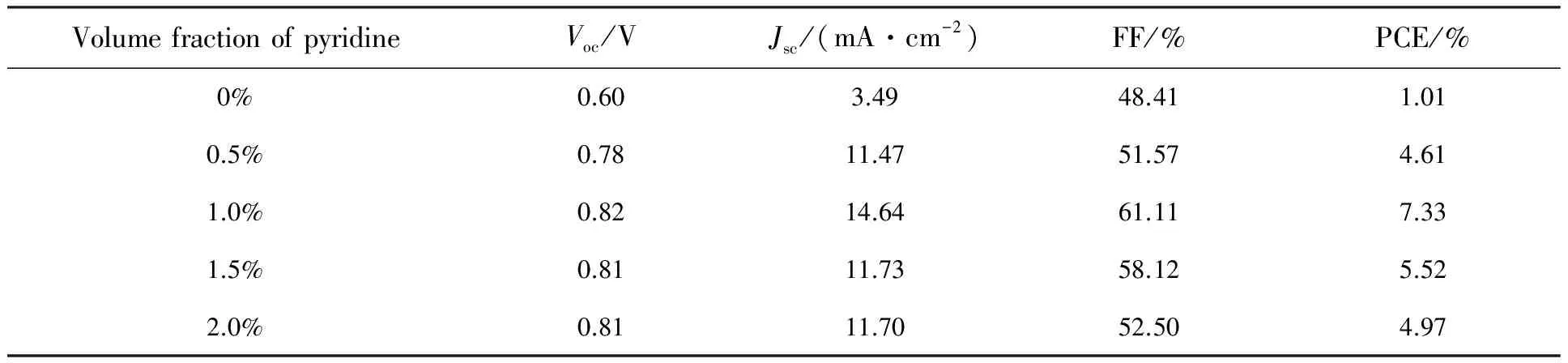
Tab.1 Summary of the device parameters for the perovskite solar cells with different volume percent of pyridine
Fig.3 shows the atomic force microscope (AFM) images of perovskite films without or with pyridine additive. TheRaand root meant-squared roughness (Rq) of the pristine perovskite film (in Fig.3(a)) is 11.1 and 14.2 nm, respectively. When the volume fraction of pyridine reached to 1.0%, theRaandRqvalues of the perovskite film reduced to 3.71 and 4.69 nm(in Fig.3(c)), respectively. It is obvious that pyridine can significantly reduce the roughness of the film. Fig.3(b) for pristine film shows a non-continuous film with void and pin-hole, which is detrimental to the coverage of PCBM layer on the top perovskite film and may result in a problem of current leaking. It may be one of the reasons for the poor performance of the control device. The perovskite layer using pyridine as additive shows a continuous and pinhole-free surface, which was supported by SEM images in Fig.3(d). As a result, improved performance of the planar heterojunction perovskite device with increasedJscand FF is achieved.

Fig.3 AFM and SEM images of the active layer with different amount of pyridine (a,b: pristine; c,d: 1%)
We also compare the XRD patterns of the pure CH3NH3PbI3and films containing 1.0% pyridine as additive. The peaks located at 14.2° and 28.5° are belonging to the (110) and (220) crystal planes of the orthorhombic lattice of CH3NH3PbI3. The Scherrer equation was used to compare the crystallinities (average crystallite sizes):
D=Kλ/βcosθ,
(1)
whereDis the average crystallite size,λis the wavelength of the X-ray irradiation (0.154 nm), andβis the line width at half maximum (in radians). The average crystal size estimated from the FWHM of the (110) peak is approximately 28 nm and 38 nm for the pristine CH3NH3PbI3films and the films with 1% pyridine as additive, respectively. The increasing in crystal size indicates that the crystallinity is enhanced, mainly due to the fact that the incorporation of pyridine affects the crystallization process. It should be noted that there is no PbI2peak at 12.65° for the films containing 1.0% pyridine additive, implying that the crystallization transformation kinetics of perovskite can be influenced to form high quality crystal films through carefully regulating the amount of the pyridine additive. However, further increasing the content of pyridine, decomposition of perovskite was observed as the main peak associated with PbI2at 12.65° is appeared. The decomposition process was speculated as follows. Pyridine, as a lewis-base with high binding affinity with the lewis-acid PbI2, can easily form complexes with perovskite and induce perovskite dissociation.

Fig.4 XRD patterns of the perovskite layer with different amount of pyridine

Fig.5 UV-Vis (a) and PL (b) spectra of the perovskite layer with different amount of pyridine
Fig.5(a) and Fig.5(b) show the UV-Vis and PL spectra of perovskite film without and with different concentration of pyridine additive. We observed higher optical densities for the films prepared with pyridine as the additive, due to better surface coverage and uniformity. The intensities of the signals in the UV-Vis and PL spectra of these films decreased when the concentration of the additive followed the order 1%>1.5%>2.0%>0.5%>0%. It proves that light absorption intensity is closely related to the quality of the perovskite film. As the concentration of the additive increases gradually, the crystallinity increases and the grains become larger. Especially, upon addition of 1% pyridine in the film, high quality perovskite film with better coverage, lower roughness and fewer defects was obtained. Consequently, the film with 1% pyridine additive exhibits the strongest absorption intensity. From the PL spectra, the pure perovskite shows a weak emission intensity, indicating more defects existing in the film. Pyridine is usually considered as electron-rich molecule and can passivate the defects in the film. Therefore, introducing pyridine into perovskite precursor solution can reduce the defects and suppress the nonradiative recombination, which is conductive to light absorption, exction generation, as well as higher PL emission. These results are also in agreement with the photovoltaic performance of the devices.
4 Conclusion
An efficient one-step solution method to prepare CH3NH3PbI3perovskite solar cells by adding pyridine to the precursor solution was investigated. The film morphologies, crystallinity, and optical properties of CH3NH3PbI3films are investigated by SEM, AFM, XRD, UV-Vis and PL. The results showed that the perovskite film properties (coverage of perovskite films and surface morphology) can be manipulated by incorporating a small amount of pyridine. The adding of pyridine is helpful to obtain a smooth, continuous and dense morphology. With optimized volume fraction of 1%, the PCE of 7.33% withJscof 14.64 mA/cm2,Vocof 0.82 V, and FF of 0.61 was obtained for the planar device structure. However, further increases the content of pyridine, decomposition of perovskite was observed due to pyridine can easily form complexes with perovskite and induce perovskite dissociation. We hope this finding will facilitate better understanding perovskite material and advance the further development of efficient and highly stable perovskite solar cells.
[1] BALL J M, LEE M M, HEY A,etal.. Low-temperature processed meso-superstructured to thin-film perovskite solar cells [J].EnergyEnviron.Sci., 2013, 6(6):1739-1743.
[2] CHEN C W, KANG H W, HSIAO S Y,etal.. Efficient and uniform planar-type perovskite solar cells by simple sequential vacuum deposition [J].Adv.Mater., 2014, 26(38):6647-6652.
[3] CHENG J W, ZHENG S T, YANG G Y. A series of lanthanide-transition metal frameworks based on 1-, 2-, and 3D metal-organic motifs linked by different 1D copper (Ⅰ) halide motifs[J].Inorgan.chem., 2007, 46(24):10261-10267.
[4] STRANKS S D, EPERON G E, GRANCINI G,etal.. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber [J].Science, 2013, 342(6156):341-344.
[5] WEHRENFENNIG C, EPERON G E, JOHNSTON M B,etal.. High charge carrier mobilities and lifetimes in organolead trihalide perovskites [J].Adv.Mater., 2014, 26(10):1584-1589.
[6] XING G, MATHEWS N, SUN S,etal.. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3[J].Science, 2013, 342(6156):344-347.
[7] LIU M, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition [J].Nature, 2013, 501(7467):395-398.
[8] SHIN S S, YEOM E J, YANG W S,etal.. Colloidally prepared La-doped BaSnO3electrodes for efficient, photostable perovskite solar cells [J].Science, 2017, 356(6334):167-171.
[9] JIANG Q, ZHANG L, WANG H,etal.. Enhanced electron extraction using SnO2for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells [J].Nat.Energy, 2017, 2(4):17060.
[10] SALIBA M, MATSUI T, DOMANSKI K,etal.. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance [J].Science, 2016, 354(6309):206-209.
[11] SALIBA M, MATSUI T, SEO J Y,etal.. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency [J].EnergyEnviron.Sci., 2016, 9(6):1989-1997.
[12] YANG W S, PARK B W, JUNG E H,etal.. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells [J].Science, 2017, 356(6345):1376-1379.
[13] IM J H, LEE C R, LEE J W,etal.. 6.5% efficient perovskite quantum-dot-sensitized solar cell [J].Nanoscale, 2011, 3(10):4088-4093.
[14] KIM H S, LEE C R, IM J H,etal.. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9% [J].Sci.Rep., 2012, 2:591.
[15] ETGAR L, GAO P, XUE Z,etal.. Mesoscopic CH3NH3PbI3/TiO2heterojunction solar cells [J].J.Am.Chem.Soc., 2012, 134(42):17396-17399.
[16] JENG J Y, CHIANG Y F, LEE M H,etal.. CH3NH3PbI3perovskite/fullerene planar-heterojunction hybrid solar cells [J].Adv.Mater., 2013, 25(27):3727-3732.
[17] JEON N J, NOH J H, KIM Y C,etal.. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells [J].Nat.Mater., 2014, 13(9):897-903.
[18] LIANG P W, LIAO C Y, CHUEH C C,etal.. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells[J].Adv.Mater., 2014, 26(22):3748-3754.
[19] XIAO M, HUANG F, HUANG W,etal.. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells [J].Angew.Chem., 2014, 126(37):10056-10061.
[20] ZUO C, DING L. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive [J].Nanoscale, 2014, 6(17):9935-9938.
[21] ZHAO Y, ZHU K. CH3NH3Cl-assisted one-step solution growth of CH3NH3PbI3: structure, charge-carrier dynamics, and photovoltaic properties of perovskite solar cells [J].J.Phys.Chem. C, 2014, 118(18):9412-9418.
[22] SONG X, WANG W, SUN P,etal.. Additive to regulate the perovskite crystal film growth in planar heterojunction solar cells [J].Appl.Phys.Lett., 2015, 106(3):033901.
[23] STRANKS S D, EPERON G E, GRANCINI G,etal.. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber [J].Science, 2013, 342(6156):341-344.
[24] NOEL N K, ABATE A, STRANKS S D,etal.. Enhanced photoluminescence and solar cell performancevialewis base passivation of organic-inorganic lead halide perovskites [J].ACSNano, 2014, 8(10):9815-9821.
[25] YUE Y F, SALIM N T, WU Y Z,etal.. Enhanced stability of perovskite solar cells through corrosion-free pyridine derivatives in hole-transporting materials [J].Adv.Mater., 2016, 28(48):10738-10743.

李志成(1991-),男,黑龙江绥化人,硕士研究生,2014年于哈尔滨理工大学获得学士学位,主要从事有机光电材料与器件的研究。

E-mail: 465304037@qq.com纪伟伟(1983-),女,山东烟台人,博士研究生,2010年于南开大学获得硕士学位,主要从事新能源材料与器件的研究。

E-mail: jww315@126.com杨利营(1973-),男,河北石家庄人,博士,研究员,2002年于天津大学获得博士学位,主要从事有机光电功能材料与器件的研究。

E-mail: liyingyang@tjut.edu.cn印寿根(1965-),男,江苏镇江人,博士,研究员,1997年南开大学获得博士学位,主要从事有机光电功能材料与器件的研究。
E-mail: sgyin@tjut.edu.cn
2017-04-18;
2017-08-24
国家自然科学基金(51402214,61504097); 天津市自然科学基金(17JCYBJC21000,14JCYBJC42800); 国家重点科学仪器设备发展项目(2014YQ120351); 天津教委项目(20140423)资助
利用吡啶添加剂提高钙钛矿太阳能电池的光伏性能
李志成1,2, 王亚凌1,2, 杨 银1,2, 曹焕奇1,2, 秦文静1,2, 杨利营1,2*, 纪伟伟3,4*, 印寿根1,2 *
(1. 天津理工大学 显示材料与光电器件教育部重点实验室, 天津 300384 ;2. 天津市光电显示材料与器件重点实验室, 天津理工大学 材料科学与工程学院, 天津 300384;3. 天津大学 化工学院, 天津 300072; 4. 中国电子科技集团第18研究所, 天津 300384)
研究了吡啶作为添加剂对一步法制备甲胺铅碘钙钛矿太阳能电池光电性能的影响。利用SEM、AFM、XRD、UV-Vis、PL等手段研究了不同吡啶掺杂浓度对制备的CH3NH3PbI3薄膜的表面形貌、结晶度和光学性能的影响。研究结果表明:少量的吡啶掺杂可以提高钙钛矿薄膜的覆盖率及降低薄膜的表面粗糙度。当在CH3NH3PbI3前驱体溶液中添加体积分数为1%的吡啶时,制备的钙钛矿太阳能电池的能量转换效率达到7.33%,而未加吡啶的对比器件效率仅为1.01%。进一步添加吡啶会导致钙钛矿材料的降解。
钙钛矿太阳能电池; 添加剂; 形貌; 吡啶
Supported by National Natural Science Foundation of China(51402214,61504097); Natural Science Foundation of Tianjin (17JCYBJC21000,14JCYBJC42800); National Key Scientific Instrument and Equipment Development Project (2014YQ120351); Project of Tianjin Education Commission (20140423)
1000-7032(2017)11-1503-07
TM914.4DocumentcodeA
10.3788/fgxb20173811.1503

