海藻多糖对H2O2诱导人胚肺成纤维细胞MRC-5氧化损伤的影响及其机制
刘骅漫,刘学,贾新华,张心月,张伟
(1山东中医药大学,济南250014;2山东省胸科医院;3山东中医药大学附属医院)
海藻多糖对H2O2诱导人胚肺成纤维细胞MRC-5氧化损伤的影响及其机制
刘骅漫1,3,刘学1,2,贾新华3,张心月1,张伟3
(1山东中医药大学,济南250014;2山东省胸科医院;3山东中医药大学附属医院)
目的探讨海藻多糖对H2O2诱导人胚肺成纤维细胞MRC-5氧化损伤的影响及机制。方法将体外培养的人胚肺成纤维细胞MRC-5随机分为空白组、H2O2组、海藻多糖组、海藻多糖+H2O2组、H2O2+海藻多糖组。空白组正常培养,H2O2组给予H2O2处理,海藻多糖组给予海藻多糖处理,海藻多糖+H2O2组先给予海藻多糖干预1 h、再给予H2O2处理,H2O2+海藻多糖组先给予H2O2干预1 h、再给予海藻多糖处理。各组H2O2浓度均为600 μmol/L,海藻多糖浓度均为0.312 5 mg/mL。各组均于干预0、24、48、72 h时采用MTT法检测细胞增殖抑制率;处理24 h时检测丙二醛(MDA)、超氧化物歧化酶(SOD)、活性氧(ROS)表达,采用免疫荧光法检测核转录因子E2相关因子2(Nrf2)蛋白表达,采用RT-PCR法检测Nrf2、Kelch样ECH联合蛋白1(Keap1)、NADP(H)醌氧化还原酶1(NQO1)、血红素氧合酶1(HO-1)、CGLC mRNA表达。结果培养24、48、72 h时,H2O2组、海藻多糖+H2O2组、H2O2+海藻多糖组细胞增殖抑制率均高于空白组及海藻多糖组,海藻多糖+H2O2组、H2O2+海藻多糖组均低于H2O2组,组间比较P均<0.05。与空白组及海藻多糖组比较,H2O2组、海藻多糖+H2O2组、H2O2+海藻多糖组MDA、ROS表达增加,SOD表达降低;与H2O2组比较,海藻多糖+H2O2组、H2O2+海藻多糖组MDA、ROS表达降低,SOD表达增加,组间比较P均<0.05。与空白组及海藻多糖组比较,H2O2组、海藻多糖+H2O2组、H2O2+海藻多糖组Nrf2 mRNA和蛋白相对表达量均降低,海藻多糖+H2O2组、H2O2+海藻多糖组均较H2O2组升高,组间比较P均<0.05。与空白组及海藻多糖组比较,H2O2组、海藻多糖+H2O2组、H2O2+海藻多糖组Keap1 mRNA相对表达量均升高,NQO1、CGLC、HO-1 mRNA相对表达量均降低;与H2O2组比较,海藻多糖+H2O2组、H2O2+海藻多糖组Keap1 mRNA相对表达量均降低,NQO1、CGLC、HO-1 mRNA相对表达量均升高;组间比较P均<0.05。结论海藻多糖可抑制H2O2诱导人胚肺成纤维细胞MRC-5的氧化应激损伤,上调 Nrf2表达、激活Nrf2/Keap1/抗氧化反应序列元件信号通路可能是其作用机制。
肺纤维化;人胚肺成纤维细胞MRC-5;海藻多糖;过氧化氢;氧化损伤;核转录因子E2相关因子2
Abstract:ObjectiveTo investigate the effects of seaweed polysaccharides on H2O2-induced oxidative damage of human embryonic lung fibroblasts MRC-5 and its mechanism.MethodsMRC-5 cells cultured in vitro were randomly divided into the blank group, H2O2group, seaweed polysaccharide group, seaweed polysaccharide+H2O2group, and H2O2+seaweed polysaccharide group. Cells in the blank group were cultured normally, cells in the H2O2group were treated with H2O2, cells in the seaweed polysaccharide group were treated with seaweed polysaccharide, cells in the seaweed polysaccharide+H2O2group were treated with seaweed polysaccharide for 1 h, then followed by H2O2, and cells in the H2O2+seaweed polysaccharide group were treated with H2O2for 1 h, then followed by H2O2. The concentration of H2O2in each group was 600 umol/L, and the concentration of seaweed polysaccharide was 0.3125 mg/mL. At 0, 24, 48 and 72 h after treatment, the cell proliferation inhibitory rate in each group was determined by MTT assay. The levels of malondialdehyde (MDA), superoxide dismutase (SOD), and reactive oxygen species (ROS) were measured at 24 h. NF-E2-related factor 2 (Nrf2) protein was detected by immunofluorescence assay. The expression levels of Nrf2, Kelch-like ECH-associated protein 1 (Keap1), NADP (H) quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), and CGLC mRNA were detected by RT-PCR.ResultsThe cell inhibition rates of the H2O2group, seaweed polysaccharide +H2O2group, and H2O2+ seaweed polysaccharide group were higher than those of the blank group and seaweed polysaccharide group at 24, 48 and 72 h, and those of the seaweed polysaccharide+H2O2group and H2O2+seaweed polysaccharide group were lower than that of the H2O2group (allP<0.05). Compared with the blank group and the seaweed polysaccharide group, the levels of MDA and ROS in the H2O2group, the seaweed polysaccharide+H2O2group, and the H2O2+seaweed polysaccharide group increased, and the SOD level decreased. Compared with the H2O2group, the MDA and ROS levels decreased and the SOD level increased in the seaweed polysaccharide+H2O2group and the H2O2+seaweed polysaccharide group (allP<0.05). Compared with the blank group and the seaweed polysaccharide group, the expression of Nrf2 mRNA and protein in the H2O2group, the seaweed polysaccharide+H2O2group, and the H2O2+seaweed polysaccharide group decreased, and the seaweed polysaccharide+H2O2group and H2O2+seaweed polysaccharide group were higher than the H2O2group (allP<0.05). Compared with the blank group and the seaweed polysaccharide group, the relative expression of Keap1 mRNA in the H2O2group, the seaweed polysaccharide+H2O2group, and the H2O2+seaweed polysaccharide group increased, and the relative expression of NQO1, CGLC, and HO-1 mRNA decreased. Compared with the H2O2group, the relative expression of Keap1 mRNA in the polysaccharide+H2O2group and H2O2+seaweed polysaccharide group decreased, and the relative expression of NQO1, CGLC, and HO-1 mRNA increased (allP<0.05).ConclusionSeaweed polysaccharide could inhibit the oxidative stress injury of MRC-5 induced by H2O2through up-regulating the expression of Nrf2 and activating Nrf2-Keap1-ARE signaling pathway.
Keywords: pulmonary fibrosis; human embryonic lung fibroblasts MRC-5; seaweed polysaccharide; H2O2; oxidative damage; nuclear transcription factor E2-related factor 2
肺纤维化是肺间质性疾病的最终结局,氧化应激诱导的细胞氧化/抗氧化失衡是其形成及进展的主要原因之一。肺纤维化主要表现为肺功能进行性下降。目前尚无确切有效的治疗药物。中药海藻味咸性寒,归脾、肝、肾经,具有消痰散结、利水消肿之功效,起主要作用的成分为海藻多糖。海藻多糖具有抗氧化作用,目前多被作为食品抗氧化剂,其作为药物用于疾病抗氧化的研究较少。2014年10月~2015年6月,本研究探讨了海藻多糖对H2O2诱导人胚肺成纤维细胞MRC-5氧化损伤的影响及其机制。
1 材料与方法
1.1 材料 细胞:人胚肺成纤维细胞MRC-5购自南京凯基生物工程有限公司。主要试剂:海藻多糖(含量>99%)购自美国Sigma公司,MEM培养基、DMSO均购自美国Gibco公司,FBS购自美国 ExCell公司;RT-PCR试剂盒购自美国 Thermo Fisher公司,丙二醛(MDA)、超氧化物歧化酶(SOD)、活性氧(ROS)、总蛋白提取试剂盒及核转录因子E2相关因子2(Nrf2)一抗均购自南京凯基生物工程有限公司。Nrf2、Kelch样ECH联合蛋白1(Keap1)、NADP(H)醌氧化还原酶1(NQO1)、血红素氧合酶1(HO-1)、CGLC、GAPDH引物均由南京凯基生物工程有限公司合成。
1.2 细胞培养及分组处理 将MRC-5细胞置于含10% FBS、100 IU/mL青霉素、100 IU/mL链霉素的DMEM培养液中,在37 ℃、5% CO2培养箱中培养,隔天换液。当培养瓶中细胞覆盖面积达80%~90%时,加入0.25%胰蛋白酶,按1∶3传代培养。取第4代细胞,随机分为空白组、H2O2组、海藻多糖组、海藻多糖+H2O2组、H2O2+海藻多糖组。空白组正常培养,H2O2组给予终浓度为600 μmol/L H2O2处理,海藻多糖组给予终浓度为0.312 5 mg/mL海藻多糖处理,海藻多糖+H2O2组给予0.312 5 mg/mL海藻多糖干预1 h后再给予600 μmol/L H2O2处理,H2O2+海藻多糖组给予600 μmol/L H2O2干预1 h后再给予0.312 5 mg/mL海藻多糖处理。
1.3 细胞增殖抑制率检测 采用MTT法。取1.2中处理24 h的各组细胞,以密度为5×104个/mL接种于96孔细胞培养板,每组设3个复孔,分别在孵育0、24、48、72 h时每孔加入20 μL MTT,继续培养4 h;弃去所有液体,每孔加入DMSO 150 μL,置于摇床上轻轻摇匀10 min;酶标仪读取波长490 nm处各孔吸光度(A)值,计算细胞增殖抑制率。细胞增殖抑制率=(A空白组-A观察组)/A空白组×100%。
1.4 MDA、SOD、ROS表达检测 取1.2中处理24 h的各组细胞,采用硫代巴比妥酸法检测MDA表达,采用氯化硝基氮蓝四唑光还原法检测SOD表达,采用DCFH-DA探针检测ROS表达(以A值表示)。具体步骤均严格参照试剂盒说明书操作。
1.5 Nrf2 mRNA和蛋白表达检测 ①Nrf2 mRNA:采用RT-PCR法。取1.2中处理24 h的各组细胞,提取细胞总RNA,逆转录为cDNA后置于PCR仪中,42 ℃、1 h,70 ℃、10 min,冰浴5 min;将样本稀释10倍,依次向0.2 mL PCR管加入2×Master Mix(SYBR Green) 10 μL、模板(进行10倍稀释后的cDNA)1 μL、引物混合物2 μL(含正、反向引物各10 μmol/L)、无RNase的双蒸水7 μL,总量20 μL。以GAPDH为内参,计算Nrf2 mRNA相对表达量。Nrf2引物:F:TCCGGGTGTGTTTGTTCCAA,R:CGCCCGCGAGATAAAGAGTT;产物长度88 bp。GAPDH引物:F:TCCTGGCTCAGCCTCAAATG,R:CGTTAAACACCTCCCTCCCC;产物长度108 bp。②Nrf2蛋白:采用免疫荧光法。将MRC-5细胞置于预先放有载玻片的24孔培养板中培养,分组及处理同1.2。处理24 h时取出细胞爬片室温下自然晾干,浸入4%多聚甲醛中,室温固定30 min或4 ℃过夜,PBS洗涤3 min×3次;血清封闭,滴加Nrf2一抗,37 ℃恒温箱中湿盒孵育2 h,PBS洗涤3 min×3次;滴加FITC二抗,37 ℃恒温箱中湿盒避光孵育1 h,PBS洗涤3 min×3次;滴加DAPI染液,封片,荧光显微镜观察。取3个高表达视野拍照留存,检测荧光强度,计算Nrf2蛋白相对表达量。
1.6 Keap1、NQO1、CGLC、HO-1 mRNA表达检测 采用RT-PCR法,具体步骤参照1.5①。 Keap1引物:F:GTCCCCTACAGCCAAGGTCC,R:ACTCAGTGGAGGCGTACATC;产物长度175 bp。NQO1引物:F:GGTTTGGAGTCCCTGCCATT,R:ACCAGTGGTGATGGAAAGCA;产物长度134 bp。CGLC引物:F:GAGGTCAAACCCAACCCAGT,R:AAGGTACTGAAGCGAG-
GGTG;产物长度92 bp。HO-1引物:F:TCCTGGCTCAGCCTCAAATG,R:CGTTAAACACCTCCCTCCCC;产物长度108 bp。以GAPDH为内参,计算Keap1、NQO1、CGLC、HO-1 mRNA相对表达量。
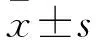
2 结果
2.1 各组细胞增殖抑制率比较 见表1。

表1 各组细胞增殖抑制率比较
注:与空白组同时间点比较,*P<0.05;与H2O2组同时间点比较,△P<0.05;与海藻多糖组同时间点比较,#P<0.05。
2.2 各组MDA、SOD、ROS表达比较 见表2。

表2 各组MDA、SOD、ROS表达比较
注:与空白组比较,*P<0.05;与H2O2组比较,△P<0.05;与海藻多糖组比较,#P<0.05。
2.3 各组Nrf2 mRNA和蛋白表达比较 见表3。

表3 各组Nrf2 mRNA和蛋白相对表达量比较
注:与空白组比较,*P<0.05;与H2O2组比较,△P<0.05;与海藻多糖组比较,#P<0.05。
2.4 各组Keap1、NQO1、CGLC、HO-1 mRNA表达比较 见表4。

表4 各组Keap1、NQO1、CGLC、HO-1 mRNA相对表达量比较
注:与空白组比较,*P<0.05;与H2O2组比较,△P<0.05;与海藻多糖组比较,#P<0.05。
3 讨论
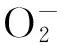
Nrf2是细胞氧化应激反应的关键因子,在氧化应激应答中发挥核心调控作用。Nrf2可与抗氧化反应序列元件(ARE)结合,发挥内源性抗氧化作用;诱导机体产生Ⅱ相解毒酶及抗氧化酶,增强对ROS的清除能力,减轻细胞氧化损伤,维持细胞内氧化还原平衡状态。当细胞被亲电子物质或氧化剂等攻击处于氧化应激状态时,Nrf2与Keap1解偶联后跨膜转运入核,激活Nrf2/Keap1/ARE信号通路。Nrf2/Keap1/ARE信号通路是目前机体最主要的内源性抗氧化信号通路,在机体抗氧化损伤中发挥至关重要的作用[11]。本研究免疫荧光观察发现,空白组Nrf2主要在细胞质内表达,细胞质内荧光反应明显增强;当细胞受到H2O2刺激时,机体产生氧化应激反应,细胞质内Nrf2向细胞核内转移,细胞核内Nrf2荧光反应增强,用海藻多糖对细胞进行预刺激后再进行H2O2诱导,细胞质内Nrf2较正常细胞内明显增多。与单用H2O2刺激组相比,海藻多糖+H2O2组、海藻多糖组、H2O2+海藻多糖组各项抗氧化指标CGLC、HO-1、NQO1、Nrf2 mRNA相对表达量均明显增高,海藻多糖组高于海藻多糖+H2O2组和H2O2+海藻多糖组;海藻多糖+H2O2组、海藻多糖组、H2O2+海藻多糖组Keap1 mRNA表达量下降,H2O2+海藻多糖组和海藻多糖+H2O2组高于海藻多糖组,提示海藻多糖抗氧化作用机制可能与Nrf2/Keap1/ARE信号通路激活有关。
综上所述,海藻多糖可抑制人胚肺成纤维细胞MRC-5的氧化应激损伤,上调 Nrf2表达、激活Nrf2/Keap1/ARE信号通路可能是其作用机制。
[1] Katzanstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification[J]. Am J Respir Crit Care Med, 1998,157(4):1301-1315.
[2] 余晶,鲍中英,徐玉敏,等.花青素抗氧化损伤及细胞凋亡的作用研究[J]中西医结合肝病杂志,2009,19(1):24-26,31.
[3] 胡婷婷.海藻多糖的生物活性研究进展[J].科技视窗,2012(36):17.
[4] Liu D, Sheng J, Li Z, et al. Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching[J]. Int J Biol Macromol, 2013(56):1-5.
[5] 秦华.海藻多糖对博来霉素诱导的大鼠肺间质纤维化模型的干预作用研究[J].齐鲁药事,2008,27(9):554-557.
[6] Ananthi S, Raghavendran HR, Sunil AG, et al. In vitroantioxidant and in vivo anti-inflammatory potential of crudepolysaccharide from Turbinaria ornate(Marine Brown Alga)[J]. Food Chem Toxicol, 2010,48(1):187-192.
[7] 冯珍鸽,王力,吴永沛,等.褐藻中岩藻聚糖的化学成分及其对超氧离子的抑制作用[J].食品研究与开发,2010,31(3):66-68.
[8] Wang J, Zhang QB, Zhang ZS, et al. Antioxidant activity of sulfated polysacchari defractions extracted from Laminaria japonica[J]. Int J Biol Macromol, 2008,42(2):127-132.
[9] Deshmukh P, Unni S, Krishnappa G, et al. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases[J]. Biophys Rev, 2017,9(1):41-56.
[10] 李研,丛建波,田晓华,等.海藻多糖抑制白细胞呼吸暴发作用研究[J].生物化学与生物理进展,1996,26(2):162-164.
[11] Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention[J]. Mutat Res, 2005,591(1-2):93-102.
Antioxidation of seaweed polysaccharides on H2O2-induced oxidative damage of human embryonic lung fibroblasts MRC-5
LIUHuaman1,LIUXue,JIAXinhua,ZHANGXinyue,ZHANGWei
(1ShandongUniversityofTraditionalChineseMedicine,Jinan250014,China)
国家自然科学基金资助项目(81273704);“泰山学者”建设工程(ts20110819)。
刘骅漫(1986-),女,主治医师,研究方向为呼吸系统疾病的中西医结合诊断及治疗。E-mail: liuhuaman@126.com
张伟(1963-),男,主任医师,研究方向为呼吸系统疾病的中西医结合诊断及治疗。E-mail: huxizhijia@126.com
10.3969/j.issn.1002-266X.2017.32.005
R56
A
1002-266X(2017)32-0017-04
2017-04-14)

