细胞源γ干扰素的表达与宫颈癌患者预后的关系研究
王新月,马 冬,张 琪,常凌雅,张丽杰,闫锡钊,王 洋,陈丽荣,陈允恩,李 鸥
王新月1,马 冬1,张 琪1,常凌雅1,张丽杰1,闫锡钊1,王 洋1,陈丽荣2,陈允恩1,李 鸥3*
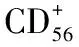
宫颈肿瘤;CD56;干扰素γ;预后
本研究背景:


1 资料与方法
1.1 临床资料 选取2008年2月—2011年11月在唐山市工人医院活检或妇产科手术切除的宫颈炎、宫颈癌前病变及宫颈癌标本共计121例,其中慢性宫颈炎患者25例(对照组)、宫颈上皮内瘤变(CIN)Ⅰ级患者11例(CINⅠ组)、CINⅡ/Ⅲ级患者45例(CINⅡ/Ⅲ组),宫颈鳞癌(SCC)患者40例(SCC组)。40例SCC患者中Ⅰ期17例,ⅡA期11例,ⅡB期12例。所有受试者术前未行化疗、放疗或生物治疗。研究样本的收集获得患者知情同意。
1.2 随访 SCC组患者自病理确诊之日起开始随访,治疗后2年内每3个月门诊复查1次,3~5年内每6个月门诊复查1次,随访截至2015年12月,共计随访5年,平均随访时间33个月。若未就诊于本院门诊复查者,进行电话随访,主要记录症状、复查情况、复发情况、生存时间(月)及随访时状态(存活、死亡或其他)等,患者因死亡或其他原因失联,则停止对其随访。
1.3 主要仪器与试剂 ABI 7500荧光定量聚合酶链式反应(PCR)仪(美国ABI公司),自动脱水机、石蜡切片机、石蜡包埋机、烤片机(上海艾测电子科技有限公司),正置显微镜(德国FLUKO公司);单克隆抗人IFN-γ抗体(1∶50 dilution;R&D Systems,Minneapolis,MN,USA),cDNA反转录试剂盒(Applied Biosystems,Foster City,CA,美国),12+2高危型HPV联合16/18基因分型检测试剂盒(广东凯普生物科技股份有限公司)。
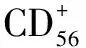
1.5 HPV检测 避开月经期,24 h不上药、不冲洗、无性生活。充分暴露宫颈外口,棉签轻拭表面分泌物,将细胞刷尖端插入宫颈口,轻轻加压并顺时针旋转5圈[7],细胞刷去除刷柄后放入专用的细胞保存液中,送病理科。HPV亚型检出项包括:HPV16、18型和其他12种高危型(即HPV31、33、35、39、45、51、52、56、58、59、66、68)。
1.6 实时荧光反转录PCR(qRT-PCR)法测定不同病理宫颈组织中IFN-γ mRNA的表达 Trizol试剂提取不同病理宫颈组织中的总RNA(Invitrogen公司),根据其在波长260/280 nm处的吸光度值,对核酸进行定量,采用cDNA反转录试剂盒将RNA反转录成cDNA。最后进行实时定量PCR分析。所需引物序列设计来自Primer 5.0,引物合成由宝生物工程(大连)有限公司合成。IFN-γ:上游引物:5′-TATAGGTGGGTATAATGGGTTT-3′,下游引物:5′-ATCAAAACAATATACTACACCTCCT-3′;GAPDH:上游引物:5′-GGAAGGTGAAGGTCGGAGTC-3′,下游引物:5′-CGTTCTCAGCCTTGACGGT-3′。使用SYBR绿色®PCR Master Mix(应用生物系统)7500实时PCR系统评价IFN-γ mRNA表达。GAPDH作为内参基因,以公式2-ΔΔCt(Ct为循环阈值)计算IFN-γ mRNA表达水平。
2 结果

注:CIN=宫颈上皮内瘤变,SCC=宫颈鳞癌

2.2 HPV阳性与IFN-γ mRNA表达水平关系 4组HPV16阳性率比较,差异有统计学意义(P<0.05);4组HPV18、其他12种高危型阳性率比较,差异均无统计学意义(P>0.05,见表2)。对照组HPV16阳性者IFN-γ mRNA表达低于HPV16阴性者,差异有统计学意义(P<0.05,见表3);CINⅠ组HPV16阳性与阴性者IFN-γ mRNA表达水平比较,差异无统计学意义(P>0.05,见表4);CINⅡ/Ⅲ和SCC组HPV16阳性者IFN-γ mRNA表达高于HPV16阴性者,差异均有统计学意义(P<0.05,见表5、6,图2)。

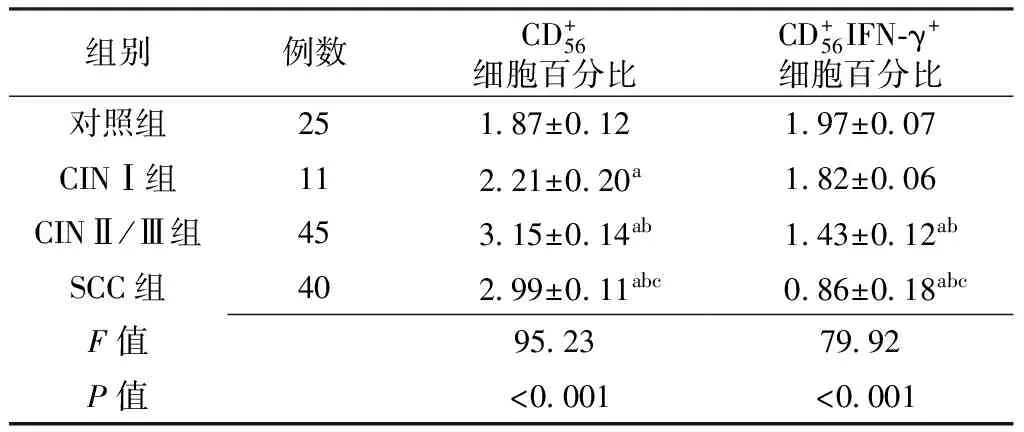
组别例数CD+56细胞百分比CD+56IFN⁃γ+细胞百分比对照组251 87±0 121 97±0 07CINⅠ组112 21±0 20a1 82±0 06CINⅡ/Ⅲ组453 15±0 14ab1 43±0 12abSCC组402 99±0 11abc0 86±0 18abcF值95 2379 92P值<0 001<0 001
注:CIN=宫颈上皮内瘤变,SCC=宫颈鳞癌;与对照组比较,aP<0.05;与CINⅠ组比较,bP<0.05;与CINⅡ/Ⅲ组比较,cP<0.05
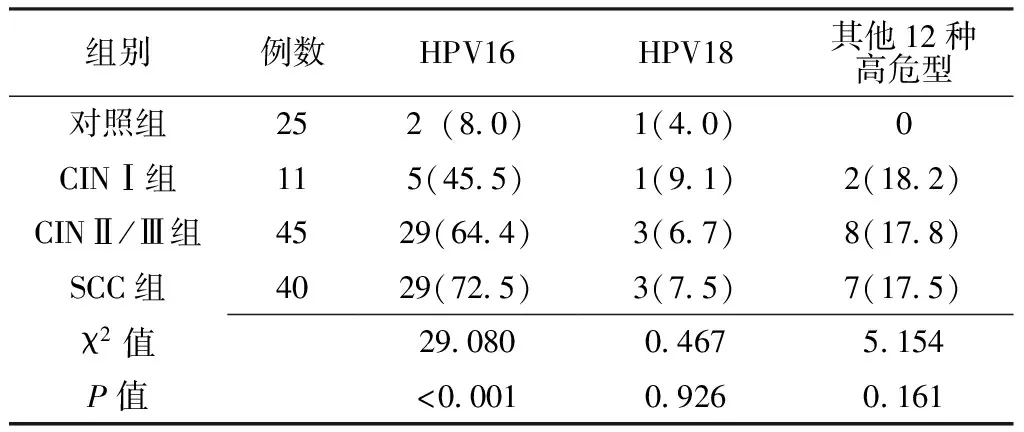
表2 4组HPV阳性率比较〔n(%)〕
注:HPV=人乳头瘤病毒

Table3 Comparison of expression levels of IFN-γ mRNA in patients with and without HPV16 infection in the control group

HPV16例数IFN⁃γmRNA阳性21 96±0 13阴性232 34±0 21t值2 491P值0 020
注:IFN-γ=γ干扰素

Table4 Comparison of expression levels of IFN-γ mRNA in patients with and without HPV16 infection in the CINⅠ group
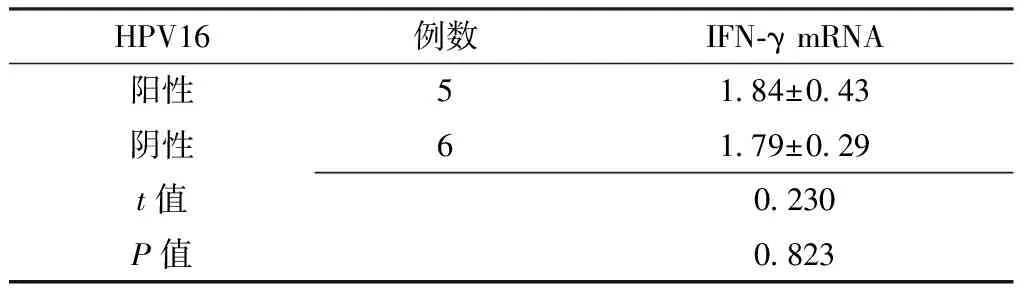
HPV16例数IFN⁃γmRNA阳性51 84±0 43阴性61 79±0 29t值0 230P值0 823

Table5 Comparison of expression levels of IFN-γ mRNA in patients with and without HPV16 infection in the CINⅡ/Ⅲ group
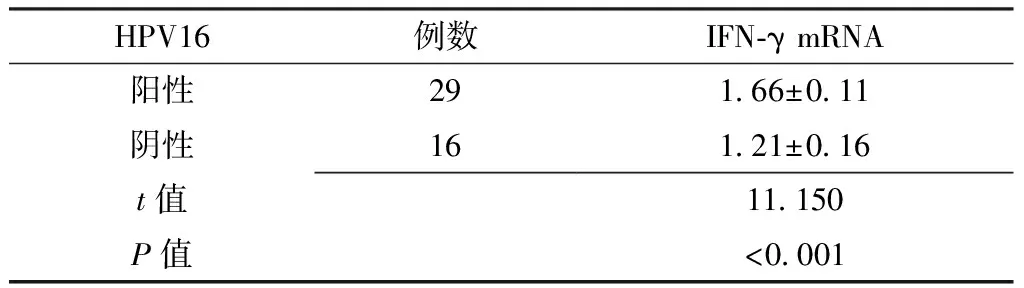
HPV16例数IFN⁃γmRNA阳性291 66±0 11阴性161 21±0 16t值11 150P值<0 001

Table6 Comparison of expression levels of IFN-γ mRNA in patients with and without HPV16 infection in the SCC group

HPV16例数IFN⁃γmRNA阳性290 89±0 12阴性110 56±0 09t值8 256P值<0 001
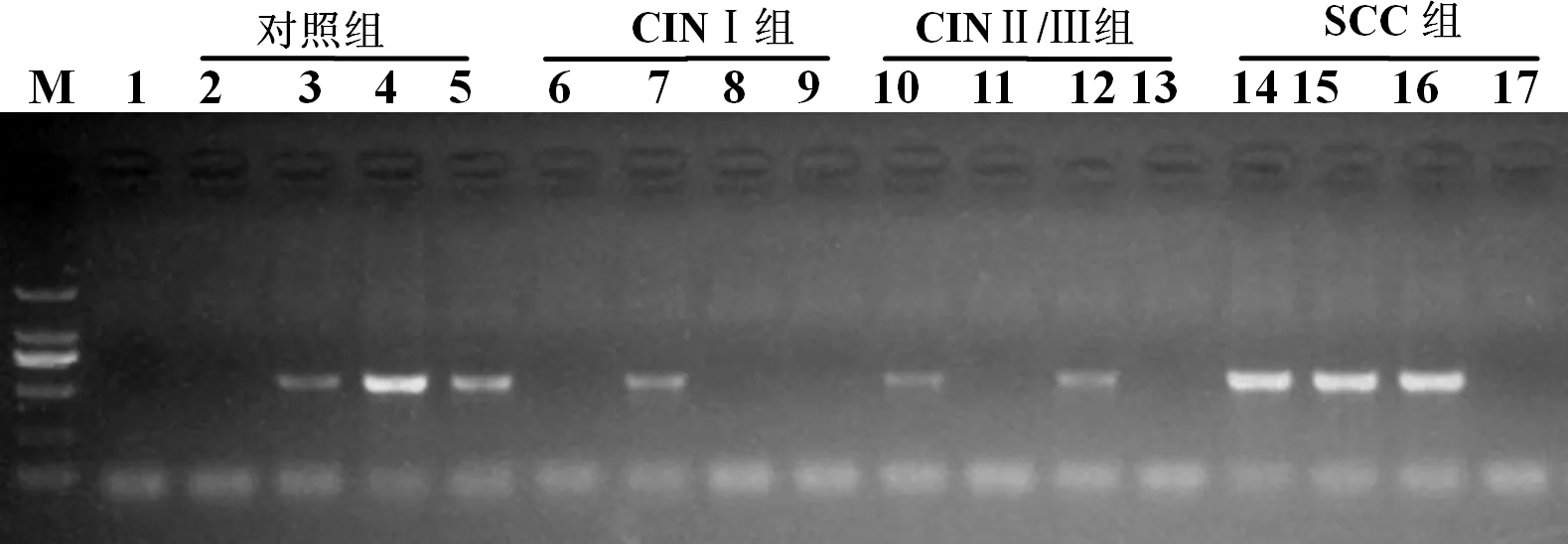
图2 不同病理宫颈组织中IFN-γ mRNA表达
Figure2 Expression levels of IFN-γ mRNA in different cervical pathological tissues


基本特征例数高表达低表达χ2值P值年龄(岁)0 4520 501 ≤50278(29 6)19(70 4) >50136/137/13初次性生活年龄(岁)-0 350 ≤1611/10 >163913(33 3)26(66 7)流产次数(次)-1 000 ≤23813(34 2)25(65 8) >221/21/2HPV16阳性1 5010 221 是298(27 6)21(72 4) 否116/115/11贫血0 0020 965 是73/74/7 否3311(33 3)22(66 7)组织学分级3 1990 074 高分化75/72/7 中低分化339(27 3)24(72 7)肿瘤大小(cm)0 0630 802 ≤43513(37 1)22(62 9) >451/54/5宫旁浸润3 8150 051 是121/1211/12 否2813(46 4)15(53 6)淋巴结转移4 4260 035 有909/9 无3114(45 2)17(54 8)FIGO分期(期)7 3760 007 Ⅰ1710/177/17 Ⅱ234(17 4)19(82 6)
注:-采用Fisher确切概率法

图3 SCC组患者宫颈组织中表达与预后生存率的关系
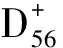
3 讨论



作者贡献:王新月进行文章的构思与设计、统计学处理、结果的分析与解释及撰写论文;张琪、常凌雅、张丽杰、闫锡钊、王洋、陈丽荣、陈允恩进行数据收集和整理;马冬、李鸥进行论文的修订、文章的质量控制及审校和对文章整体负责,监督管理。
本文无利益冲突。
[1] MOSCICKI A B,SCHIFFMAN M,BURCHELL A,et al.Updating the natural history of human papillomavirus and anogenital cancers[J].Vaccine,2012,30(Suppl 5):F24-33.DOI:10.1016/j.vaccine.2012.05.089.
[2] 姬清华,李国胜,张汝一,等.大肠癌患者外周血T细胞亚群和NK细胞活性的检测[J].山东医药,2010,50(4):94-96.DOI:10.3969/j.issn.1002-266X.2010.04.048. JI Q H,LI G S,ZHANG R Y,et al.Detection of T lymphocyte subsets and NK cell activity in peripheral blood of patients with colorectal carcinoma[J].Shandong Medical Journal,2010,50(4):94-96.DOI:10.3969/j.issn.1002-266X.2010.04.048.
[3] 姬清华,李国胜,张汝一,等.大肠癌患者T细胞亚群和NK细胞活性检测的意义[J].贵阳医学院学报,2010,35(1):47-49.DOI:10.3969/j.issn.1000-2707.2010.01.013. JI Q H,LI G S,ZHANG R Y,et al.Determination of T cell subgroups and NK cells in peripheral blood of colon cancer patients and its clinical significance[J].Journal of Guiyang Medical College,2010,35(1):47-49.DOI:10.3969/j.issn.1000-2707.2010.01.013.
[4] 张彩,田志刚.NK细胞受体群谱偏移与肿瘤免疫逃逸及逆转[J].中国免疫学杂志,2016,32(5):609-614.DOI:10.3969/j.issn.1000-484X.2016.05.001. ZHANG C,TIAN Z G.Imbalance of NK cell receptors and tumor immune escape as well as reversal strategies[J].Chinese Journal of Immunology,2016,32(5):609-614.DOI:10.3969/j.issn.1000-484X.2016.05.001.
[5] QUILLAY H,EL COSTA H,DURIEZ M,et al.NK cells control HIV-1 infection of macrophages through soluble factors and cellular contacts in the human decidua[J].Retrovirology,2016,13(1):39.DOI:10.1186/s12977-016-0271-z.
[6] ISHIGAMI S,NATSUGOE S,TOKUDA K,et al.Prognostic value of intratumoral natural killer cells in gastric carcinoma[J].Cancer,2000,88(3):577-583.
[7] 薛国勇,张居芬.TCT检测在宫颈病变中的应用[J].中国妇幼保健,2009,24(32):4639. XUE G Y,ZHANG J F.Application of TCT detection in cervical lesions[J].Maternal and Child Health Care of China,2009,24(32):4639.
[8] 吴意,陆学东,刘键,等.膜杂交多重检测技术在HPV基因分型中的应用[J].中国皮肤性病学杂志,2006,20(4):245-246.DOI:10.3969/j.issn.1001-7089.2006.04.029. WU Y,LU X D,LIU J,et al.Human papillomavirus genotyping by multiplex menbrane hybridization technique[J].The Chinese Journal of Dermatovenereology,2006,20(4):245-246.DOI:10.3969/j.issn.1001-7089.2006.04.029.
[9] 李广太.HPV检测在子宫颈癌筛查中的意义[J].中华妇产科杂志,2015,50(4):241-245.DOI:10.3760/cma.j.issn.0529-567x.2015.04.001. LI G T.Significance of HPV detection in cervical cancer screening[J].Chinese Journal of Obstetrics and Gynecology,2015,50(4):241-245.DOI:10.3760/cma.j.issn.0529-567x.2015.04.001.
[10] 徐明堂,何春年,许长田,等.宫颈鳞状上皮病变中人乳头状瘤病毒16/18存在状态的研究[J].中华病理学杂志,2013,42(6):400-401.DOI:10.3760/cma.j.issn.0529-5807.2013.06.011. XU M T,HE C N,XU C T,et al.Study on the status of HPV16/18 in cervical squamous epithelium lesions[J].Chinese Journal of Pathology,2013,42(6):400-401.DOI:10.3760/cma.j.issn.0529-5807.2013.06.011.
[11] MILLER C H,MAHER S G,YOUNG H A.Clinical use of interferon-gamma[J].Ann NY Acad Sci,2009,1182(1):69-79.DOI:10.1111/j.1749-6632.2009.05069.x.
[12] RENOUX V M,BISIG B,LANGERS I,et al.Human papillomavirus entry into NK cells requires CD16expression and triggers cytotoxic activity and cytokine secretion[J].Eur J Immunol,2011,41(11):3240-3252.DOI:10.1002/eji.201141693.
[13] 李曼,姚嫣,高月求.慢性乙型肝炎病毒感染者外周血自然杀伤细胞免疫效应分子的表达[J].中华肝脏病杂志,2010,18(2):96-100.DOI:10.3760/cma.j.issn.1007-3418.2010.02.005. LI M,YAO Y,GAO Y Q.Expression profile of immune effector molecules in natural killer cells in patients with chronic hepatitis B[J].Chinese Journal of Hepatology,2010,18(2):96-100.DOI:10.3760/cma.j.issn.1007-3418.2010.02.005.
[14] TREGONING J S,WANG B L,MCDONALD J U,et al.Neonatal antibody responses are attenuated by interferon-γ produced by NK and T cells during RSV infection[J].Proc Natl Acad Sci U S A,2013,110(14):5576-5581.DOI:10.1073/pnas.1214247110.
[15] LANIER L L.NK cell receptors[J].Annu Rev Immunol,1998,16(1):359-393.DOI:10.1146/annurev.immunol.16.1.359.
[16] LONG E O.Regulation of immune responses through inhibitory receptors[J].Annu Rev Immunol,1999,17(1):875-904.DOI:10.1146/annurev.immunol.17.1.875.
[17] UNANUE E R.Inter-relationship among macrophages,natural killer cells and neutrophils in early stages of Listeria resistance[J].Curr Opin Immunol,1997,9(1):35-43.
[18] COLMENARES V,NOYOLA D E,MONSIVIS-URENDA A,et al.Human papillomavirus immunization is associated with increased expression of different innate immune regulatory receptors[J].Clin Vaccine Immunol,2012,19(7):1005-1011.DOI:10.1128/CVI.00043-12.
[19] LEE S J,CHO Y S,CHO M C,et al.Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells[J].J Immunol,2001,16(1):497-504.
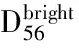
[21] RABINOVICH G A,GABRILOVICH D,SOTOMAYOR E M.Immunosuppressive strategies that are mediated by tumor cells[J].Annu Rev Immunol,2007,25:267-296.DOI:10.1146/annurev.immunol.25.022106.141609.
[22] BALSAMO M,SCORDAMAGLIA F,PIETRA G,et al.Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity[J].Proc Natl Acad Sci U S A,2009,106(49):20847-20852.DOI:10.1073/pnas.0906481106.
[23] PIETRA G,MANZINI C,RIVARA S,et al.Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity[J].Cancer Res,2012,72(6):1407-1415.DOI:10.1158/0008-5472.CAN-11-2544.
[24] MCGILVRAY R W,EAGLE R A,WATSON N F S,et al.NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting[J].Clin Cancer Res,2009,15(22):6993-7002.DOI:10.1158/1078-0432.CCR-09-0991.
[25] CARLSTEN M,NORELL H,BRYCESON Y T,et al.Primary human tumor cells expressing CD155impair tumor targeting by down-regulating DNAM-1 on NK cells[J].J Immunol,2009,183(8):4921-4930.DOI:10.4049/jimmunol.0901226.
[26] FAURIAT C,LONG E O,LJUNGGREN H G,et al.Regulation of human NK-cell cytokine and chemokine production by target cell recognition[J].Blood,2010,115(11):2167-2176.DOI:10.1182/blood-2009-08-238469.
[27] COOPER M A,FEHNIGER T A,CALIGIURI M A.The biology of human natural killer-cell subsets[J].Trends Immunol,2001,22(11):633-640.
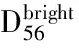
[29] 陈香丽,王连才,刘苏虎,等.干扰素-γ在肿瘤免疫编辑过程中的作用[J].国际肿瘤学杂志,2007,34(12):890-893.DOI:10.3760/cma.j.issn.1673-422X.2007.12.004. CHEN X L,WANG L C,LIU S H,et al.Effects of interferon-γ on tumor immunoeditting[J].Journal of International Oncology,2007,34(12):890-893.DOI:10.3760/cma.j.issn.1673-422X.2007.12.004.
[30] 蒋永林.子宫颈癌患者手术前后血清IFN-γ,D-D,TK1和sFas测定的临床价值[J].放射免疫学杂志,2013,26(5):565-568.DOI:10.3969/j.issn.1008-9810.2013.05.009. JIANG Y L.Clinical value on determination of serum IFN-γ,D-D,TK1 and sFas levels in postoperative patients with uterine cervix malignancies[J].Journal of Radioimmunology,2013,26(5):565-568.DOI:10.3969/j.issn.1008-9810.2013.05.009.
[31] 胡必成,姜祥兵,马威,等.细胞因子诱导的杀伤细胞对宫颈癌细胞的体内外杀伤效应[J].中国肿瘤生物治疗杂志,2010,17(3):327-332.DOI:10.3872/j.issn.1007-385X.2010.03.016. HU B C,JIANG X B,MA W,et al.Anti-tumor activity of cytokine-induced killer cells against cervical cancer cells in vivo and in vitro[J].Chinese Journal of Cancer Biotherapy,2010,17(3):327-332.DOI:10.3872/j.issn.1007-385X.2010.03.016.
[32] 吴伟.培美曲塞联合顺铂对肺腺癌炎性因子与免疫功能的影响[J].深圳中西医结合杂志,2016,26(14):18-20.DOI:10.16458/j.cnki.1007-0893.2016.14.009. WU W.Effect of pemetrexed combined with cisplatin on inflammatory factors and immune function in lung adenocarcinoma[J].Shenzhen Journal of Integrated Traditional Chinese and Western Medicine,2016,26(14):18-20.DOI:10.16458/j.cnki.1007-0893.2016.14.009.
(本文编辑:陈素芳)

WANG Xin-yue1,MA Dong1,ZHANG Qi1,CHANG Ling-ya1,ZHANG Li-jie1,YAN Xi-zhao1,WANG Yang1,CHEN Li-rong2,CHEN Yun-en1,LI Ou3*
1.North China University of Science and Technology,Tangshan 063000,China 2.Qianxi Hospital of Traditional Chinese Medicine,Qianxi 063000,China 3.Tangshan Workers′ Hospital,Tangshan 063000,China
*Corresponding author:LI Ou,Professor;E-mail:tsliou@163.com
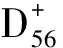
Uterine cervical neoplasms;CD56;Interferon-gamma;Prognosis
国家自然科学基金资助项目(81541149);河北省自然科学基金资助项目(H2016209046)
R 737.33
A
10.3969/j.issn.1007-9572.2017.00.007
2017-01-24;
2017-06-15)
1.063000河北省唐山市,华北理工大学
2.063000河北省唐山市迁西县中医院
3.063000河北省唐山市,唐山工人医院
*通信作者:李鸥,教授;E-mail:tsliou@163.com

