一类六核三价铁簇化合物的合成及结构表征
田菊梅崔正张景萍
一类六核三价铁簇化合物的合成及结构表征
田菊梅*,1崔正2张景萍2
(1厦门医学院口腔医学系,厦门361023)
(2东北师范大学化学学院,长春130024)
选择3种不同尺寸含氮配体(哌嗪、咪唑和三氮唑)与三羟甲基丙烷(H3tmp)和FeCl3采用溶剂热反应合成3例六核Fe合物:(C5H14N2)[Fe6(μ6-O)Cl6(tmp)4]·2H2O·CH3OH(1)、(C3H5N2)2[Fe6(μ6-O)Cl6(tmp)4](2)和(C4H8N3)3(C2H4N3)[Fe12(μ6-O)2Cl12(tmp)8]·3CH3OH (3),并对它们的结构进行表征。发现三元醇配体有利于合成高核金属簇。3个化合物具有相同的阴离子簇[Fe6(μ6-O)Cl6(tmp)4]2-。通过晶体学参数,元素分析,红外等手段证实,在化合物1和3的体系中,氮杂环配体经历了N-和C-烷基化反应。
六核铁簇;晶体结构;抗衡离子
0 Introduction
The polynuclear transition metal clusters have atracted numerous attention since they simultaneously displaypleasingarchitecturalaestheticsand fascinating magnetic properties[1-6].The investigation of such magnetic materials is helpful in understanding the magnetio-structural correlations and provides a fertile platform for the research of the attractive magnetic characteristics,such as ferromagnetic ordering[7], antiferromagnetic ordering[8],ferrimagnetic ordering[9-11], spin-canting[12-13],metamagnetictransition[14],spinglass[15-17],spin-flop[18-20],and spin-frustrated[21-23],etc[24-26]. Although there is great success made in the practice, the design and synthesis of such materials still remain an elusive goal and a major challenge to systema-tically study the structure-property relationship[27].To obtain a magnetic polynuclear cluster,incorporation of paramagnetic transition-metal ions with appropriate multidentate chelating ligands is a general strategy.In this respect,we chose Feion,with S=5/2,as the paramagnetic center due to the exchange anisotropy and the large spin ground state achieved[28-30].As for the main organic ligand,we selected tripodal alcohol ligand,i.e.,1,1,1-tris(hydroxymethyl)propane(abbreviated H3tmp),which is a very attractive candidate for the construction of multinuclear clusters,due to its flexibility and diverse coordination modes as well as superexchangecapacityreflectedininteresting magnetic properties[31].It is well known that nitrogencontaining heterocyclic groups generally act as a role of bridging ligands,due to their strong σ-electron donors to metals[32-33].Furthermore,they can also perform counter cations because they are weak base and prone to protonate.Therefore,we have concentrated our effortsonthecombinationofnitrogen-containing heterocyclic ligands,which act as assistant role,with Fe-H3tmp system to explore the structures and investigate the resultant magnetic properties.
In this paper,we have successfully assembled and investigated three hexanuclear compounds,(C5N2H14) [Fe6(μ6-O)Cl6(tmp)4]·2H2O·CH3OH(1),(C3N2H5)2[Fe6(μ6-O)Cl6(tmp)4](2),and(C4N3H8)3(C2N3H4)[Fe12(μ6-O)2Cl12(tmp)8]·3CH3OH(3).These three compounds possess different protonated nitrogen-containing heterocyclic counter cations,despite the same[Fe6(μ6-O)Cl6(tmp)4]2-core.Within the system of compounds 1 and 3,the nitrogen-containing heterocyclic ligands experience N-and C-alkylation reactions,which is validated by the crystallographic parameters,elemental analyses,and IR.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of reagent grade and used as commercially purchased without further purification.
Infrared spectra(400~4 000 cm-1)were measured on a Perkin-Elmer Fourier transform infrared(FTIR) spectrophotometer using KBr pellets(Fig.S1,Supporting information).The elemental analyses(C,H and N) wereperformedonaPerkin-ElmerModel240C elemental analyzer.The Fe and Cl elemental contents were determined by the Leaman inductively coupled plasma(ICP)spectrometer.Thermogravimetric analyses (TGA)wereperformedonaPerkin-ElmerTG-7 analyzer in flowing nitrogen atmosphere from room temperature to 800℃with a heating rate of 10℃· min-1.Powder X-ray diffraction(PXRD)measurements were assessed on a Siemens D5005 diffractometer with CuKα(λ=0.154 184 nm)in 2θ range of 5°~50°at room temperature.The XRD accelerating voltage and emission current were 40 kV and 30 mA,respectively.
1.2 Syntheses of complexes 1~3
1.2.1 Synthesis of(C5N2H14)[Fe6(μ6-O)Cl6(tmp)4]·2H2O ·CH3OH(1)
A mixture of FeCl3(0.049 g,0.3 mmol),H3tmp (0.027 g,0.2 mmol),piperazine(0.026 g,0.3 mmol), and methanol(15 mL)was placed in a 20 mL Teflonlined autoclave and heated under autogenous pressure at 120℃for 72 h.Then,the autoclave was cooled to ambient temperature at 3℃·h-1.Block-shaped red crystals of 1 were collected in~80%yield(based on Fe).Anal.Calcd.for C30H66Cl6Fe6N2O16(%):C,28.62; H,5.25;N,2.23;Fe,26.64;Cl,16.91.Found(%):C, 28.98;H,5.14;N,2.33;Fe,26.51;Cl,17.11.IR (KBr,cm-1):3 489(m),2 966(m),2 914(m),2 868(m), 1 623(w),1 472(m),1 453(m),1 398(w),1 356(w), 1 304(w),1 198(w),1 111(s),1 034(s),941(s),771 (w),593(m),527(s),473(m),437(w).
1.2.2 Synthesis of(C3N2H5)2[Fe6(μ6-O)Cl6(tmp)4](2)
A mixture containing FeCl3(0.081 g,0.5 mmol), H3tmp(0.027 g,0.2 mmol),imidazole(0.014 g,0.2 mmol),and methanol(15 mL)was heated in a Teflonlined autoclave at 120℃for 72 h and then allowed to cool to room temperature at the rate of 3℃·h-1. Needle-like red crystals of 2 were obtained in~85% yield(based on Fe).Anal.Calcd.for C30H54Cl6Fe6N4O13(%):C,29.36;H,4.41;N,4.57;Fe,27.34;Cl,17.35. Found(%):C,29.05;H,4.66;N,4.39;Fe,27.84;Cl, 17.64.IR(KBr,cm-1):3 148(m),2 972(m),2 915(m), 2 868(m),1 585(m),1 472(m),1 455(w),1 399(w),1 303(w),1 199(w),1 110(s),1 033(s),990(w), 941(s),821(w),794(w),771(w),755(w),628(m), 591(m),528(s),474(m),438(w).
1.2.3 Synthesis of(C4N3H8)3(C2N3H4)[Fe12(μ6-O)2Cl12(tmp)8]·3CH3OH(3)
A mixture of FeCl3(0.081 g,0.5 mmol),H3tmp (0.041 g,0.3 mmol),triazole(0.138 g,2 mmol),and methanol(15 mL)was heated in Teflon-lined autoclave at 140℃for 72 h.After the autoclave was cooled to room temperature at 3℃·h-1,red block-shaped crystals of 3 were afforded(Yield:75%based on Fe).Anal. Calcd.for C65H128Cl12Fe12N12O29(%):C,29.59;H,4.856; N,6.37;Fe,25.43;Cl,16.14.Found(%):C,28.31;H, 4.921;N,6.12;Fe,25.49;Cl,16.78.IR(KBr,cm-1): 3 464(w),3 075(m),2 964(m),2 911(m),2 866(m), 1 628(w),1 584(m),1 470(m),1 452(m),1 398(m), 1 302(w),1 198(w),1 165(w),1 112(s),1 038(s), 942(s),771(w),733(w),659(w),625(m),592(m), 526(s),473(m).
1.3 Details of X-ray crystallography
Single-crystal X-ray diffraction data for complexes 1~3 were measured on a Bruker SMART APEX CCD area detector diffractometer using graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at room temperature.Structure solution(direct methods)and the refinement of full-matrix least-squares were carried out using the SHELXTL software package[34].All the non-hydrogen atoms were refined with anisotropic thermal parameters.The hydrogen atoms attached to carbon and nitrogen atoms were placed in geometrically calculated positions.For complex 1,the hydrogen atoms on the non-coordinated MeOH molecule,that is O(14),and on water molecules,that are O(1W)and O(2W),were not located.The atoms C(30),O(14), O(1W)and O(2W)were found disordered over two sites.For complex 2,the atom C(1)was highly disordered over two sites.For complex 3,the hydrogen atom on one non-coordinated MeOH molecule,that is O(14), was not located.The atoms C(13)and C(14)were highly disordered over two sites.The protonated degrees of all the counter cations are according to the charge balance.Crystallographic and refinement details for all compounds are summarized in Table 1.Selected bond lengths and angles are listed in Table S1~S3.
CCDC:980726,1;980727,2;980728,3.
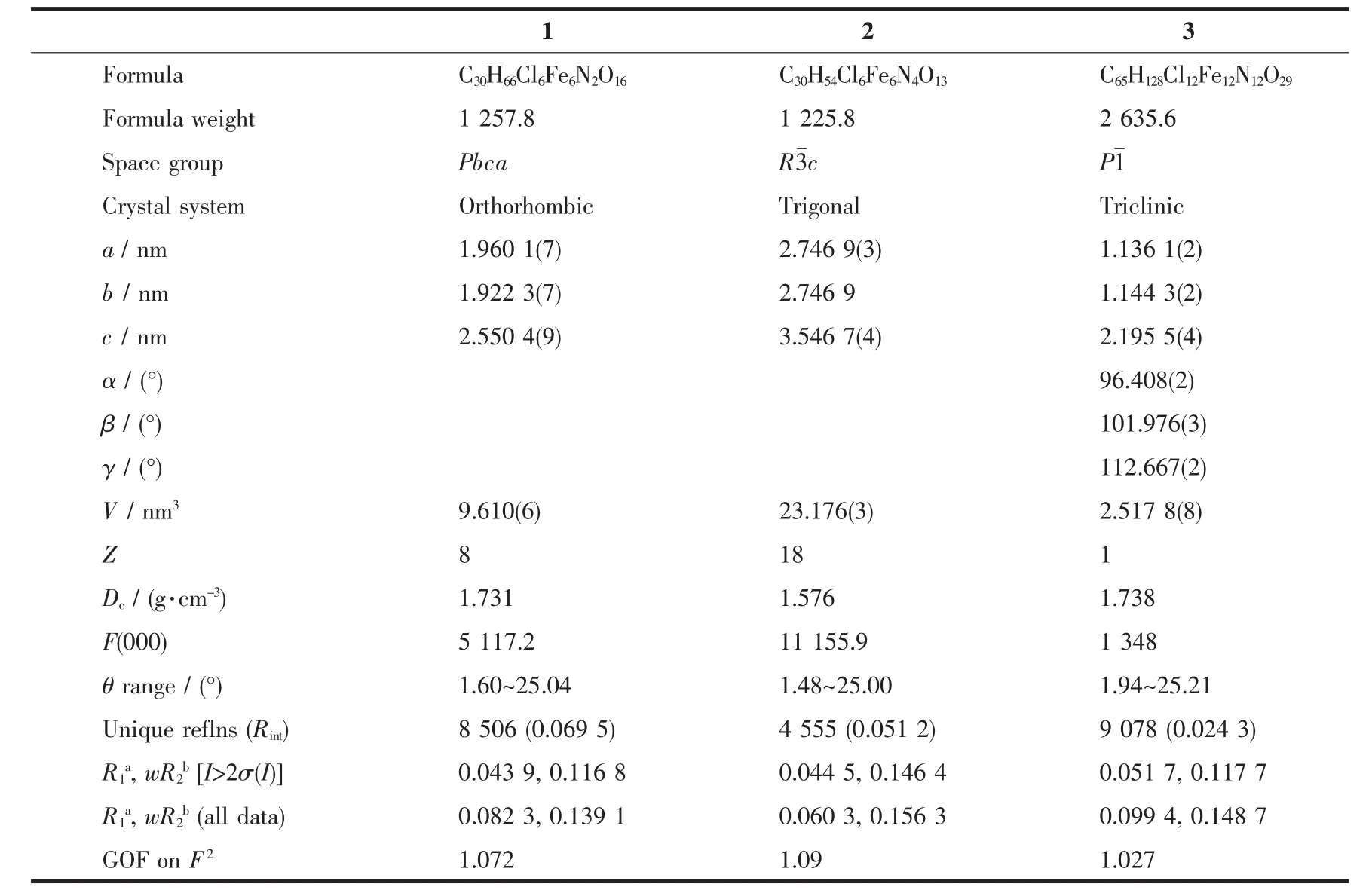
Table1 Crystallographic data and structure refinement for complexes 1~3
2 Results and discussion
2.1 Synthesis
Complex 1 was prepared by the solvothermal reaction of FeCl3,H3tmp,and piperazine in a 3∶2∶3 molar ratio in MeOH at 120℃for 72 h.Interestingly, the piperazine ligand in such system experienced N-alkylation reaction to form methyl substituent piperazine(abbreviated as L1).Complex 3 was synthesized by the solvothermal reaction of FeCl3,H3tmp,and triazole in a 5∶3∶20 molar ratio in MeOH at 140℃for 72 h.Part of triazole in such system went through C-and N-alkylation reactions to form dimethyl substituent triazole(abbreviated as L2).The reasons for such N/C-alkylation reactions in the systems 1 and 3 are unclear due to the complexity under the solvothermal condition.Complex 2 was obtained by the reaction of FeCl3with H3tmp and imidazole in a 5∶2∶2 molar ratio in MeOH at 120℃for 72 h.
2.2 X-ray crystal structures
Single-crystal X-ray diffraction results of 1~3 reveal that each compound possesses same anionic structure unit composed of[Fe6(μ6-O)Cl6(tmp)4]2-core (Fig.1),which exhibits marked similarities to previously reported compounds contained 1,1,1-tris(hydroxymethyl)ethane(H3thme)[35-36].In the anionic core,the ligand H3tmp possesses the level of deprotonation tmp3-and adopts the η2∶η2∶η2∶μ3coordinated mode[31].The central μ6-Oapproximatelylocatesinthecenterofan octahedron formed by six Featoms.The Fe ions are all in+3 oxidation state,as determined from a combination of charge balance considerations,inspection of bond lengths,and BVS calculations[37-39].Each Fe atom exhibits distorted octahedral geometry,which coordinates to four oxygen atoms from two different tmp3-ligands,one μ6-O atom,and terminal chloride atom.It is worth notice that the charge balance is provided by the protonated nitrogen-containing heterocyclic cations. These counter cations provide not only the charge balance but also the hydrogen bond interactions to glue the counterpart of the structure.More structural detailsinvolvingthepackingpatternsandthe intracluster/interclusterdistancesarediscussedas follows.The ellipsoid for the complexes 1~3 are shown in Fig.2~4,respectively.
Compound 1 crystallizes in the orthorhombic system Pbca.The asymmetric unit consists of one [Fe6(μ6-O)Cl6(tmp)4]2-unit,one doubly-protonated L1ligand,one non-coordinated methanol molecule,and two non-coordinated water molecules.The bond distances of Fe-O(0.197 2~0.227 7 nm)and Fe-Cl(0.226 6~0.229 4 nm)are similar to those reported[Fe6]clustercontaining H3thme ligand[35-36].The intracluster Fe…Fe distances are observed in the range of 0.315 4~0.322 3 nm(Fig.S2a).
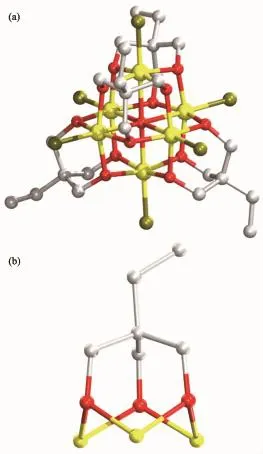
Fig.1 (a)Anion unit[Fe6(μ6-O)Cl6(tmp)4]2-of complex 1; (b)Bridging mode of the tmp3-ligand
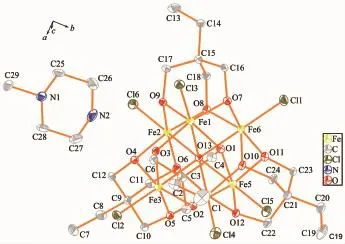
Fig.2 Ellipsoid for the complex 1 with probability of 30%
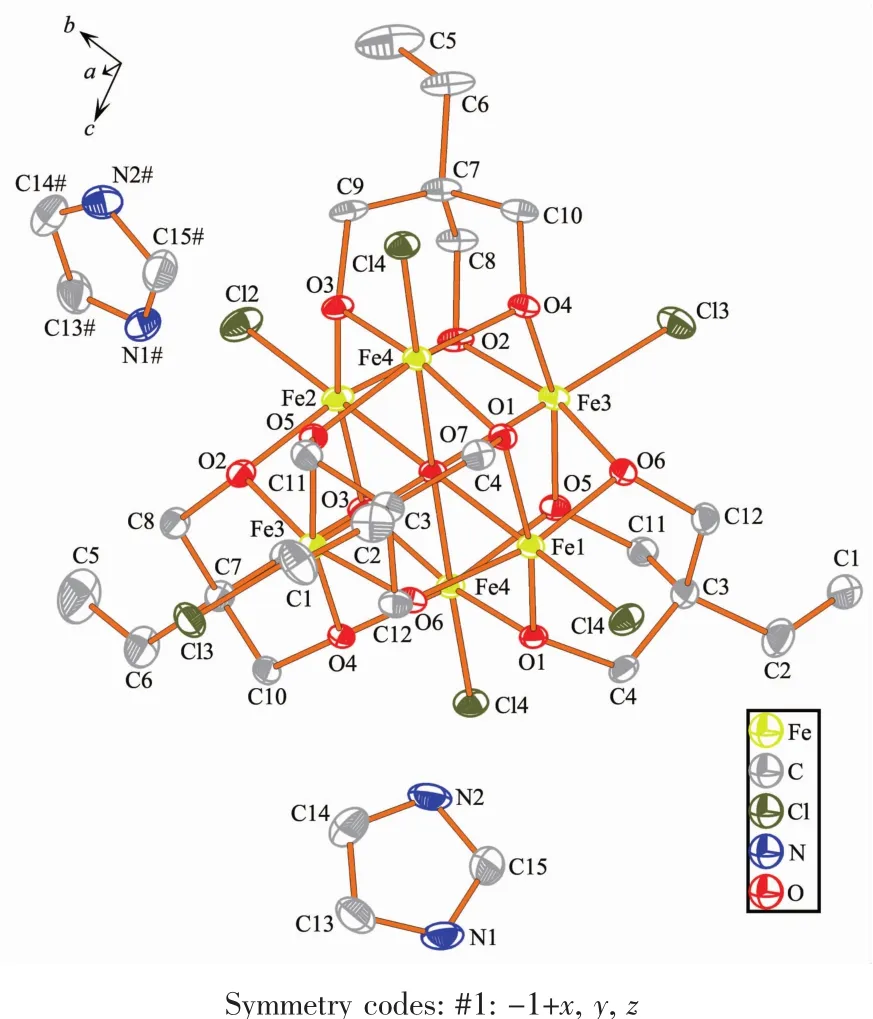
Fig.3 Ellipsoid for the complex 2 with probability of 30%
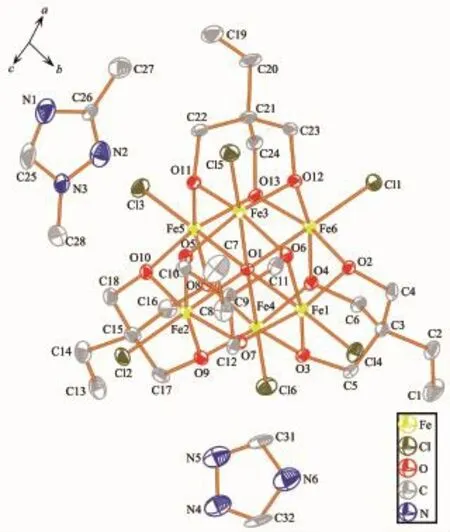
Fig.4 Ellipsoid for the complex 3 with probability of 30%
Compound 2 belongs to the trigonal system R3c. The asymmetric unit consists of half of[Fe6(μ6-O)Cl6(tmp)4]2-unit,and one mono-protonated imidazole cation. The Fe-O and Fe-Cl bond distance is in the range of 0.198 2~0.230 1 nm and 0.224 7~0.228 7 nm,respectively.The intracluster Fe…Fe distances fall in the range of 0.318 5~0.321 9 nm(Fig.S2b).The[Fe6]units within the lattice of 2 connect to each other into a hydrophobic channel along c axis.
Compound 3 crystallizes in triclinic system P1. The asymmetric unit consists of one[Fe6(μ6-O)Cl6(tmp)4]2-unit,one and half of mono-protonated L2ligands,half of mono-protonated triazole ligand,one and half of non-coordinated methanol molecules.The Fe-O and Fe-Cl bond distance is in the range of 0.198 0~0.228 2 nm and 0.227 1~0.230 6 nm,respectively.The intracluster Fe…Fe distances are within the range of 0.316 9~0.321 7 nm(Fig.S2c).
2.3 Thermal stability and PXRD analysis of 1~3
Thethermogravimetricanalyses(TGA)were performed to investigate the thermal stabilities of compounds 1~3(Fig.S3).The TGA trace of compound 1 exhibits a weight loss of 5.13%from room temperature to 200℃corresponding to the release of lattice water and methanol molecules(Calcd.5.41%).Then two continuous weight loss steps are attributed to the loss of the counter-cation and the anion[Fe6]core.The remaining 37.84%above 735℃corresponds to the final product of Fe2O3(Calcd.38.09%).The TGA profile of 2 does not lose any weight below 110℃indicating there are no solvent molecules in the lattice.Then the weight loss is attributed to the gradualdecompositionofthecounter-cationand ligands.Finally,a plateau region is observed from 443 to 800℃.The final residue of 38.95%corresponds to the formation of Fe2O3(Calcd.39.08%).Compound 3 displays the first weight loss of approximately 3.76% from room temperature to 144℃corresponding to the release of three methanol molecules(Calcd.3.64%). And then,the release of the counter-cation and the decomposition of organic ligands occur through several continuous weight loss steps after 200℃.The final residuals were not characterized,which may be owing to the corrosive reactions of the final residuals with the TGA baskets made of Al2O3[40].Viewing from the trend of the TGA curve,it is known that the decom-posing process is not achieved at the operating temperature limit(800℃)of our instrument.
The phase purity is confirmed by powder X-ray diffraction(PXRD)at room temperature.The experimental patterns of 1~3 are in fairly good agreement with the simulated ones generated from single-crystal diffraction data(Fig.S4~S6).
3 Conclusions
In summary,the use of different counter ions has led to the synthesis of a family of hexanuclear ironclusters 1~3.They have same anionic[Fe6(μ6-O)Cl6(tmp)4]2-core structures and different counter cations. The improved magnetic properties are in progress.
Supporting information is available at http://www.wjhxxb.cn
[1]Milway V A,Tuna F,Farrell A R,et al.Angew.Chem.Int. Ed.,2013,52:1949-1952
[2]Mannini M,Pineider F,Danieli C,et al.Nature,2010,468: 417-421
[3]Engelhardt L P,Muryn C A,Pritchard R G,et al.Angew. Chem.Int.Ed.,2008,47:924-927
[4]Xiang S C,Hu S M,Sheng T L,et al.J.Am.Chem.Soc., 2007,129:15144-15146
[5]Han S D,Song W C,Zhao J P,et al.Chem.Commun.,2013, 49:871-873
[6]ZHAO Su-Qin(赵素琴),GU Jin-Zhong(顾金忠).Chinese J. Inorg.Chem.(无机化学学报),2016,32(9):1611-1618
[7]Seki H,Hosaka Y,Saito T,et al.Angew.Chem.Int.Ed., 2016,55:1360-1363
[8]Fondo M,Doejo J,Garcia-Deibe A M,et al.CrystEngComm, 2016,18:6673-6682
[9]Zhang Y J,Liu T,Kanegawa S,et al.J.Am.Chem.Soc.,2009, 131:7942-7943
[10]Ohba M,Kaneko W,Kitagawa S,et al.J.Am.Chem.Soc., 2008,130:4475-4484
[11]Numata Y H,Inoue K,Baranov N,et al.J.Am.Chem.Soc., 2007,129:9902-9909
[12]Zheng Y Z,Tong M L,Xue W,et al.Angew.Chem.,2007, 119:6188-6192
[13]Tian J M,Li W L,Li B,et al.Chem.Eur.J.,2013,19:5097-5103
[14]Wöhlert S,Boeckmann J,Wriedt M,et al.Angew.Chem. Int.Ed.,2011,50:6920-6923
[15]Ouellette W,Prosvirin A V,Whitenack K,et al.Angew. Chem.Int.Ed.,2009,48:2140-2143
[16]Mahata P,Natarajan S,Panissod P,et al.J.Am.Chem. Soc.,2009,131:10140-10150
[17]Ting L X,Yi W X,Xin Z W,et al.Adv.Mater.,2006,18: 2852-2856
[18]Folven E,Scholl A,Young A,et al.Nano Lett.,2012,12: 2386-2390
[19]Pękała M,Wolff-Fabris F,Fagnard J F,et al.J.Magn.Magn. Mater.,2013,335:46-52
[20]Zhao J P,Zhao R,Yang Q,et al.Dalton Trans.,2012,41: 4852-4858
[21]Yoshida H,Yamaura J-i,Isobe M,et al.Nat.Commun., 2012,3:860-864
[22]Kenneth R P,Masaki A.Nat.Chem.,2011,3:758-759
[23]Aidoudi F H,Aldous D W,Goff R J,et al.Nat.Chem.,2011, 3:801-806
[24]Lennartson A,Bond A D,Piligkos S,et al.Angew.Chem. Int.Ed.,2012,51:11049-11052
[25]Hoshino N,Iijima F,Newton G N,et al.Nat.Chem.,2012, 4:921-926
[26]Miller J S.Chem.Soc.Rev.,2011,40:3266-3296
[27]Bi Y F,Wang X T,Liao W P,et al.J.Am.Chem.Soc., 2009,131:11650-11651
[28]Sanudo E C,Font-Bardia M,Solans X,et al.New J.Chem., 2011,35:842-848
[29]Kershaw Cook L J,Kulmaczewski R,Mohammed R,et al. Angew.Chem.Int.Ed.,2016,55:4327-4331
[30]Li Z Y,Ohtsu H,Kojima T,et al.Angew.Chem.Int.Ed., 2016,55:5184-5189
[31]Brechin E K.Chem.Commun.,2005:5141-5153
[32]Yang F,Li B,Xu W,et al.Inorg.Chem.,2012,51:6813-6820
[33]WU Xiao-Shuo(吴小说),WANG Peng-Fei(汪鹏飞),LU Peng -Peng(路朋朋),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(9):1667-1675
[34]Sheldrick G M.SHELXS-97 and SHELXL-97,University of Göttingen,Germany,1997.
[35]Batchelor L J,Shaw R,Markey S J,et al.Chem.Eur.J., 2010,16:5554-5557
[36]Jiang G Q,Bai J F,Xing H,et al.Cryst.Growth Des.,2006, 6:1264-1266
[37]Dobson K D,McQuillan A J.Spectrochim.Acta Part A,1999, 55:1395-1405
[38]Sun Z,Gantzel P K,Hendrickson D N.Inorg.Chem.,1996, 35:6640-6641
[39]Liu W,Thorp H H.Inorg.Chem.,1993,32:4102-4105
[40]Song J L,Yi F Y,Mao J G.Cryst.Growth Des.,2009,9: 3273-3277
Syntheses and Structures Characterization of a Family of Hexanuclear IronClusters
TIAN Ju-Mei*,1CUI Zheng2ZHANG Jing-Ping2
(1Department of Stomatology,Xiamen Medical College,Xiamen,Fujian 361023,China)
(2Faculty of Chemistry,Northeast Normal University,Changchun 130024,China)
Solvothermal reactions of 1,1,1-tris(hydroxymethyl)propane(H3tmp)and FeCl3by using different counter ions resulted in three hexanuclear ironclusters(C5N2H14)[Fe6(μ6-O)Cl6(tmp)4]·2H2O·CH3OH(1),(C3N2H5)2[Fe6(μ6-O)Cl6(tmp)4](2),and(C4N3H8)3(C2N3H4)[Fe12(μ6-O)2Cl12(tmp)8]·3CH3OH(3),whose structures were characterized. The tripodal alcohols ligands are very useful in constructing high-nuclearity metal clusters.In all cases,similar anionic cluster[Fe6(μ6-O)Cl6(tmp)4]2-is formed by six Feions,four tmp3-ligands,one center O2-ion,and six Clions.Within the system of compounds 1 and 3,the nitrogen-containing heterocyclic ligands experience N-and C-alkylation reactions,which is validated by the crystallographic parameters,elemental analyses,and IR.CCDC: 980726,1;980727,2;980728,3.
hexanuclear ironclusters;crystal structure;counter ions
O614.81+1
A
1001-4861(2017)09-1625-06
10.11862/CJIC.2017.187
2017-03-04。收修改稿日期:2017-07-08。
厦门医学高等专科学校自然科学研究项目(No.K2015-13)和福建省卫生计生青年科研课题(No.2017-2-115)资助。*
。E-mail:tjm@xmmc.edu.cn

