脑活素治疗Alzheimer’s病的Meta分析
李艳,贺电,李娅,楚兰,刘芳,方旭明,田田
·论著·
脑活素治疗Alzheimer’s病的Meta分析
李艳,贺电,李娅,楚兰,刘芳,方旭明,田田
目的 评价脑活素治疗Alzheimer’s病(AD)的绝对有效性和安全性。方法 检索CENTRAL、MEDLINE、EMBASE、PsycINFO、CBMdisc(检索截至2015年9月30日)关于脑活素单药治疗AD痴呆或AD源性轻度认知障碍的随机、安慰剂平行对照临床试验。采用Cochrane协作网的偏倚风险评价工具评价原始研究方法学质量,GRADEpro 3.2软件评价原始研究的局限性、结果的不一致性和不精确性、证据的间接性、发表偏倚对主体证据质量的影响。结果 共纳入6项研究,共950例AD痴呆患者。亚组分析结果显示脑活素30 ml可改善轻中度AD痴呆患者随访1月时或随访6个月时认知功能[标准均数差(SMD)=-0.360,95%CI:-0.600 ~ -0.130,P=0.003;SMD=-0.350,95%CI:-0.590~-0.100,P=0.005)]。随访6个月时精神行为症状改善(SMD=-0.290,95%CI:-0.540~-0.050,P=0.020)。脑活素不能改善随访1个月时或随访6个月时日常能力(SMD=-0.160,95%CI:-0.350~0.040,P=0.120;SMD=-0.210,95%CI:-0.450~0.030,P=0.080)。随访1个月和6个月时脑活素组和安慰剂组不良事件的发生率无差异,无严重不良事件发生。所有研究均存在方法学局限性,主要缺陷为测量偏倚风险不清楚,且制药企业资助或参与研究,潜在偏倚风险较高。绝大多数结局指标的总样本量不足,并且部分结果的95%CI过宽,导致证据质量下降。结论 低/极低级别证据表明,脑活素对轻中度AD痴呆患者的认知功能和精神行为症状有轻微的改善作用,但不能改善患者的日常能力。
Alzheimer’s病;脑活素;随机对照试验;Meta分析
Alzheimer’s病(AD)是痴呆最常见的病因,目前全球范围内约有20×106例患者,预计到2050年,全球将有1.35×108例患者[1]。AD的病理生理学过程始于临床诊断为痴呆前多年,疾病过程可以分为无症状的临床前期[2]、有症状的痴呆前期[轻度认知障碍(MCI)期][3]和临床痴呆期3个阶段[4]。目前尚无有效治疗方法。随着对AD分子生物学研究的深入,治疗方向逐渐从单一的认知功能改善转为疾病修正治疗,目的在于防止或减少β-淀粉样蛋白(Aβ)和tau蛋白形成、集聚或沉积,减轻神经炎症程度,减缓神经变性过程,阻止认知功能恶化。脑活素是一种多肽混合物的水溶液,具有多种生物学活性,如神经营养[5]、抗Aβ[6]和抗tau蛋白[7]。迄今已有多项临床试验评价脑活素疗效,但样本量较小且结果不尽一致。Wei 等[8]的Meta分析显示,脑活素可以显著改善轻中度AD患者总体印象评分。Gauthier等[9]的Meta分析显示,脑活素可以改善轻中度AD患者总体印象评分和认知功能。然而,上述两项研究未充分考虑各研究间的异质性和潜在偏倚,且未全面分析和评价证据质量,有必要再次对这些临床试验进行系统评价,以探讨脑活素治疗AD的绝对有效性和安全性。
1 对象与方法
1.1 对象 (1)AD痴呆患者的诊断符合国际疾病分类法-10(ICD-10)[10]、美国精神障碍诊断与统计手册第3版修订版(DSM-Ⅲ-R)和第4版(DSM-Ⅳ)[11-12]、美国国立神经病学、语言障碍和卒中研究所-AD及相关疾病协会(NINCDS-ADRDA)[4,13]制定的很可能痴呆标准。(2)AD源性MCI患者的诊断符合Petersen等[14]、Winblad等[15]和NINCDS-ADRDA制定的标准[3],同时须有证据支持系AD所致,且有认知功能随年龄增长逐渐下降的证据;排除脑血管病、颅脑创伤或其它疾病所致的认知功能下降,排除其它神经变性疾病。
1.2 方法
1.2.1 文献检索 以“cerebrolysin”“cerebroprotein hydrolysate”“Alzheimer” “dementia”“mild cognitive impairment”为英文检索词;以“脑活素”“脑蛋白水解物” “阿尔茨海默病” “老年性痴呆”“轻度认知障碍”为中文检索词。计算机检索CENTRAL、MEDLINE、EMBASE、PsycINFO、CBMdisc(检索截至日期:2015年9月30日)等国内外数据库关于脑活素单药治疗AD痴呆或MCI的随机、安慰剂平行对照临床试验,并查阅相关综述和研究的参考文献以补充可能遗漏的相关临床试验。
1.2.2 文献纳入及排除标准 选择脑活素单药治疗AD痴呆或AD源性MCI的随机、安慰剂平行对照试验。试验组为脑活素静脉滴注,给药频率和治疗时间无限制。对照组为安慰剂静脉滴注,给药频率和治疗时间与试验组一致。主要结局包括:(1)认知功能,采用AD评估量表-认知分量表(ADAS-Cog)[16-17]、MMSE[18]、连线测验(TMT)[19]。(2)临床总体印象:采用临床医师整体印象变化量表(CGI)[20]、 临床医师访谈时对病情变化的印象补充量表(CIBIC+)[21]。(3)基线时为AD源性MCI的患者,随访中进展为AD痴呆的病例数。(4)不良事件的病例数、发生严重不良事件的病例数。次要结局包括:(1)日常能力:采用日常生活活动能力量表(ADL)[22]、工具性日常生活活动能力量表(IADL)[23]、痴呆残疾评估(DAD)[24]、纽伦堡活动问卷(NAI)[25]。(2)精神行为症状:采用神经精神症状问卷(NPI)[26]、AD病理行为评定量表(BEHAVE-AD)[27]、AD评估量表-非认知分量表(ADAS-nonCog)[16]。
1.2.3 文献质量评价 根据Cochrane 系统评价手册5.1.0版[28],由两位评价员采用Cochrane协作网的偏倚风险评价工具对各项研究的方法学质量独立进行评价,包括随机分配方法、分配方案隐藏方法、受试者和研究人员设盲情况,结局测量者的设盲情况、结局数据的完整性、选择性报告研究结果和其他偏倚情况。如果一项研究退出率>20%或干预组组间退出原因不平衡,则该项研究判定为具有较高的随访偏倚。采用GRADEpro软件[29]进一步评价各项临床试验设计和实施过程中的局限性和偏倚风险、研究结果的不一致性(研究结果间无法解释的异质性)和不精确性(总样本量过小或95%CI过宽)、研究证据的间接性,以及发表偏倚对证据质量的影响。
1.2.4 资料提取 由两位评价员独立筛选研究、提取资料,包括研究基本信息、研究方法和可能存在的偏倚、研究对象特征、干预措施、结局指标、研究结果、资助机构以及潜在利益冲突等。
1.2.5 统计学方法 采用RevMan 5.3.3[30]进行数据合成,选择脑活素30 ml组与安慰剂对照组进行Meta分析,并分析随访短期(≤1个月)、中期(1~6个月)、长期(>6个月)的结局数据。若异质性检验显示存在中度或中度以上统计学异质性(I2>30.000%),根据痴呆严重程度、同一结局所测量的量表差异、偏倚风险程度进行亚组分析,以分析异质性来源。根据一种假设(可能情况)进行敏感性分析,以评价退出病例对主要结局的影响。
2 结 果
2.1 文献检索和研究质量评价 初筛共获得相关文献95篇,经阅读文题和摘要筛选出10篇文献,进一步阅读原文,剔除1项开放性临床试验[31],最终纳入9篇文献[32-40]共6项随机对照临床试验,共950例轻中度AD痴呆患者。特征见表1;研究质量和偏倚风险评价结果见表2。
2.2 效应量的合并
2.2.1 认知功能 有5项研究[32-36]报道了随访1个月时的认知功能,其中3项研究[33-35]间无明显的异质性(P=0.300,I2=16.000%),采用随机效应模型进行合并,结果显示,脑活素治疗轻中度AD痴呆患者,可改善随访1个月时认知功能[标准均数差(SMD)=-0.360,95%CI:-0.600~-0.130,P=0.003)]。有3项研究[35-37]报道随访6个月时的认知功能,其中2项[35,37]研究间无异质性(P=0.690,I2=0.000%),用随机效应模型进行合并显示,脑活素治疗可改善轻中度AD痴呆患者随访6个月时认知功能(SMD=-0.350,95%CI:-0.590~-0.100,P=0.005)。
2.2.2 临床总体印象 有5项研究[32-36]报道随访1个月时临床总体印象;3项研究[35-37]报道随访6个月时临床总体印象,各研究间均存在异质性,故未进行数据合并。
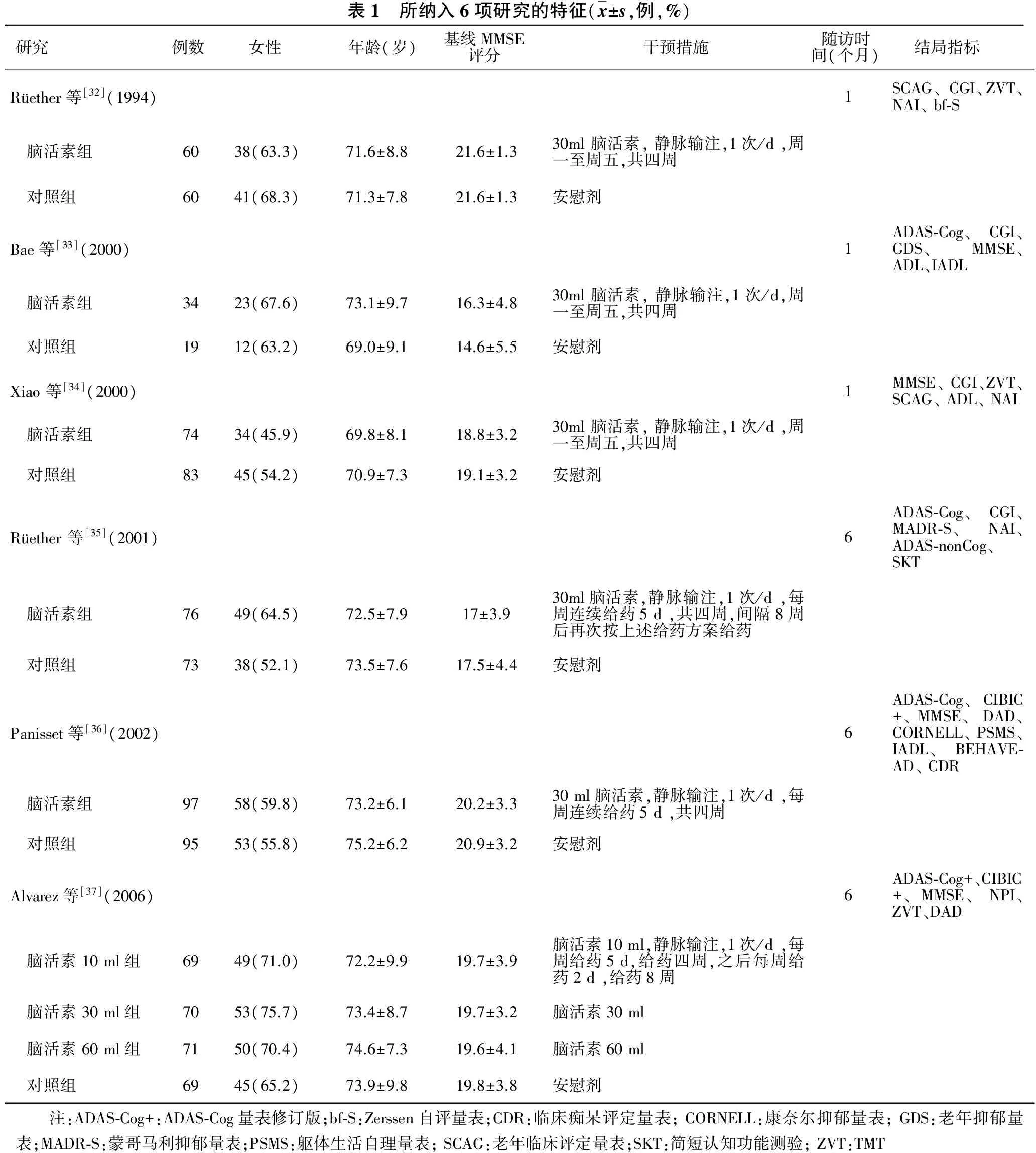
表1 所纳入6项研究的特征(x±s,例,%)研究 例数 女性 年龄(岁) 基线MMSE 评分 干预措施 随访时 间(个月)结局指标Rüether等[32](1994)1SCAG、CGI、ZVT、NAI、bf⁃S 脑活素组6038(63.3)71.6±8.821.6±1.330ml脑活素,静脉输注,1次/d,周一至周五,共四周 对照组6041(68.3)71.3±7.821.6±1.3安慰剂Bae等[33](2000)1ADAS⁃Cog、CGI、GDS、MMSE、ADL、IADL 脑活素组3423(67.6)73.1±9.716.3±4.830ml脑活素,静脉输注,1次/d,周一至周五,共四周 对照组1912(63.2)69.0±9.114.6±5.5安慰剂Xiao等[34](2000)1MMSE、CGI、ZVT、SCAG、ADL、NAI 脑活素组7434(45.9)69.8±8.118.8±3.230ml脑活素,静脉输注,1次/d,周一至周五,共四周 对照组8345(54.2)70.9±7.319.1±3.2安慰剂Rüether等[35](2001)6ADAS⁃Cog、CGI、MADR⁃S、NAI、ADAS⁃nonCog、SKT 脑活素组7649(64.5)72.5±7.917±3.930ml脑活素,静脉输注,1次/d,每周连续给药5d,共四周,间隔8周后再次按上述给药方案给药 对照组7338(52.1)73.5±7.617.5±4.4安慰剂Panisset等[36](2002)6ADAS⁃Cog、CIBIC+、MMSE、DAD、CORNELL、PSMS、IADL、BEHAVE⁃AD、CDR 脑活素组9758(59.8)73.2±6.120.2±3.330ml脑活素,静脉输注,1次/d,每周连续给药5d,共四周 对照组9553(55.8)75.2±6.220.9±3.2安慰剂Alvarez等[37](2006)6ADAS⁃Cog+、CIBIC+、MMSE、NPI、ZVT、DAD 脑活素10ml组6949(71.0)72.2±9.919.7±3.9脑活素10ml,静脉输注,1次/d,每周给药5d,给药四周,之后每周给药2d,给药8周 脑活素30ml组7053(75.7)73.4±8.719.7±3.2脑活素30ml 脑活素60ml组7150(70.4)74.6±7.319.6±4.1脑活素60ml 对照组6945(65.2)73.9±9.819.8±3.8安慰剂 注:ADAS⁃Cog+:ADAS⁃Cog量表修订版;bf⁃S:Zerssen自评量表;CDR:临床痴呆评定量表;CORNELL:康奈尔抑郁量表;GDS:老年抑郁量表;MADR⁃S:蒙哥马利抑郁量表;PSMS:躯体生活自理量表;SCAG:老年临床评定量表;SKT:简短认知功能测验;ZVT:TMT

表2 所纳入6项研究的方法学质量与偏倚风险评价(例,%)研究随机分配方法(选择性偏倚)分配方案隐藏(选择性偏倚)受试者和研究人员的设盲(实施偏倚)结果测量者的设盲(测量偏倚)结果数据的完整性(随访偏倚)脑活素组安慰剂组选择性报告研究结果(报告偏倚)其他偏倚来源Rüether等[32](1994)不清楚偏倚风险低偏倚风险低不清楚00偏倚风险低偏倚风险高Bae等[33](2000)不清楚偏倚风险低偏倚风险低不清楚00偏倚风险低偏倚风险高Xiao等[34](2000)偏倚风险低偏倚风险低偏倚风险低不清楚2(2.7)0偏倚风险低偏倚风险高Ruether等[35](2001)偏倚风险低偏倚风险低偏倚风险低不清楚6(7.9)7(9.6)偏倚风险低偏倚风险高Panisset等[36](2002)偏倚风险高偏倚风险低偏倚风险低不清楚9(9.3)12(12.6)偏倚风险低偏倚风险高Alvarez等[37](2006)偏倚风险低不清楚偏倚风险低不清楚脑活素10ml:11(15.9)脑活素30ml:9(12.9)脑活素60ml:6(8.6)16(23.2)偏倚风险低偏倚风险高
2.2.3 精神行为症状 有3项研究[35-37]报道随访6个月时精神行为症状,其中2项[35,37]研究间无异质性(P=0.870,I2=0.000%),采用随机效应模型进行合并,结果显示,脑活素治疗轻中度AD痴呆患者,可改善随访6个月时精神行为症状(SMD=-0.290,95%CI:-0.540~-0.050,P=0.020)。
2.2.4 日常能力 有5项研究[32-36]报道随访1个月时日常能力,其中3项[34-36]研究间无明显异质性(P=0.300,I2=16.000%),采用随机效应模型进行合并,结果显示,脑活素治疗轻中度AD痴呆患者,不能改善随访1个月时日常能力(SMD=-0.160,95%CI:-0.350~0.040,P=0.120)。有3项研究[35-37]报道随访6个月日常能力,其中2项[35,37]研究间无异质性(P=0.580,I2=0.000%),采用随机效应模型进行合并显示,脑活素治疗轻中度AD痴呆患者,不能改善随访6个月时日常能力(SMD=-0.210,95%CI:-0.450~0.030,P=0.080)。
2.2.5 安全性 有3项[32-34]报道随访1个月时不良事件发生率,各研究间无异质性(P=0.710,I2=0.000%),采用随机效应模型进行合并,结果显示,与安慰剂比较,脑活素治疗轻中度AD痴呆不增加随访1个月时不良事件发生率(RR=2.940,95%CI:0.720~11.990;P=0.130)。3项研究[32-34]报道随访1个月时无一例发生严重不良事件。3项研究[35-37]报道随访6个月时不良事件和严重不良事件发生率,各项研究间无异质性(P=0.430,I2=0.000%;P=0.790,I2=0.000%),采用随机效应模型进行合并,结果显示,与安慰剂比较,脑活素治疗轻中度AD痴呆不增加随访6个月时不良事件和严重不良事件发生率(RR= 0.920,95%CI:0.790~1.070,P=0.280 ;RR= 1.090,95%CI:0.620~1.910,P=0.760)。
2.3 主要结局的证据质量 所有经Meta分析的结局证据等级因原始研究局限性或(和)结果的不精确性而降低,评为极或极低级别证据。由于本系统评价纳入的研究数目较少,未能进行发表偏倚评价。
3 讨 论
本系统评价共纳入6项脑活素单药治疗AD的随机、安慰剂平行对照临床试验,所有试验在方法学上均存在局限性,主要为测量偏倚风险不清楚,并且制药企业资助或参与了研究。因此,所有试验均判定为高偏倚风险的研究。其中1项研究使用计算机产生随机化序列,但因区组长度过大,导致两组患者在年龄和病程上不具有可比性,选择性偏倚风险较高。
本研究结果显示,静脉滴注脑活素30 ml治疗轻中度AD痴呆可改善患者短中期认知功能;此外,精神行为症状亦有所改善;但不能改善日常能力。脑活素30 ml治疗轻中度AD痴呆安全性较好。
然而,由于所纳入研究方法学的缺陷(主要为测量偏倚风险不清楚、制药企业资助或参与研究)或/和结果的不精确性(由于总样本量不足或/和结果95%CI过宽),上述结果的真实性受影响,证据质量降低。总的来说,使用量表评估的结局,包括认知功能、精神行为症状以及日常能力,受测量偏倚的影响较大。本系统评价所纳入试验的测量偏倚风险不清楚,并且制药企业资助或参与了所有研究,潜在偏倚风险较高,上述量表评估结局的证据质量下调2个等级,此外,除随访1个月日常能力外,其余结局总样本量均不足400例,并且部分结果的95%CI过宽,证据质量进一步下调1个等级,被评定为极低级别证据。虽不良事件结局的证据受测量偏倚影响小,但仍受潜在高偏倚风险(制药企业资助或参与研究)的影响,因此,证据质量下调1个等级。此外,不良事件结局的证据质量还因样本量不足或/和结果95%CI过宽进一步下调1个等级,被评定为低级别证据。在临床总体印象方面,由于各项研究间存在统计学异质性,未能进行Meta分析。
低/极低级别证据表明,脑活素对轻中度AD痴呆患者的认知功能和精神行为症状有轻微的改善作用,但不能改善患者的日常能力。
[1]Alzheimer ’s Association. 2014 Alzheimer’s disease facts and figures[J]. Alzheimers Dement, 2014, 10:e47.
[2]Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the national institute on aging- Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease[J]. Alzheimers Dement, 2011, 7:280.
[3]Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease[J]. Alzheimers Dement, 2011, 7:270.
[4]McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease[J]. Alzheimers Dement, 2011, 7:263.
[5]Ubhi K, Rockenstein E, Vazquez-Roque R, et al. Cerebrolysin modulates pronerve growth factor/nerve growth factor ratio and ameliorates the cholinergic deficit in a transgenic model of Alzheimer’s disease[J]. J Neurosci Res, 2013, 91:167.
[6]Rockenstein E, Torrance M, Mante M, et al. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease[J]. J Neurosci Res, 2006, 83:1252.
[7]Rockenstein E, Ubhi K, Trejo M, et al. CerebrolysinTMefficacy in a transgenic model of tauopathy: role in regulation of mitochondrial structure[J]. BMC Neurosci, 2014,15:90.
[8]Wei ZH, He QB, Wang H, et al. Meta-analysis: the efficacy of nootropic agent Cerebrolysin in the treatment of Alzheimer’s disease[J]. J Neural Transm (Vienna), 2007, 114:629.
[9]Gauthier S, Proao JV, Jia J, et al. Cerebrolysin in mild-to-moderate Alzheimer’s disease: a meta-analysis of randomized controlled clinical trials[J]. Dement Geriatr Cogn Disord, 2015, 39:332.
[10]World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical description and diagnostic guidelines[M]. Geneva: World Health Organization, Division of Mental Health, 1992.
[11]American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders[M]. Third Edition, Revised. American Psychiatric Association, 1989.
[12]American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders[M]. 4th Edition. American Psychiatric Association, 1994.
[13]McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease[J]. Neurology, 1984, 4:939.
[14]Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome[J]. Arch Neurol,1999,56:303.
[15]Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment[J]. J Intern Med, 2004,256:240.
[16]Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease[J]. Am J Psychiatry, 1984,141:1356.
[17]Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s disease assessment scale that broaden its scope. The Alzheimer’s disease cooperative study[J]. Alzheimer Dis Assoc Disord, 1997,11:s13.
[18]Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician[J]. J Psychiatr Res,1975,12:189.
[19]Oswald WD. Der Zahlen-Verbindungs-Test im hoheren lebensalter[M]. In: Aspekte Psychologischer Forschung. Hogrefe, Gottingen: 1986.
[20]Guy W. Clinical Global Impressions (CCI)[M]. In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health and Human Services, 1976.
[21]Knopman DS, Knapp MJ, Gracon SI, et al. The Clinician Interview-Based Impression (CIBI): a clinician’s global change rating scale in Alzheimer’s disease[J]. Neurology, 1994,44:2315.
[22]Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in aged. The index of ADL: a standardized measure of biological and psychosocial funtion[J]. JAMA, 1963, 185:914.
[23]Lawton MP, Brody EM. Assessment of older people: self-maintaining andinstrumental activities of daily living[J]. Gerontologist, 1969, 9:179.
[24]Gélinas I, Gauthier L, McIntyre M, et al. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia[J]. Am J Occup Ther, 1999,53:471.
[25]Oswald WD, Fleischmann UM. Nürnberger-Alters-Inventar NAI[M]. Testkasten und Manual, 3. Aufl. Universität Erlangen-Nürnberg, Nürnberg. Hogrefe (Testzentrale Stuttgart), Göttingen. 1992.
[26]Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia[J]. Neurology, 1994,44:2308.
[27]Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment[J]. J Clin Psychiatry,1987,48:9.
[28]Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011][DB/OL]. Available at: www.cochrane-handbook.org.
[29]Schünemann H, Brozek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009][DB/OL]. Available at: http://www.cc-ims.net/gradepro.
[30]Review Manager (RevMan). Version 5.3 [CP]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
[31]Muresanu DF, Rainer M, Moessler H. Improved global function and activities of daily living in patients with AD: a placebo-controlled clinical study with the neurotrophic agent Cerebrolysin[J]. J Neural Transm Suppl, 2002,62:277.
[32]Rüether E, Ritter R, Apecechea M, et al. Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the lzheimer type (SDAT) [J]. Pharmacopsychiatry,1994,27:32.
[33]Bae CY, Cho CY, Cho K, et al. A double-blind, placebo-controlled, multicenter study of erebrolysin for Alzheimer’s disease[J]. J Am Geriatr Soc,2000,48:1566.
[34]Xiao SF, Yan HQ, Yao PF, et al. Efficacy of FPF 1070 (Cerebrolysin) in atients with Alzheimer ’s disease: A multicentre, randomised, double-blind, lacebo-controlled trial[J]. Clin Drug Invest, 2000,9:43.
[35]Rüether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease[J]. Int Clin Psychopharmacol, 2001,16:253.
[36]Panisset M, Gauthier S, Moessler H, et al. Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent[J]. J Neural Transm (Vienna),2002,109:1089.
[37]Alvarez XA, Cacabelos R, Laredo M, et al. A 24-week, double-blind, placebo-controlled study of three dosages of cerebrolysin in patients with mild to moderate Alzheimer’s disease[J]. Eur J Neurol,2006, 13:43.
[38]Rüether E, Ritter R, Apecechea M, et al.Sustained improvements in patients with dementia of Alzheimer’s type (DAT) 6 months after termination of cerebrolysin therapy[J]. J Neural Transm (Vienna),2000,107:815.
[39]Rüether E, Alvarez XA, Rainer M, et al. Sustained improvement of cognition and global function in patients with moderately severe Alzheimer’s disease: a double-blind, placebo-controlled study with the neurotrophic agent cerebrolysin[J]. J Neural Transm Suppl, 2002,62:265.
[40]Alvarez XA, Cacabelos R, Sampedro C, et al. Efficacy and safety of cerebrolysin in moderate to moderately severe Alzheimer’s disease: results of a randomized, double-blind, controlled trial investigating three dosages of cerebrolysin[J]. Eur J Neurol, 2011,18:59.
Meta-analysis of cerebrolysin for Alzheimer’s disease
LIYan,HEDian,LIYa,etal.
DepartmentofNeurology,AffiliatedHospitalofGuizhouMedicalUniversity,Guiyang550004,China
Objective To assess the absolute efficacy and safety of cerebrolysin for patients with Alzheimer’s disease(AD). Methods CENTRAL, MEDLINE、EMBASE, PsycINFO, CBMDisc(up to September 30,2015) were searched for the randomized, placebo-controlled, parallel-group clinical trials evaluating cerebrolysin as monotherapy for patients with AD dementia or mild cognitive impairment due to AD. The methodological quality of the original studies were evaluated by using the Cochrane collaboration’s tool. The influences of study limitations, inconsistency of results, imprecision of effect estimates, indirectness of evidence and publication bias on the quality of the body of evidence were assessed by using GRADEpro software (version 3.2).Results Six studies were included, involving 950 patients with AD dementia. Subgroup analyses indicated cerebrolysin at a dose of 30 ml improved cognitive function in patients with mild-to-moderate AD dementia at one month or six months of follow-up [standard mean difference (SMD)=-0.360,95%CI:-0.600--0.130,P=0.003;SMD =-0.350,95%CI:-0.590--0.100,P=0.005)]. There was improvement in behavioural and psychiatric symptoms at six months of follow-up(SMD=-0.290,95%CI:-0.540--0.050,P=0.020).Cerebrolysin did not improve activities of daily living at one month or six months of follow-up (SMD=-0.160,95%CI:-0.350-0.040,P=0.120;SMD=-0.210,95%CI:-0.450-0.030,P=0.080). The incidence of adverse events (AEs) at one month or six month of follow-up was not different between the cerebrolysin group and the placebo group. No serious AEs occurred in both groups. All studies had methodological limitations, mainly on an unclear risk of detection bias. The pharmaceutical company funded or participated in the clinical trials, therefore,the potential risk of bias was high.Furthermore, the total sample size for most outcomes was insufficient, and the 95%CIof most results was wide. All these factors contributed to a decreased quality level of the evidence.Conclusions There is low/very low-quality evidence to show that cerebrolysin has slight effects in improving cognitive function and behavioural and psychiatric symptoms in patients with mild-to-moderate AD dementia. It has no effects in improving activities of daily living.
Alzheimer’s disease; cerebrolysin; randomized controlled trial; meta-analysis
贵州省科技计划项目(黔科合重大专项字[2014]6008号)
550004 贵阳,贵州医科大学附属医院神经内科
贺电
R749.1
A
1004-1648(2017)03-0179-06
2016-03-29
2016-05-19)

