基于高内涵筛选技术研究生首乌和制首乌醇提物的肝毒性机制
李丹丹,汤响林,龙隆,许龙龙,谭洪玲,梁乾德,肖成荣,王宇光,马增春,王莉莉,高月
(1.广西医科大学药理学系,广西南宁530000;军事医学科学院2.放射与辐射医学研究所药理学研究室,
3.毒物药物研究所药物化学研究室,北京100850;4.安徽医科大学药理学系,安徽合肥230032)
基于高内涵筛选技术研究生首乌和制首乌醇提物的肝毒性机制
李丹丹1,2,汤响林2,龙隆3,许龙龙4,谭洪玲2,梁乾德2,肖成荣2,王宇光2,马增春2,王莉莉3,高月1,2
(1.广西医科大学药理学系,广西南宁530000;军事医学科学院2.放射与辐射医学研究所药理学研究室,
3.毒物药物研究所药物化学研究室,北京100850;4.安徽医科大学药理学系,安徽合肥230032)
目的 应用高内涵筛选技术研究生首乌醇提物(RPM)和制首乌醇提物(RPMP)的肝毒性及其可能机制。方法RPM(终浓度10,25,50,100,200和300 mg·L-1)和RPMP(终浓度10,50,100,300,600和1200 mg·L-1)作用于HepG2细胞3~24 h。采用CellTiter-GloTM荧光细胞活性检测试剂盒检测HepG2细胞存活率;应用高内涵筛选技术进行HepG2细胞计数,并检测线粒体内活性氧(ROS)、线粒体膜电位(MMP)、细胞内谷胱甘肽(GSH)、超氧化物歧化酶2(SOD2)、转录激活因子4(ATF4)水平及细胞凋亡和细胞周期阻滞;Western蛋白质印迹法验证HepG2细胞SOD2和ATF4蛋白表达水平。结果 与细胞对照组相比,RPM 300 mg·L-1作用24 h使HepG2细胞存活率下降约48%(P<0.01),而相同浓度RPMP对细胞存活率无显著影响;RPM和RPMP均能降低MMP(P<0.05),并升高GSH,ROS,SOD2和ATF4水平(P<0.05)。与细胞对照组相比,RPM 200 mg·L-1作用3 h SOD2水平显著升高(P<0.05),6 h ATF4水平显著升高(P<0.05);RPMP 300 mg·L-1作用6 h ATF4水平显著升高(P<0.05),24 h SOD2水平显著升高(P<0.05)。结论RPM和RPMP均具有一定的细胞毒性,RPM的细胞毒性强于RPMP,两者的肝毒性可能主要与氧化应激和内质网应激导致的细胞凋亡有关。
何首乌;肝毒性;细胞,HepG2;高内涵筛选技术;氧化应激;内质网应激
DOl:10.3867/j.issn.1000-3002.2017.06.019
何首乌是蓼科多年生缠绕藤本植物何首乌(Polygonum multiflorum Thunb.),又名紫乌藤或夜交藤的干燥块根,性温,味苦涩,是中医传统的补益类中药[1-2]。生首乌和制首乌是何首乌在临床上常用的2种剂型。生首乌主要用于解毒、消痈和润肠通便等,制首乌主要用于补肝肾、乌须发和强筋骨等[3]。何首乌自古以来一直被认为安全无毒,但自20世纪90年代开始,有关何首乌及含何首乌制剂的毒性报道逐渐增多,甚至出现中毒致死的案例[4-11]。何首乌肝毒性的问题引起了国内外的高度关注,但何首乌的肝毒性机制及其毒性物质基础至今还不完全清楚,严重制约了何首乌的临床应用并威胁患者的用药安全。
高内涵筛选技术是药物筛选的新技术,通过与不同的荧光指示剂、荧光抗体或配体结合,在细胞水平实现对生物体多系统、多途径、多靶标的动态筛选,通过观察细胞形态预测药物的毒性,实现毒性的早期、快速和高通量检测[12-13]。本研究通过高内涵筛选技术检测与肝毒性密切相关的细胞毒性参数及毒性反应通路,如细胞数目、线粒体内活性氧(reactive oxygen species,ROS)水平、线粒体膜电位(mitochondrial membrane potential,MMP)、细胞内谷胱甘肽(glutathione,GSH)含量、氧化应激反应、内质网应激反应、凋亡和细胞周期阻滞等,对比研究了RPM和RPMP的肝毒性及其可能的毒性机制,并应用Western蛋白质印迹法进一步确证高内涵分析技术所得结果 的可靠性。因何首乌的肝毒性成分主要存在于醇提物中[14-16],故本研究采用何首乌的醇提物进行毒性机制研究。
1 材料与方法
1.1 药物、主要试剂和仪器
生首乌及同一批次制首乌均购自北京同仁堂,产地河南,批号20120707;多聚甲醛,广东西陇化工股份有限公司;DMEM培养基、磷酸盐缓冲液(phosphate-buffered saline,PBS)、Hank平衡液(Hank balance sodium solution,HBSS)、0.25%胰蛋白酶溶液、青霉素-链霉素双抗液(100×)和1× GlutaMAXTM-I CTSTM,美国Gibco公司;牛血清白蛋白(bovine serum albumin,BSA),美国Sigma公司;CellTiter-GloTM荧光细胞活性检测试剂盒,美国Promega公司;荧光染料Hoechst 33342,Mitotracker Red CMXRos,MitoSOX red,monochlorobimane(mBCI),碘化丙啶(propidium iodide,PI)和AnnexinⅤ,西班牙Invitrogen公司;兔抗人转录激活因子4(activating transcription factor 4,ATF4)单克隆抗体(一抗),美国Cell Signaling公司;小鼠抗人超氧化物歧化酶2(superoxide dismutase 2,SOD2)单克隆抗体(一抗),英国Abcam公司;Aexa Fluor 488荧光标记驴抗小鼠IgG抗体和Aexa Fluor 488荧光标记驴抗兔IgG抗体(二抗),美国Life公司;GAPDH兔单克隆抗体(一抗)、HRP标记山羊抗兔IgG抗体和HRP标记山羊抗小鼠IgG抗体(二抗)、抗体稀释液、RIPA裂解液、蛋白酶抑制剂、电泳液、转膜液、封闭液和TBS Tween-20(TBST,10×),北京康为世纪公司;HRP发光液和蛋白质分子质量标准,美国Thermo公司;二苯乙烯苷(2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside,TSG)、大黄素和大黄素-8-O-β-D-葡萄吡喃糖苷对照品(纯度均>98%),上海一飞生物科技有限公司。CO2培养箱,美国Thermo公司;IN CELL 2000高内涵分析仪,美国GE HealthCare公司;超高效液相色谱(UPLC)-四级杆飞行时间(qTOF)-质谱(MS)仪,美国Waters公司。
1.2 生首乌和制首乌醇提物制备
称取生首乌和制首乌各100 g,分别加入10倍体积无水乙醇浸泡30 min后冷凝回流煎煮3次,每次1 h,合并3次滤液,经低压抽滤、浓缩后干燥成粉末。生首乌醇提物(ethanol extract of Radix Polygoni Multiflori,RPM)得率为12.71%,制首乌醇提物(ethanol extract of Radix Polygoni Multiflori Praeparata,RPMP)得率为24.54%。称取RPM和RPMP粉末,溶于DMSO中配制成浓度分别为100和400 g·L-1的母液。
1.3 细胞培养和给药
HepG2细胞购自北京协和细胞库,生长于含有10%胎牛血清、1×105U·L-1青霉素、100 mg·L-1链霉素和1×GlutaMAXTM-I CTSTM的DMEM高糖培养基中,在含5%CO2的37℃恒温培养箱中培养。待培养于T-25培养瓶中的HepG2细胞生长至80%~90%融合时,用胰酶将细胞消化下来,添加适量完全培养基调整细胞密度至5×107L-1;将HepG2细胞接种于底透壁黑的96孔板中,每孔200 μL细胞悬液,约1×104细胞。RPM终浓度为10,25,50,100,200和300 mg·L-1;RPMP则为10,50,100,300,600和1200 mg·L-1;细胞对照组为0.3%DMSO。
1.4 CellTiter-GloTM荧光法检测HepG2细胞存活率
给药处理24 h后,采用CellTiter-GloTM荧光细胞活性检测试剂盒检测细胞存活率。先将CellTiter-GloTM的底物和缓冲液在室温平衡1 h;检测开始前,将缓冲液转移至底物瓶中,混匀制成底物混合物。给药结束后,取出96孔板平衡至室温,每孔吸弃100 μL细胞培养基,添加100 μL底物混合液,将96孔板置于摇床上振摇2 min,室温放置10 min后,用酶标仪检测荧光强度(fluorescence intensity,FI)值,计算细胞存活率。细胞存活率(%)=(待测药物组FI-空白对照组FI)/(细胞对照组FI-空白对照组FI)×100%。
1.5 高内涵筛选技术检测GSH和ROS水平
给药处理24 h后,用预热至37℃的1×HBSS配制含有细胞核染料Hoechst 33342,GSH染料mBCI和线粒体ROS染料MitoSOX red的混合活细胞染料,各染料的终浓度分别为1,100和5 μmol·L-1。HepG2细胞用每孔200 μL的1×HBSS轻轻洗涤1次,加入50 μL混合细胞染料,37℃避光染色40 min,再用200 μL的1×HBSS小心洗涤1次,加入200 μL 1×HBSS,采用高内涵分析仪进行检测。
1.6 高内涵筛选技术检测MMP,SOD2和ATF4蛋白水平
给药处理24 h后,用预热至37℃的1×HBSS配制含有细胞核染料Hoechst 33342和线粒体红色荧光探针染料MitoTracker Red CMXRos(其红色荧光染料的积累取决于膜电位)的混合细胞染料,终浓度分别为1和0.25 μmol·L-1。吸弃96孔板中的培养基,每孔细胞用200 μL的1×HBSS轻轻洗涤1次,加入50 μL混合细胞染料,于37℃避光染色40 min。染色结束后,吸弃96孔板中的混合细胞染料,每孔加入100 μL含4%甲醛的PBS溶液,室温避光固定20 min;吸弃甲醛溶液,加入200 μL的1×透膜液(含0.1%Triton X-100的PBS溶液),室温避光孵育30 min;吸弃透膜液,用200 μL PBS清洗细胞3次,加入200 μL的1×封闭液(含5%BSA的PBS溶液)室温孵育1 h;吸弃封闭液,加入40 μL SOD2或ATF4一抗工作液(小鼠抗人SOD2单克隆抗体用1×封闭液按1∶500稀释,兔抗人ATF4单克隆抗体用1×封闭液按1∶200稀释),4℃避光孵育过夜;吸弃一抗工作液,用100 μL的1×封闭液清洗细胞3次,加入50 μL相对应的二抗工作液(Aexa Fluor 488驴抗兔IgG二抗与Aexa Fluor 488驴抗鼠IgG二抗均用1×封闭液按1∶500稀释)室温避光孵育2 h。吸弃二抗工作液,用200 μL PBS清洗细胞3次,加入100 μL PBS,采用高内涵分析仪进行检测。
1.7 高内涵筛选技术检测细胞凋亡和细胞周期
给药处理24 h后,HepG2细胞用PBS清洗1次,每孔加入2 μL AnnexinⅤ-FITC,2 μL PI溶液和40 μL 1×Annexin结合缓冲液,室温避光孵育15 min,补加156 μL的1×Annexin结合缓冲液,再加入100 μL用1×Annexin结合缓冲液配制的12%甲醛溶液,室温避光固定20 min;吸弃固定液,加入100 μL含1 μmol·L-1Hoechst 33342的PBS,室温避光染色1 h后,采用高内涵分析仪进行检测。
1.8 Western蛋白质印迹法检测ATF4和SOD2水平
将HepG2细胞接种于6孔板中,每孔2×105细胞,放入CO2培养箱中培养18~24 h后进行给药处理。RPM终浓度为50,100和200 mg·L-1;RPMP为300,600和1200 mg·L-1;对照组为0.3%DMSO。给药处理24 h后,使用RIPA裂解液将细胞裂解,取上清,采用BCA法测定蛋白质浓度,取30 μg蛋白质样品经10%SDS-PAGE电泳和转印,使用凝胶成像系统进行图像采集,Image J软件分析各组条带的积分吸光度(integrated absorbance,IA)值,目的蛋白的相对表达水平用IA目的蛋白/IAGAPDH比值表示。
1.9 UPLC-qTOF-MS技术检测RPM和RPMP化学成分
色谱柱Waters HSS T3(2.1 mm×100 mm,1.8 μm);流动相:A为含0.1%甲酸水溶液,B为含0.1%甲酸乙腈溶液;流速:0.5 mL·min-1;进样量:5 μL;柱温:30℃。流动相采用梯度洗脱:0~1 min,2%B;1~2 min,2%~5%B;2~5 min,5%~12%B;5~7 min,12%~20%B;7~10 min,20%~30%B;10~12 min,30%~50%B;12~14 min,50%~80% B;14~16 min,80%~85%B;16~18 min,85%~100%B;18~22 min,2%B。DAD全波长扫描范围:200~400 nm。采用电喷雾离子源(ESI),应用飞行时间(TOF)Ⅴ模式进行质量检测。负离子扫描范围为m/z 100~1500。
称取RPM和RPMP粉末各10 mg,置于10 mL容量瓶中,加入50%甲醇溶解并定容,12 000×g离心15 min,取上清经0.22 μm微孔滤膜过滤后得到供试品溶液。称取适量的TSG、大黄素和大黄素-8-O-β-D-葡萄吡喃糖苷对照品,置于10 mL容量瓶中,加入50%甲醇溶解并定容,经0.22 μm微孔滤膜过滤后得到相应的对照品溶液。
1.10 统计学分析
实验结果 数据以x±s表示,使用SPSS 18.0统计软件,采用单因素方差分析和LSD检验进行统计处理,P<0.05为差异具有统计学意义。
2 结果
2.1 RPM和RPMP对HepG2细胞存活率的影响
如图1所示,RPM和RPMP作用于HepG2细胞24 h后,与细胞对照组相比,RPM 300 mg·L-1使HepG2细胞存活率明显下降(P<0.01);而相同浓度RPMP对细胞存活率无明显影响;当RPMP浓度升高至600和1200 mg·L-1时,HepG2细胞存活率明显下降(P<0.01)。
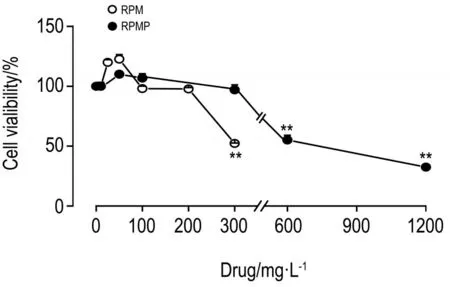
Fig.1Effect of ethanol extract of Radix Polygoni Multiflori(RPM)and Radix Polygoni Multiflori Praeparata(RPMP)on HepG2 cell vialibility by CellTiter-GloTMluminescent cell viability assay.Cells were incubated with RPM(10,25,50,100,200 and 300 mg·L-1)and RPMP(10,50,100,300,600 and 1200 mg·L-1)for 24 h,respectively.x±s,n=3.**P<0.01,compared with corresponding cell control(0)group.
2.2 RPM和RPMP对HepG2细胞数目的影响
RPM和RPMP作用于HepG2细胞24 h后,与细胞对照组相比,RPM 300 mg·L-1使细胞数目显著减少(P<0.01),而相同浓度RPMP对细胞数目无明显影响;当RPMP浓度升高至600和1200 mg·L-1时细胞数目显著减少(P<0.05,P<0.01)(图2)。

Fig.2 Effect of RPM and RPMP on HepG2 cell count by high-content screen assay.See Fig.1 for the cell treatment.x±s,n=3.*P<0.05,**P<0.01,compared with corresponding cell control(0)group.
2.3 RPM和RPMP对GSH,ROS和MMP水平的影响
如图3和4所示,RPM和RPMP 100 mg·L-1作用24 h,HepG2细胞内GSH水平显著升高(P<0.05);RPM 200 mg·L-1和RPMP 600 mg·L-1组 ROS水平开始显著升高(P<0.01,P<0.05);RPM 100 mg·L-1和RPMP 300 mg·L-1组MMP水平显著降低(P<0.05,P<0.01),使线粒体发生损伤。说明RPM和RPMP对肝毒性相关参数GSH,ROS和MMP均有一定的影响;与RPMP相比,RPM在较低浓度时即可对线粒体造成一定损伤。
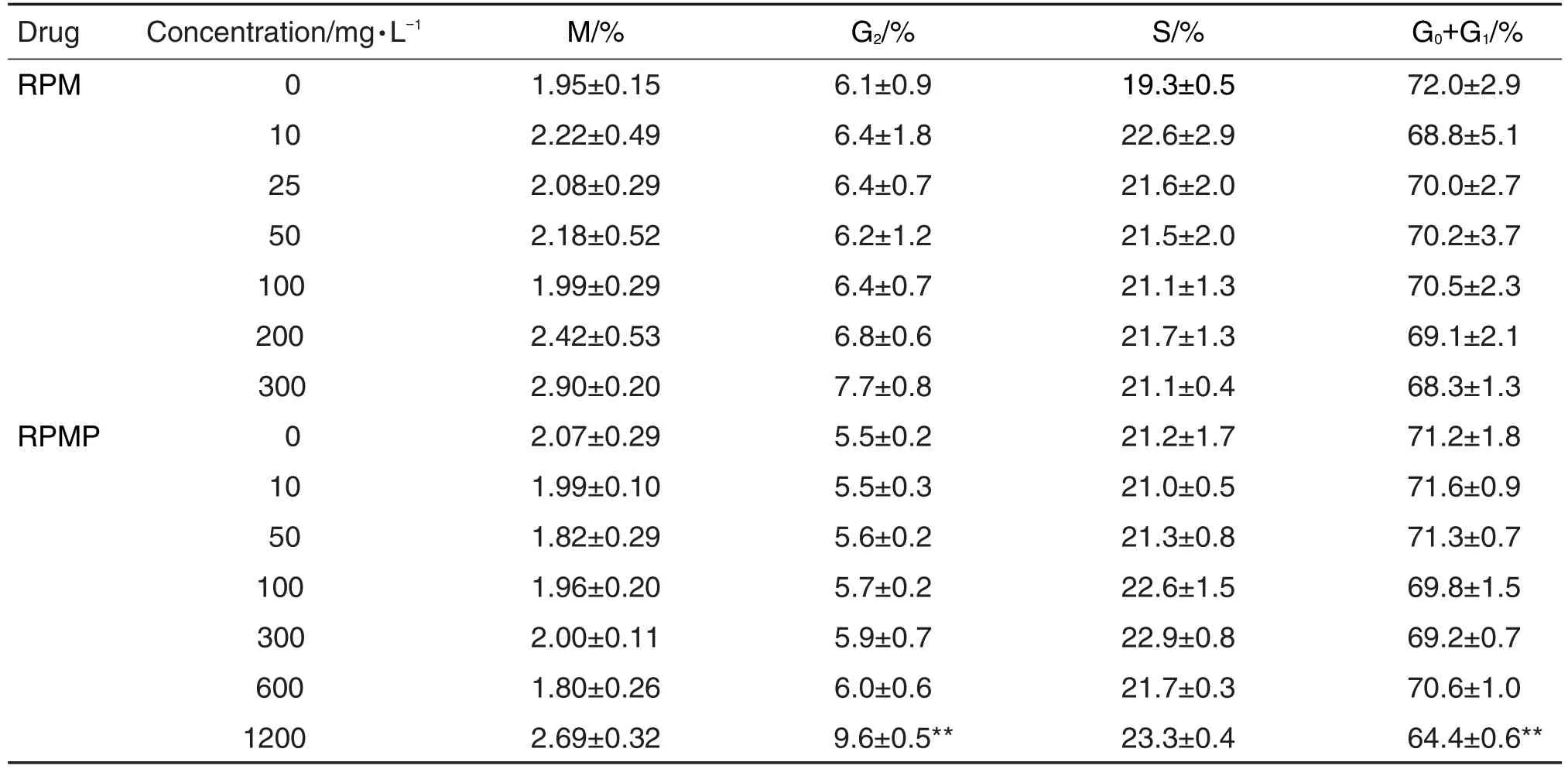
2.4 RPM和RPMP对SOD2和ATF4蛋白水平的影响
如图5和6所示,不同浓度的RPM和RPMP作用24 h均能引起HepG2细胞内氧化应激相关蛋白SOD2和内质网应激相关蛋白ATF4水平显著升高(P<0.05)。从图7可见,RPM 200 mg·L-1在3 h时即能引起SOD2水平升高(P<0.05),6 h时引起ATF4水平升高(P<0.05)。RPMP 300 mg·L-1在 6 h时引起ATF4水平升高(P<0.05),24 h时引起SOD2水平升高(P<0.05)。
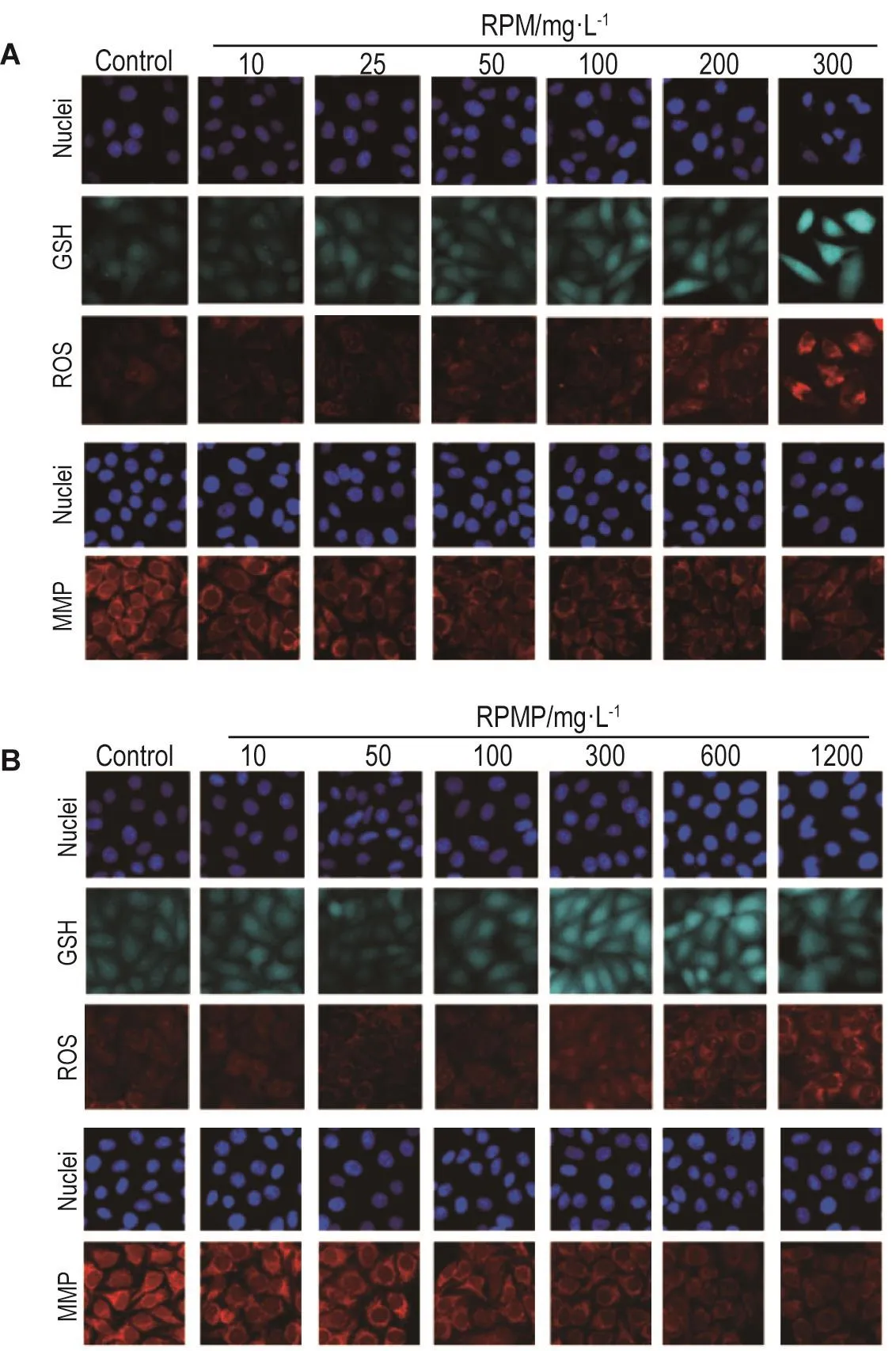
Fig.3 Representative images of effect of RPM(A)and RPMP(B)on levels of glutathione(GSH),reactive oxygen species(ROS)and mitochondrial membrane potential(MMP)of HepG2 cells by high-content screen assay.See Fig.1 for the cell treatment.The levels of GSH,ROS and MMP were determined by relative fluorescence intensity(FI)of mBCI,Mito-SOX red and Mitotracker Red CMXRos,respectively.
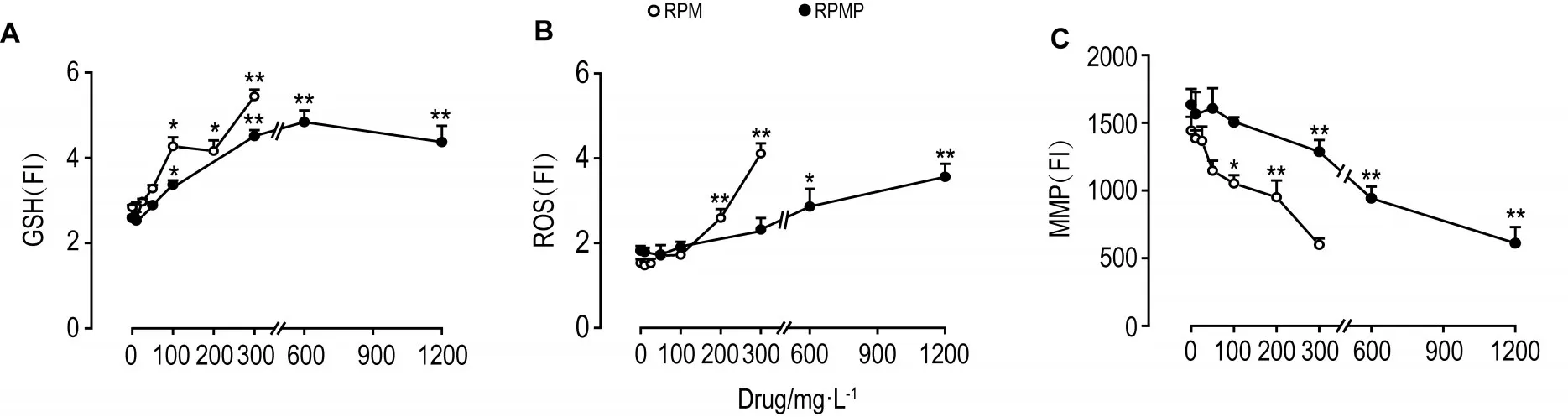
Fig.4 Effect of RPM and RPMP on levels of GSH(A),ROS(B)and MMP(C)of HepG2 cells by high-content screen assay.See Fig.1 and Fig.3 for the cell treatment.FI:fluorescence intensity.x±s,n=3.*P<0.05,**P<0.01,compared with corresponding cell control(0)group.
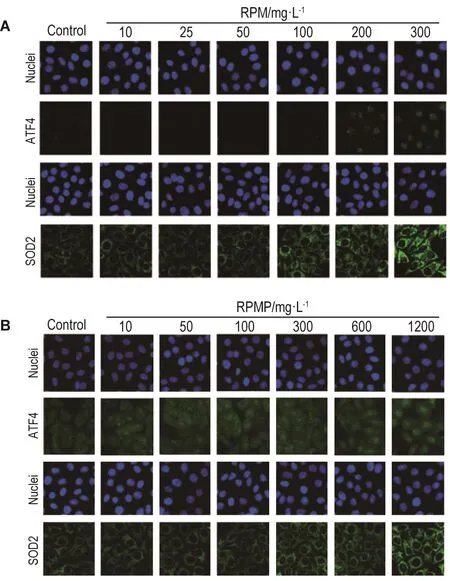
Fig.5 Representative images of effect of RPM(A)and RPMP(B)on levels of activating transcription factor 4(ATF4)and superoxide dismutase 2(SOD2)of HepG2 cells by high-content screen assay.See Fig.1 for the cell treatment.The expression of SOD2 and ATF4 was determined by relative FI of SOD2 and ATF4,respectively.
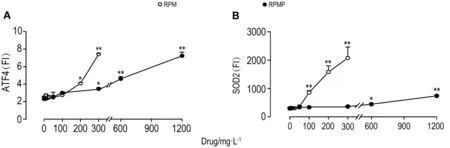
Fig.6 Effect of RPM and RPMP on levels of ATF4(A)and SOD2(B)of HepG2 cells by high-content screen assay.See Fig.1 and Fig.5 for the cell treatment.x±s,n=3.*P<0.05,**P<0.01,compared with corresponding cell control(0)group.
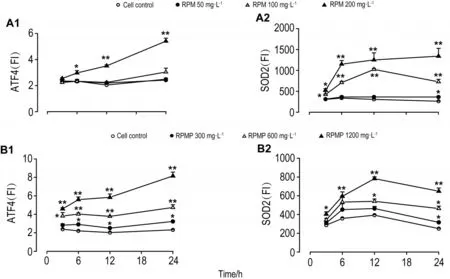
Fig.7 Effect of RPM(A)and RPMP(B)on expression of ATF4(A1 and B1)and SOD2(A2 and B2)protein in HepG2 cells at different time points by high-content screen assay.Cells were treated with RPM and RPMP for 3,6,12 and 24 h,respectively.x±s,n=3.*P<0.05,**P<0.01,compared with corresponding cell control group.
2.5 RPM和RPMP对细胞凋亡和细胞周期的影响
RPM和RPMP作用24 h均可使HepG2细胞发生不同程度的凋亡(表1和图8)。RPM诱导细胞凋亡的作用明显强于RPMP。RPM 300 mg·L-1使细胞早期凋亡率、晚期凋亡率和死亡率明显升高(P<0.01),相同浓度的RPMP对HepG2细胞凋亡无明显诱导作用。此外,RPM 100 mg·L-1引起细胞凋亡作用与RPMP 1200 mg·L-1接近。细胞周期分析结果 (图8和表2)表明,RPM对细胞周期无明显影响,RPMP在最高浓度1200 mg·L-1时使HepG2细胞G2期发生明显的阻滞。
2.6 Western蛋白质印迹法验证RPM和RPMP对SOD2和ATF4蛋白表达的影响
Western蛋白质印迹法检测结果 表明,RPM和RPMP作用24 h均能显著升高SOD2和ATF4蛋白水平(图9)。RPM引起SOD2和ATF4含量显著升高的最低浓度分别为50和200 mg·L-1(P<0.05,P<0.01),RPMP则分别为1200和300 mg·L-1(P<0.05),与高内涵技术所得实验结果 基本一致。
2.7 RPM和RPMP的化学成分
RPM和RPMP中主要含有TSG、大黄素和大黄素-8-O-β-D-葡萄吡喃糖苷3种主要的二苯乙烯苷类、蒽醌类及蒽醌糖苷类化合物。根据各单体成分的峰面积比(图10)可知,RPM经炮制后化学成分发生了明显的改变,TSG和大黄素-8-O-β-D-葡萄吡喃糖苷含量分别下降约37%和78%,大黄素含量上升约337%,与文献[6,10]报道一致。
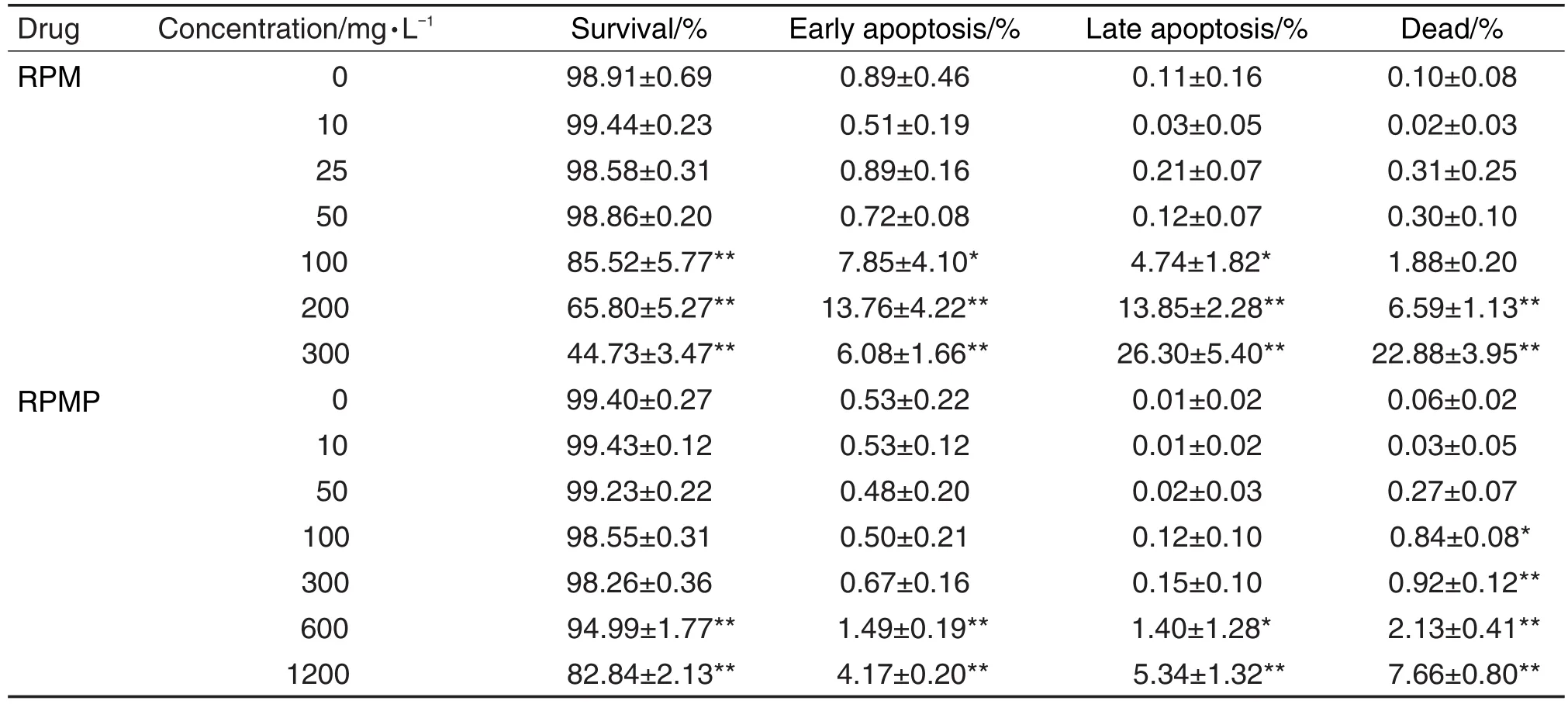
Tab.1 Effect of RPM and RPMP on HepG2 cell apopotosis by high-content screen assay
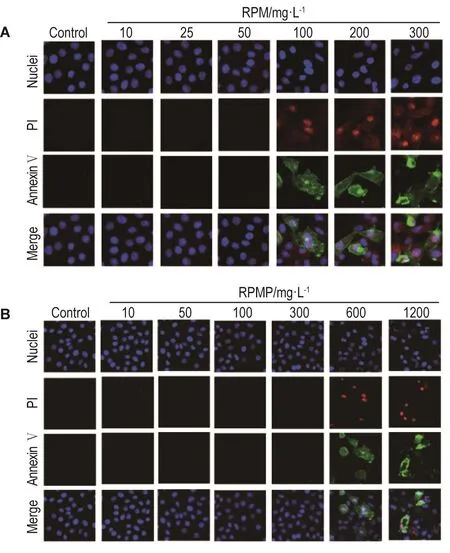
Fig.8 Representative images of effect of RPM(A)and RPMP(B)on apoptosis and cell cycles of HepG2 cells by high-content screen assay.See Fig.1 for the cell treatment.
Tab.2 Effect of RPM and RPMP on HepG2 cell cycle by high-content screen assay
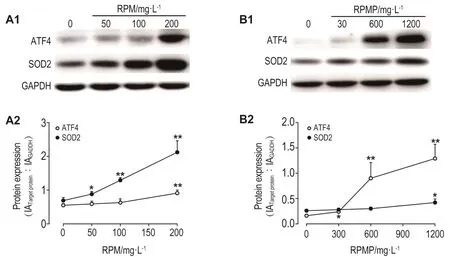
Fig.9 Effect of RPM(A)and RPMP(B)on expression of SOD2 and ATF4 proteins in HepG2 cells detected by Western blotting.Cells were treated with RPM and RPMP for 24 h,respectively.A2 and B2 was the semi-quantitative results of A1 and B1,respectively.x±s,n=3.*P<0.05,**P<0.01,compared with corresponding cell control(0)group.
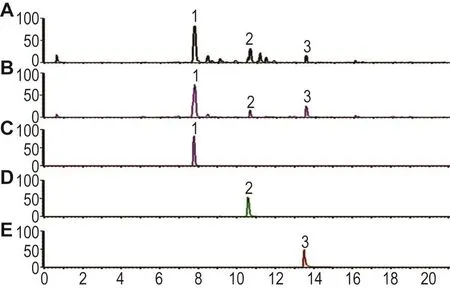
Fig.10 Negative ion mass spectra of RPM(A)and RPMP(B)by UPLC-qTOF-MS.C:2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside;D:emodin-8-O-β-D-glucoside;E:emodin.Peak 1:TSG;peak 2:emodin-8-O-β-D-glucoside;peak 3:emodin.
3 讨论
本研究采用高内涵筛选技术较为系统的研究了RPM和RPMP对肝毒性相关参数及信号通路的影响,并应用Western蛋白质印迹技术证实了高内涵筛选结果 的可靠性。研究发现,高内涵筛选技术测得细胞计数结果 与CellTiter-GloTM荧光法检测HepG2细胞存活率相近,说明应用高内涵筛选技术检测药物对细胞数量的影响可在一定程度上反映药物的细胞毒性。RPM和RPMP都能引起一定的氧化应激和内质网应激反应,并能通过降低线粒体膜电位对线粒体功能造成损伤,最终导致细胞凋亡。虽然RPM和RPMP均能使HepG2细胞发生明显的氧化应激与内质网应激反应,但应激反应的强弱及引起应激反应所需要的药物浓度和作用时间有很大区别,RPM主要以氧化应激为主,在较低浓度和(或)较短作用时间内就能使氧化应激相关指标GSH,ROS和SOD2水平明显升高,而内质网应激相关指标ATF4发生明显变化的时间较晚且所需药物浓度较高;RPMP则与RPM正相反。吴宇等[17]通过高内涵筛选技术同样检测到何首乌70%乙醇提取物能引起HepaRG细胞发生明显的内质网应激反应,但并未检测到其对活性氧和线粒体膜电位的影响,与本研究结果 不一致,其原因可能是何首乌乙醇提取物的制备工艺不同导致了其中的主要成分有差异所致。
药物的肝毒性与氧化应激和内质网应激密切相关,能引起肝损伤的药物通常会刺激线粒体和内质网,进而激活氧化应激和内质网应激等信号通路使细胞恢复稳态,但当损伤无法修复时,长期的氧化应激与内质网应激将导致细胞凋亡并引发肝损伤[18-20]。此外,氧化应激产生的ROS能扰乱内质网上蛋白质的正确折叠并引起内质网应激,反之,内质网应激也能引起线粒体功能的失调并引起线粒体ROS的产生,所以内质网应激与氧化应激不仅能单独影响细胞功能导致细胞死亡,还可通过相互促进作用形成一个正反馈的循环,共同干扰细胞的功能并激活促凋亡等信号通路[21-23]。
RPM经炮制后其主要成分TSG的含量明显降低,而大黄素含量显著升高。实验室前期研究发现,TSG可引起ROS含量升高并导致强烈的氧化应激反应,大黄素则可以通过内质网应激反应途径引发HepG2细胞凋亡(待发表)。RPM引起的应激反应主要以氧化应激为主,这可能与其含有大量的TSG有关;而RPMP经炮制后升高的大黄素可能是其能引起强烈内质网应激的主要原因。考虑到氧化应激与内质网应激存在协同促进的关系,何首乌中同时存在能导致氧化应激的TSG和导致内质网应激的大黄素可能是其引起肝毒性的潜在危险因素,故炮制虽然在一定程度上减弱了何首乌的细胞毒性,但并未完全消除何首乌致肝毒性的可能性。
[1]Ma ZJ.A preliminary study on the objectivity,clinical biomarkers and injury mechanism of Polygonum multiflorum-induced hepatotoxicity(何首乌肝毒性客观性、临床标志物及损伤机制的初步研究)[D]. Chengdu:Chengdu University of TCM(成都中医药大学),2013.
[2]He YZ,Chen J,Shen SL.Research progress in relationship between Polygonum multiflorum and liver injury[J].Med Recapit(医学综述),2013,19(12):2206-2208.
[3]Yan LC,Zhao JN,Qiu X.The research progress on the safety of Polygonum multiflorum[J].Pharmacol Clin Chin Mater Med(中药药理与临床),2009,25(3):77-81.
[4]Dong H,Slain D,Cheng J,Ma W,Liang W.Eighteen cases of liver injury following ingestion of Polygonum multiflorum[J].Complement Ther Med,2014,22(1):70-74.
[5]Lei X,Chen J,Ren J,Li Y,Zhai J,Mu W,et al. Liver damage associated with Polygonum multiflorum Thunb.:A systematic review of case reports and case series[J].Evid Based Complement Alternat Med,2015,2015:459749.
[6]Liang Z,Chen H,Yu Z,Zhao Z.Comparison of raw and processed Radix Polygoni Multiflori(Heshouwu)by high performance liquid chromatography and mass spectrometry[J].Chin Med,2010,5:29.
[7]Lin L,Ni B,Lin H,Zhang M,Li X,Yin X,et al. Traditional usages,botany,phytochemistry,pharmacology and toxicology of Polygonum multiflorum Thunb.:a review[J].J Ethnopharmacol,2015,159:158-83.
[8]Lv GP,Meng LZ,Han DQ,Li HY,Zhao J,Li SP. Effect of sample preparation on components and liver toxicity of Polygonum multiflorum[J].J Pharm Biomed Anal,2015,109:105-111.
[9]Ma J,Zheng L,He YS,Li HJ.Hepatotoxic assessment of Polygoni Multiflori Radix extract and toxicokinetic study of stilbene glucoside and anthraquinones in rats[J].J Ethnopharmacol,2015,162:61-68.
[10]Wu X,Chen X,Huang Q,Fang D,Li G,Zhang G. Toxicity of raw and processed roots of Polygonum multiflorum[J].Fitoterapia,2012,83(3):469-475.
[11]Yu J,Xie J,Mao XJ,Wang MJ,Li N,Wang J,et al.Hepatotoxicity of major constituents and extractions of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata[J].J Ethnopharmacol,2011,137(3):1291-1299.
[12]O′Brien PJ,Irwin W,Diaz D,Howard-Cofield E,Krejsa CM,Slaughter MR,et al.High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening[J].Arch Toxicol,2006,80(9):580-604.
[13]Gasparri F.An overview of cell phenotypes in HCS:limitations and advantages[J].Expert Opin Drug Discov,2009,4(6):643-657.
[14]Li Y,Xu L,Liu RN,Lin LF,Shi L.Influence of ethanol extract of raw and processed Polygonum multiflorum on mice liver[J].J Hainan Med Univ(海南医学院学报),2011,17(4):452-455.
[15]Li Q,Zhao KJ,Zhao YL,Wang JB,Fang F,Lv Y,et al.High dosage administration of Polygonum multiflorum alcohol extract caused the multi-organ injury in rats[J].Global Tradit Chin Med(环球中医药),2013,6(1):1-7.
[16]LV Y,Wang JB,Ji Y,Zhao YL,Ma ZJ,Li Q,et al.Influence of extracting solvent on hepatocytes toxicity of Polygonum multiflorum[J].Chin J Exp Tradit Med Form(中国实验方剂学杂志),2013,19(20):268-272.
[17]Wu Y.Screening of in vitro model in drug-induced liver injury and preliminary investigation of Polgonum multiflorum induced liver injury(药物性肝损伤体外筛选模型和何首乌致肝损伤的初步研究)[D].Chinese Academy of Medical Sciences&Peking Union Medical College(中国医学科学院北京协和医学院),2016.
[18]Adachi T,Kaminaga T,Yasuda H,Kamiya T,Hara H.The involvement of endoplasmic reticulum stress in bile acid-induced hepatocellular injury[J]. J Clin Biochem Nutr,2014,54(2):129-135.
[19]Han D,Dara L,Win S,Than TA,Yuan L,Abbasi SQ,et al.Regulation of drug-induced liver injury by signal transduction pathways:critical role of mitochondria[J].Trends Pharmacol Sci,2013,34(4):243-353.
[20]Pereira CV,Nadanaciva S,Oliveira PJ,Will Y. The contribution of oxidative stress to drug-inducedorgan toxicity and its detection in vitro and in vivo[J].Expert Opin Drug Metab Toxicol,2012,8(2):219-237.
[21]Cheville NF.Ultrastructural pathology and interorganelle cross talk in hepatotoxicity[J].Toxicol Pathol,2013,41(2):210-226.
[22]Cao SS,Kaufman RJ.Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease[J].Antioxid Redox Signal,2014,21(3):396-413.
[23]Chen S,Melchior WB Jr,Guo L.Endoplasmic reticulum stress in drug-and environmental toxicantinduced liver toxicity[J].J Environ Sci Health C Environ Carcinog Ecotoxicol Rev,2014,32(1):83-104.
High-content screen assay for studying hepatotoxicity mechanisms of ethanol extract of Radix Polygoni Multiflori and Radix Polygoni Multiflori Praeparata
LI Dan-dan1,2,TANG Xiang-lin2,LONG Long3,XU Long-long4,TAN Hong-ling2,LIANG Qian-de2,
XIAO Cheng-rong2,WANG Yu-guang2,MA Zeng-chun2,WANG Li-li3,GAO Yue1,2
(1.Department of Pharmacology,Guangxi Medical University,Nanning 530021,China;2.Department of Pharmacology,Institute of Radiation Medicine,3.Department of Medicinal Chemistry,Institute of Pharmacology and Toxicology,Academy of Military Medical Sciences,Beijing 100850,China;
4.Department of Pharmacology,Anhui Medical University,Hefei 230032,China)
OBJECTlVETo investigate the hepatotoxicity mechanisms of ethanol extract of Radix Polygoni Multiflori(RPM)and Radix Polygoni Multiflori Praeparata(RPMP)by high-content screen assay.METHODSHepG2 cells were treated with RPM(10,25,50,100,200 and 300 mg·L-1)and RPMP(10, 50,100,300,600 and 1200 mg·L-1)for 3-24 h,respectively.The cell viability was detected by a CellTiter-GloTMluminescent cell viability assay kit.Cell count,reactive oxygen species(ROS),mitochondrial membrane potential(MMP),glutathione(GSH),superoxide dismutase 2(SOD2),activating transcription factor 4(ATF4),apoptosis,and cell cycles were investigated by high-content screen assay.Besides, SOD2 and ATF4 levels were confirmed by Western blotting.RESULTSRPM 300 mg·L-1showed nearly 48%reduction in cell viability compared with cell control(P<0.01),while RPMP had no significant effect at the same concentration.Both RPM and RPMP decreased the level of MMP(P<0.05)but incresed levels of GSH,ROS,SOD2 and ATF4 significantly(P<0.05).Besides,RPM 200 mg·L-1significantly increased the expression of SOD2(P<0.05)at 3 h by high-content screen assay,and the enhanced expression of ATF4 was shown at 6 h(P<0.05).RPMP 300 mg·L-1markedly increased the expression of ATF4 at 6 h(P<0.05),while the expression of SOD2 significantly increased at 24 h(P<0.05).CONCLUSlONBoth RPM and RPMP have some cytotoxicity,and the cytotoxicity of RPM is stronger than that of RPMP.The hepatotoxicity mechanisms of RPM and RPMP may be related to cell apoptosis caused by long-term oxidative stress and endoplasmic reticulum stress.
Polygonum multiflorum Thunb.;hepatotoxicity;cells,HepG2;high-content screen; oxidative stress;endoplasmic reticulum stress
The project supported by Natural Science Foundation of Beijing City(7164291);National Science and Techonology Major Project of China(2014ZX09304307-001-003);National Science and Techonology Major Project of China(2015ZX 09501004-003-003);and Special Scientific Research for Traditional Chinese Medicine of State Administrortion of Traditional Chinese Medicine of China(201507004)
GAO Yue,E-mail:gaoyue@bmi.ac.cn;TANG Xiang-ling,E-mail:tangxianglin@139.com
R285
A
1000-3002-(2017)06-0626-10
2016-11-10接受日期:2017-01-25)
(本文编辑:齐春会)
北京市自然科学基金(7164291);国家科技重大专项(2014ZX09304307-001-003);国家科技重大专项(2015ZX-09501004-003-003);中医药行业科研专项(201507004)
李丹丹,硕士研究生,主要从事中药药理学和毒理学研究;高月,博士,研究员,主要从事中药药理学和毒理学研究;汤响林,博士,助理研究员,主要从事中药药理学和毒理学研究。
高月,E-mail:gaoyue@bmi.ac.cn;汤响林,E-mail:tangxianglin@139.com

