Determinationof29ChemicalConstituentsintheQingGanSanJieDecoctionbyAcceleratedSolventExtractionandHPLC-ESI-TOFMS
WANG Hui, LI Yun-qing,2, LI Yang, QIAN Xian, CHAI Yi-feng, ZHANG Guo-qing, LV Lei, ZHAO Liang
(1.Department of Pharmacy, Eastern Hepatobiliary Surgery Hospital,Second Military Medical University, Shanghai 200438, China; 2.Department of Pharmacy,The Second Hospital of Shanxi Medical University, Taiyuan 030000, China; 3.School of Pharmacy, Second Military Medical University, Shanghai 200433, China)
Determinationof29ChemicalConstituentsintheQingGanSanJieDecoctionbyAcceleratedSolventExtractionandHPLC-ESI-TOFMS
WANG Hui1, LI Yun-qing1,2, LI Yang1, QIAN Xian1, CHAI Yi-feng3, ZHANG Guo-qing1, LV Lei1, ZHAO Liang1
(1.DepartmentofPharmacy,EasternHepatobiliarySurgeryHospital,SecondMilitaryMedicalUniversity,Shanghai200438,China; 2.DepartmentofPharmacy,TheSecondHospitalofShanxiMedicalUniversity,Taiyuan030000,China; 3.SchoolofPharmacy,SecondMilitaryMedicalUniversity,Shanghai200433,China)
QingGanSanJie decoction (QGSJ), which consists of 14 different herbs, is a multiherbal formula for liver cancer that is administered in different combinations based on experimental observations and clinical experiences from long-term applications. A method based on accelerated solvent extraction (ASE) followed by high-performance liquid chromatography (HPLC) coupled with electrospray time-of-flight mass spectrometry (ESI-TOF MS) were established for simultaneous determination of 29 chemical components in QGSJ. The operational parameters of ASE, including the extraction solvent (70% ethanol), extraction temperature (100 ℃), static extraction time (5 min), and extraction cycles (two cycles) were optimized by orthogonal design and principal component analysis. HPLC separation was performed on a Kromasil C18 HPLC column with a linear gradient mobile phase system. TOF MS was set in negative ion mode with a detector range ofm/z100-1 100. The results show that 29 compounds in the QGSJ have good linearity (r>0.994), and intra-day and inter-day precision (relative standard deviation) are less than 5%. The extraction recoveries of the 29 compounds range from 96.5% to 104.5%. The method is rapid and reliable, which is suitable for the quantitative evaluation of multiherbal traditional Chinese medicine.
accelerated solvent extraction (ASE); high-performance liquid chromatography coupled with electrospray time-of-flight mass spectrometry (HPLC-ESI-TOF MS); QingGanSanJie decoction; simultaneous determination
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third largest cause of cancer-related deaths due to its high mortality and recurrence rates[1-3]. Traditional Chinese medicine (TCM) makes use of a synergistic therapeutic approach for healing liver cancer, which differs greatly from Western medicine approaches. It also has certain advantages with regard to alleviating patient symptoms and enhancing the survival rate. QingGanSanJie decoction (QGSJ) is a multiherbal decoction that is administered in different combinations based on experimental observations and clinical experiences from long-term applications in liver cancer. It is composed of 14 herbs, including the 4 monarchsActinidiavalvataDunn (contains ursolic acid and asiatic acid),SalviaChinensisBenth (contains oleanolic acid),HerbaHedyotidisDiffusae (contains oleanolic acid, ursolic acid, kaempferol, and quercetin), andPortulacagrandifloraHook (contains scutellarin, baicalein, apigenin) with the effects of heat and dampness, stasis removal, and detoxification; the 3 minister herbsPoria,RhizomaAtractylodisMacrocephalae, andGanodermalucidumwith the effects of supporting vital qi, dispelling pathogenic qi, fortifying the spleen, and regulating qi; the 6 assistantsAkebiaquinataDecne (contains oleanolic acid),ArdisiajaponicaBlume (contains bergenin),BupleurmchinenseDC (contains saikosaponin A, B1, B2C, D),RadixScutellariaeBaicalensis (contains flavonoids such as baicalin, wogonoside, baicalein, and wogonin),ArtemisiacapillariesThunb (contains coumarins), andHerbaSediSarmentosi (contain chlorogenic acid and luteolin) with the effects of soothing and rectifying the liver, and the guideRadixGlycyrrhizae(liquiritin, isoliquritin, glycyrrhizic acid, and glycyrrhetinic acid) with the effects of reconciling the various drugs and reducing toxicity. QGSJ has mainly been used for the treatment of primary liver cancer. Based on years of research and observation, it is found to significantly improve liver function, relieve symptoms in patients, inhibit the growth of HCC, inhibit tumor metastasis and recurrence, and improve the survival rate of patients. The efficacy of TCM is generally determined by its chemical composition, therefore, it is very important to develop a sensitive and effective method for qualitatively and quantitatively analyzing the major constituents in QGSJ. This can reflect the overall distribution of each component in the Chinese herbal compound. In addition, using pharmacodynamics studies to clarify the specific role of individual components will be extremely valuable for the development of simpler and more effective Chinese herbal prescriptions.
The traditional liquid chromatographic separation method can be used to identify a single compound in a multiherbal therapy or several components in a single herb, which does not meet the requirements of multiple components analysis in the QGSJ prescription. With the advantages of accurate mass measurement and fragmented ion abundance information, time-of-flight mass spectrometry (TOF MS) has received increasing attention. Importantly, most of the compounds to be detected in TCM exhibit high sensitivity in TOF MS analysis. As a consequence, the combination of HPLC with TOF MS has commonly been considered an appropriate method for the qualitative and quantitative analysis of multiherbal TCMs[4-7].
Accelerated solvent extraction (ASE) is thought to be an attractive and preferred extraction method because it has higher extraction efficiency with lower solvent volumes and a shorter extraction time compared to other conventional extraction technologies, such as liquid-liquid extraction and Soxhlet extraction. In addition, the solvent can penetrate into the matrix more easily under conditions of high pressure and temperature. Moreover, it has variable parameters including extraction temperature, static extraction time, extraction cycle, extraction volume, flush volume, and nitrogen purge time. This procedure has been used for the extraction of a variety of compounds in recent years, including in environmental and food studies[8-12]. However, very few researches reported its utilization in combination with modern analytical techniques in the analysis of TCM[13-16].
The aim of the present study was to establish a reliable HPLC-TOF MS method for the quantitative determination of 29 compounds in QGSJ using an optimized ASE approach (e.g., composition of extraction solvent, temperature, pressure, and number of cycles), with the goal of providing a practical approach for the simultaneous analysis of components in multiherbal therapies.
1 Materials and Methods
1.1MaterialsandReagents
QGSJ consists of 14 herbs, includingActinidiavalvataDunn,SalviachinensisBenth,AkebiaquinataDecne,ArdisiajaponicaBlume,HerbaHedyotidisDiffusae,PortulacagrandifloraHook,BupleurmchinenseDC,RadixScutellariaeBaicalensis,Poria,RhizomaAtractylodisMacrocephalae,ArtemisiacapillarisThunb,HerbaSediSarmentosi,Ganodermalucidum,RadixGlycyrrhizae, all of which were purchased from Shanghai Qingpu Chinese medicine decoction pieces Company (Shanghai, China) in compliance with the China Pharmacopoeia standards (2010). The purchases were authenticated by Professor Sun from the Department of Pharmacognosy at the Second Military Medical University.
29 component standards including chlorogenic acid, rosmarinic acid, 3,4-dihydroxy-benzoic acid, catechin, epicatechin, catechin gallate, oleanolic acid, ursolic acid, asiatic acid, quercetin, kaempferol, apigenin, luteolin, naringin, naringenin, bergenin, baicalin, wogonoside, scutellarin, baicalein, wogonin, saikosaponin A, saikosaponin B2, saikosaponin C, saikosaponin D, liquiritigenin, glycyrrhizic acid, liquiritin, isoliquiritin, and three internal standards (puerarin, ginsenoside Re, ginsenoside Rb1) were purchased from Chengdu Must Bio-Technology Co., Ltd (Sichuan, China, purity is greater than 98%). Methanol and acetonitrile (HPLC grade, Honeywell, USA), formic acid (HPLC grade, Sigma, USA) and ultrapure-water were used for all of the analyses.
1.2SamplePreparation
1.2.1Standards Preparation Mixed standard stock solutions were prepared by dissolving 18 pure compounds in 50 mL methanol, including 3,4-dihydroxybenzoic acid, catechin, liquiritin, and glycyrrhizic acid. Bergenin, chlorogenic acid, baicalin, ursolic acid, and an additional eleven compounds were prepared by dissolving each pure compound (accurately weighted) in 10 mL methanol or 70% ethanol. The standard working solutions used for calibration were constructed by diluting the above standard stock solutions into 25 mL methanol to the desired concentrations. All of the solutions were stored at 4-10 ℃.
1.2.2Internal Standard Solution Preparation Stock solutions of the three internal standards, puerarin, ginsenoside Re, and ginsenoside Rb1were prepared by dissolving 10 mg (accurately weighed) of each compound in 10 mL methanol. Mixed internal standard solutions, including 99.50 mg/L puerarin, 88.80 mg/L ginsenoside Re, and 108.5 mg/L ginsenoside Rb1, were prepared by mixing the above internal standard stock solution in 10 mL methanol, all of which were stored at 4-10 ℃.
1.2.3Extraction of QGSJ Sample An ASE 350 System (Diane, USA) was used for pressurized liquid extraction. Following the recipe of QGSJ, 220 g mixed herbs (one prescription dose) was crushed and grinded. The resulting mixed powder (5 g) was placed into a 66 mL stainless steel ASE vessel with a cellulose fiber filter capped at both ends. The sample was extracted with 70% ethanol using the preset conditions: 11.7 MPa pressure, 5 min static extraction time, 100 ℃ extraction temperature, two cycles, 60% flush, 90 s purge. The extracted solution was diluted to 100 mL with 70% ethanol, and then filtered through a 0.22 μm microporous membrane. A total of 9.5 mL filtrated solution was mixed homogeneously with 0.5 mL internal standard solution as a sample to inject into the HPLC system.
1.3LC-TOFMSConditions
Quantitative analyses were performed using an 1 100 series HPLC (Agilent Technologies, USA) equipped with an online degasser, quaternary pump, autosampler, and column compartment. The TOF MS (6220 series, Agilent Technologies) was equipped with a standard electrospray ionization source and masshunter workstation B02.00.
Chromatographic separation was achieved under the following conditions. The separation column was Kromasil C18 (2.1 mm×100 mm×3.5 μm) maintained at 25 ℃. The mobile phase was composed of A (0.1% formic acid in water) and B (0.1% formic acid in methanol) with a solvent flow rate of 0.2 mL/min. The LC gradient condition was as follows: 0-4 min (85%-60%A), 4-30 min (60%-17%A). The injection volume was 1 μL with 10 min as the re-equilibration time among each run. Chromatograms of 29 mixed ingredients with internal standards were illustrated in Figure 1. The TOF MS conditions were set as follows:276 kPa nebulizer, 10 L/min drying gas flow rate, 350 ℃ drying gas temperature, 3 500 V capillary voltage, and 200 V fragmentor voltage. The MS spectra were acquired in full scan mode over a range ofm/z110-1 000 through an extended dynamic range in negative ion mode, and the mass axis was calibrated by the reference solutions includingm/z112.985 5 andm/z1 033.988 1.

Fig.1 BPC of standards (a) and Qinggansanjie extract (b) by LC-TOF MS in negative mode
1.4StatisticalAnalysis
To explore different extracting factors of ASE, PASW 18 Predictive Analytics Software 18 (PASW 18) was used to design the orthogonal experiment, evaluate the effects, and optimize the conditions. One-way analysis of variance (ANOVA) was utilized to evaluate significant differences (p<0.05) among parameters. Principal component analysis (PCA) was applied to investigate the variations within this large data set (29 chemical constituents in the QGSJ extract) by reducing the raw sample data size[17]. The equation of total principal component score was defined as:
Y=a1F1+a2F2+a3F3+…+aiFi
whereYis the total score,aiis the contribution coefficient of every principal component,Fiis the obtained principal component by PCA as noted below:
Fi=m1iZ1+m2iZ2+…+m29iZ29
wheremiis coefficient of variance, andZis the standardized content of 29 compounds in the QGSJ extract determined using PASW 18.
2 Results and Discussion
2.1OptimizationofASEConditions
An orthogonal experiment was employed to optimize the ASE conditions by choosing seven factors, including extraction solvent (A), extraction temperature (B), static extraction time (C), extraction cycle (D), extraction volume (E), flush volume (F), and nitrogen purge time (G). The above mentioned factors, corresponding levels, and orthogonal designs L18(37) were shown in Table 1. The mixed herbal powder (5 g) was extracted according to the orthogonal design conditions, and the combined extracts were dissolved in 100 mL of 70% ethanol. The internal standard solution (0.5 mL) and extraction solution (9.5 mL) were added to a 10 mL volumetric flask for LC-TOF MS analysis. Because of the complexity of the chemical constituents in QGSJ, which consists of triterpenoids, flavonoids, saponins, and other chemical components with similar structures and solubilities, PCA was applied to reduce dimensions of raw data. A total of 18 experiments were designed by the orthogonal method, as noted in Table 1.

Table 1 Factors in the orthogonal design for the optimization of extraction conditions
2.2StatisticalAnalysis
The results of the 29 variables and 18 samples obtained from PCA analysis showed that 4 principal components explained 89.77 of the total variance, component 1 (F1) explained 58.08, component 2 (F2) explained 18.63, component 3 (F3) explained 8.19, and component 4 (F4) explained 4.86. The last equation wasY=0.647F1+0.208F2+0.091F3+0.054F4,F1=0.034Z1+0.047Z2+0.231Z3+…+0.221Z29,F2=0.212Z1+0.360Z2-0.021Z3+…-0.153Z29,F3=0.231Z1-0.048Z2-0.11Z3+…-0.026Z29,F4=-0.515Z1+1.142Z2-0.041Z3+…+0.26Z29.
Figure 2 is the loading scatter plot from the first two PCs, which explained 76.71% of the total variance, indicating that variables far from the original point play an important role in the classification of various samples. The results showed that flavonoids, including wogonoside, scutellarin, baicalein, chlorogenic acid, and rosmarinic acid, are located in the upper right quadrant of the plot and are more related to the positive side of PC1 and PC2. In addition, triterpenoid acids, including oleanolic acid, ursolic acid, and asiatic acid, are located in the lower right quadrant and are highly oriented towards the positive side of PC1.
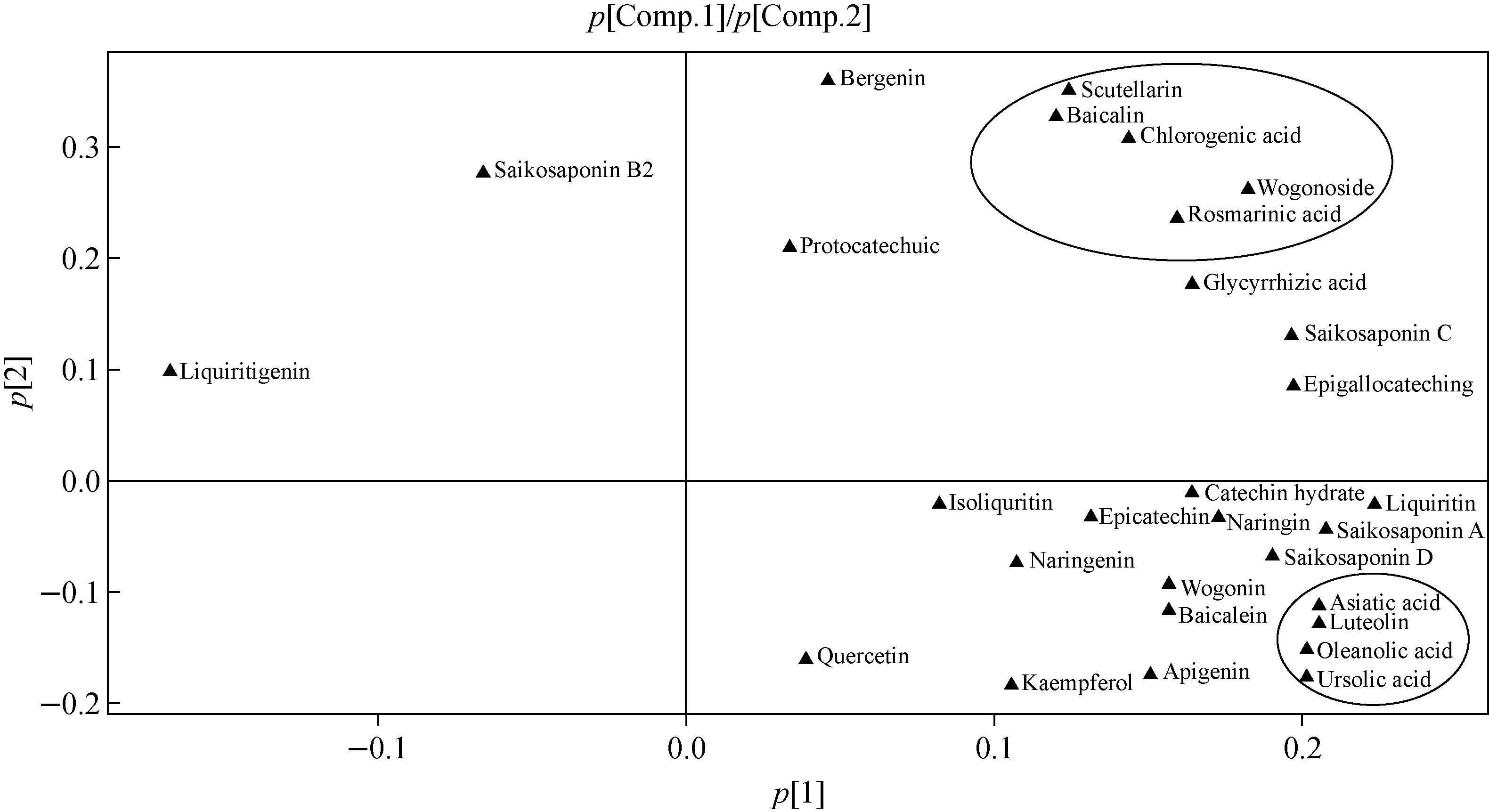
Fig.2 Loading scatter plot of the first two PCs
According to the statistical analysis from PASW software, factors A and B were the most significant (p<0.05) among the seven factors, followed by factors E and F; other factors had less influence. Considering the fact that extraction cycles 2 and 3 had no significant difference, the optimum extraction conditions were determined to be as follows: 70% ethanol as extraction solvent, 66 mL extraction volume, 5 min static extraction time, 100 ℃ extraction temperature, two extraction cycle, 60% flush volume, and 90 s nitrogen purge time.
2.3OptimizationoftheLC-TOFMSConditions
TOF MS does not have the ability to separate isomers in QGSJ such as catechin and epicatechin, liquiritin and isoliquiritin, apigenin and baicalein, luteolin and kaempferol, and oleanolic acid and ursolic acid. Thus, in order to efficiently separate these isomers, four different chromatographic columns were compared, including Agilent SB C18 (3.0 mm×100 mm×3.5 μm), Waters Xterra Rp-C18 (3.0 mm×100 mm×3.5 μm), MG C18 (3.0 mm×100 mm×3.0 μm), and Kromasil C18 (2.1 mm×100 mm×3.5 μm). Eventually, the Kromasil C18 column was selected to be the most suitable chromatographic column for separation of all isomers. Oleanolic acid and ursolic acid could achieve baseline separation after 50 min when the mobile phase was 0.1% formic acid in methanol and 0.1% formic acid in water with gradient elution.
MS signals from the target compounds determined in negative ion mode were better than those in the positive mode. Because oleanolic acid and ursolic acid were difficult to deprotonate under the electrospray ionization source, three operating parameters were optimized, including 276 kPa nebulizer gas, 10 L/min drying gas flow rate, and 200 V fragmentor voltage to ensure high responses from both oleanolic acid and ursolic acid.
2.4SelectionoftheInternalStandard
Because of the complex constitution, wide polarity distribution, and different MS responses from target compounds in the QGSJ decoction, there was a possibility that various known or unknown factors could interfere with the quantitative analysis. Therefore, selection of the internal standard was very important for eliminating interference and errors. Puerarin, ginsenoside Re, and ginsenoside Rb1had a good mass response in the negative ion mode, and their retention times were distributed in three time periods (0-12 min, 12-24 min, and 24-55 min), which were in exact accordance with the retention time of 29 analytes. Thus, they were chosen as standard compounds for our analysis.
2.5MethodValidation
2.5.1Linearity and Sensitivity Mixed standard solutions were diluted to the desired concentrations with methanol after adding 0.5 mL of the mixed internal standard solutions. The concentration ranges of the calibration curve were listed in Table 2. According to the LC-TOF MS above mentioned conditions, each calibration curve was constructed by running samples under different concentrations in triplicate, and the standard total ion spectrum was observed, as shown in Figure 1a. The standard curve was expressed asy=ax+b, wherexrepresents the concentration logarithm of baicalin, asiatic acid, oleanolic acid, and ursolic acid; andyrepresents the peak area ratio logarithm of the above standards to the corresponding internal standard. For other standards,xrepresents the concentration of these compounds, andyrepresents the peak area ratio of these compounds to the corresponding internal standard. Eventually, the correlation coefficientrwas used to evaluate the correlation of the standard curve, showing a good linear relationship (r>0.994) for the test compounds within the above concentration ranges. The limit of quantification (LOQ) was determined as the lowest concentration of the standard curve with a signal-to-noise (S/N) ratio>10, and the limit of detection (LOD) was defined as the concentration of each compound with a S/N ratio>3; the results are shown in Table 2.
2.5.2Precision The intra-day and inter-day precision were determined by testing the 29 quality control samples at high, median, and low concentration levels. Each sample was analyzed in triplicate on the same day for determination of intraday precision. Inter-day precision was calculated by testing each sample on three different validation days. The overall intra-day and inter-day variabilities were lower than 5% relative standard deviation (RSD) for all of the compounds.
2.5.3Reproducibility The reproducibility of the method was measured by running six samples, which were extracted six times using the same method. The RSD of reproducibility was less than 5% for all of the components.
2.5.4Stability The mixed herbal powder (5 g) was extracted and analyzed for stability according to the protocols in sections 2.2.3 and 2.3 at 0, 2, 4, 8, 12, 24 h. The RSD was less than 5%, indicating good stability within 24 h of extraction.
2.5.5Recovery The recoveries were performed by adding a known amount (80%, 100% and 120% of the original content) of all reference compounds into 2.5 g of the mixed herbal powder, after which the mixture was extracted and analyzed according to the procedure described in sections 2.2.3 and 2.3; this was repeated three times at every level. The recoveries using the developed analytical method ranged from 96.5% to 104.5% for the 29 compounds.

2.6SampleDetermination
The mixed herbal powder (5 g) was extracted and analyzed using the validated method, and the amounts of the 29 compounds in the extracted solution were calculated, as shown in table 3. The amounts of oleanolic acid, ursolic acid, and asiatic acid as triterpenoids are high (227.4 μg/g, 428.2 μg/g, and 148.2 μg/g, respectively) in monarch herbs includingActinidiaValvataDunn,SalviachinensisBenth, andHerbaHedyotidisDiffusae. Flavonoids like scutellarin (174.9 μg/g), baicalin (4 440 μg/g), wogonoside (1 161 μg/g), baicalein (669.0 μg/g), and wogonin (208.6 μg/g) are mainly fromPortulacagrandifloraHook andRadixScutellariaeBaicalensis. Triterpenoid saponins like saiko-saponin A (131.1 μg/g) and saikosaponin D (79.60 μg/g) are fromBupleurmchinenseDC. Other chemical constituents such as chlorogenic acid (174.6 μg/g) are fromHerbaSediSarmentosi, and bergenin (830.5 μg/g) is fromArdisiajaponicaBlume. Due to the small ratio ofRadixglycyrrhizaein the extract, the amounts of liquiritigenin (3.6 μg/g) and isoliquiritin (7.7 μg/g) are the smallest.

Table 3 Contents of 29 components in Qinggansanjie decoction determined by LC-TOF MS
LC-TOF MS has good resolving capability and high sensitivity for the simultaneous quantification of compounds in complex samples. A comparison between TOF MS and UV detection in the quantitative analysis of extracts was performed by Ref, and showed that TOF MS detection had better sensitivity. The mass response was also significantly influenced by a number of factors due to its higher sensitivity. However, the precision of TOF MS detection was inferior to that of UV detection, and ranged from 2% to 5% due to the low concentration of the analytes. Finally, the TOF MS detector showed excellent ion selectivity so that some unknown interference in the multicomponent detection could be avoided. Generally speaking, TOF MS detection provided an accurate and reliable method for the simultaneous quantitative analysis of the QGSJ decoction.
3 Concluding Remarks
In this study, a rapid and sensitive method was established by combining LC-TOF MS and ASE for the simultaneous determination of QGSJ components with high selectivity. In addition, seven ASE extracting conditions were optimized using an orthogonal design, and PCA was used to reduce large variations in the data. The validation process demonstrated a rapid and simple method with good linearity, accuracy, repeatability, selectivity, and recovery. Therefore, the method is helpful for the identification and quantification of the multiple components of TCM.
Thanks: We are grateful to Guo-rong Fan from Department of Pharmaceutical Analysis of Second Military Medical University for providing ASE instrument and technical support in this study.
Reference:
[1] LEE S C, TAN H T, CHUNG M C. Prognostic biomarkers for prediction of recurrence of hepatocellular carcinoma: current status and future prospects[J]. World Journal of Gastroenterol, 2014, 20(12) : 3 112-3 124.
[2] SOERJOMATARAM I, LORTET-TIEULENT J, PARKIN D M, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions[J]. Lancet, 2012, 380(9 856) : 1 840-1 850.
[3] PATEL M, SHARIFF M I, LADEP N G, et al. Hepatocellular carcinoma: diagnostics and screening[J]. Journal of Evaluation in Clinical Practice, 2012, 18(2) : 335-342.
[4] ZHANG F, QI P, XUE R, et al. Qualitative and quantitative analysis of the major constituents inAcorustatarinowiiSchott by HPLC/ESI-Q TOF-MS/MS[J]. Biomedical Chromatography, 2015, 29(6): 890-901.
[5] JIA W, WANG C, WANG Y, et al. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-Q-TOF MS[J]. Scientific World Journal, 2015, 2015(6): 731-765.
[6] CAO W, YE L H, CAO J, et al. Quantitative analysis of flavanones from citrus fruits by using mesoporous molecular sieve-based miniaturized solid phase extraction coupled to ultra high-performance liquid chromatography and quadrupole time-of-flight mass spectrometry[J]. Journal of Chromatography A, 2015, 1 406(7) : 68-77.
[7] LI Y W, QI J, WEN Z, et al. Determination and fingerprint analysis of steroidal saponins in roots ofLiriopemuscari(Decne.) L. H. Bailey by ultra high performance liquid chromatography coupled with ion trap time-of-flight mass spectrometry[J]. Journal of Separation Science, 2014, 37(14): 1 762-1 772.
[8] OTTONELLO G, FERRARI A, MAGI E. Determination of polychlorinated biphenyls in fish: optimisation and validation of a method based on accelerated solvent extraction and gas chromatography-mass spectrometry[J]. Food Chemistry, 2014, 142(1): 327-333.
[9] HUANG X, ZHAO X, LU X, et al. Simultaneous determination of 50 residual pesticides inFlosChrysanthemiusing accelerated solvent extraction and gas chromatography[J]. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 2014, 967(15): 1-7.
[10]TEN D G, PARDO O, TRAAG W, et al. Simultaneous extraction and determination of HBCD isomers and TBBPA by ASE and LC-MS/MS in fish[J]. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 2012, 898(898): 101-110.
[11]TAO Y, YU G, CHEN D, et al. Determination of 17 macrolide antibiotics and avermectins residues in meat with accelerated solvent extraction by liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 2012, 897(1): 64-71.
[12]CHITESCU C L, OOSTERINK E, DE J J, et al. Ultrasonic or accelerated solvent extraction followed by U-HPLC-high mass accuracy MS for screening of pharmaceuticals and fungicides in soil and plant samples[J]. Talanta, 2012, 88(1): 653-662.
[13]GAO F, HU Y, YE X, et al. Optimal extraction and fingerprint analysis ofCnidiifructusby accelerated solvent extraction and high performance liquid chromatographic analysis with photodiode array and mass spectrometry detections[J]. Food Chemistry, 2013, 141(3): 1 962-1 971.
[14]CHOI J H, LAMSHOFT M, ZUHLKE S, et al. Determination of sedatives and adrenergic blockers in blood meal using accelerated solvent extraction and Orbitrap mass spectrometry[J]. Journal of Chromatography A, 2012, 1 260(20): 111-119.
[15]CHEN J, WANG F, LIU J, et al. Analysis of alkaloids inCoptischinensisFranch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections[J]. Analytica Chimica Acta, 2008, 613(2): 184-195.
[16]CHEN J, LI W, YANG B, et al. Determination of four major saponins in the seeds ofAesculuschinensisBunge using accelerated solvent extraction followed by high-performance liquid chromatography and electrospray-time of flight mass spectrometry[J]. Analytica Chimica Acta, 2007, 596(2): 273-280.
[17]CAI M, CHEN Y, LIU X S, et al. Optimizing process of four-effect countercurrent extraction of danshensu fromSalviamiltiorrhiza[J]. China Journal of Chinese Materia Medica, 2006, 31(18): 1 499-1 502.
加速溶剂萃取法结合HPLC-ESI-TOFMS测定清肝散结汤中29种化学成分
王 慧1,李云青1,2,李 洋1,钱 跹1,柴逸峰3,张国庆1,吕 磊1,赵 亮1
(1.第二军医大学东方肝胆外科医院药材科,上海 200438;2.山西医科大学第二医院药材科,山西 太原 030000;3.第二军医大学药学院,上海 200433)
清肝散结汤(QGSJ)是由14味不同中药组成的复方制剂,基于长期的实验观察和临床经验以不同组合成方用于肝癌的治疗。本研究建立了一种基于加速溶剂萃取(ASE)结合高效液相色谱-电喷雾飞行时间质谱(HPLC-ESI-TOF MS)的方法同时测定清肝散结汤中29种化学成分。使用70%乙醇作为提取溶剂,提取温度100 ℃,静态萃取时间5 min,提取2次,以上条件采用正交设计和主成分分析进行优化。HPLC分离采用Kromasil C18色谱柱,梯度洗脱。TOF MS在负离子模式下检测,质量扫描范围m/z100~1 100。结果表明:清肝散结汤中29种成分具有良好的线性关系(r>0.994)和日内、日间精密度(RSD<5%);提取回收率在96.5%~104.5%之间。该方法快速、可靠,适用于复方中药的定量评价。
加速溶剂萃取(ASE);高效液相色谱-电喷雾飞行时间质谱(HPLC-ESI-TOF MS);清肝散结汤(QGSJ);同时测定
Date:2016-11-14;Accepted Date:2017-03-08
Funding Support:National Natural Science Foundation of China-Study on toxic constituents and poisoning mechanism of Langdu(81303300)
WANG Hui (1978—), female (Han nationality), associate chief pharmacist, contributed in researches on LC-TOF MS techniques in TCM. E-mail: wang_ehbh@126.com
ZHAO Liang(1980—), male (Han nationality), associate chief pharmacist, contributed in researches on quality control of TCM. E-mail: zhaoliangphar@163.com
LV Lei (1984—), male (Han nationality), pharmacist in charge, contributed in researches on LC-TOF MS techniques in TCM. E-mail:k_owen2002@126.com
O657.63Documentcode: AArticleIC: 1004-2997(2017)04-0503-12
10.7538/zpxb.2016.0181

