OTUB1在90例卵巢黏液性肿瘤中的表达分析
朱 勤,陆铃慧,王懿琴
复旦大学附属妇产科医院病理科,上海 200011
OTUB1在90例卵巢黏液性肿瘤中的表达分析
朱 勤,陆铃慧,王懿琴
复旦大学附属妇产科医院病理科,上海 200011
背景与目的:卵巢黏液性肿瘤是卵巢上皮性肿瘤的主要亚型之一,其恶变机制目前尚未明确。泛素化是人体主要的翻译后修饰机制,并与肿瘤密切相关。去泛素化酶可通过逆转泛素化过程从而影响肿瘤的发生、发展。该研究旨在分析编码人体最主要的去泛素化酶的癌基因泛素醛结合物1(OTU deubiquitinase,ubiquitin aldehyde binding 1,OTUB1)在卵巢黏液性肿瘤中的蛋白表达及临床应用。方法:收集2010—2015年复旦大学附属妇产科医院病理科诊断的卵巢良性黏液性囊腺瘤、卵巢交界性黏液性肿瘤及卵巢原发性黏液性癌病例共90例,收集临床病理资料,完成OTUB1的免疫组织化学法表达实验并作统计分析。结果:90例卵巢黏液性肿瘤中,卵巢良性黏液性囊腺瘤有14例,卵巢交界性黏液性肿瘤有17例,卵巢原发性黏液性癌有59例。OTUB1在卵巢原发性黏液性癌的蛋白表达率及阳性程度显著高于卵巢良性黏液性囊腺瘤(P<0.01);OTUB1在卵巢交界性黏液性肿瘤中上皮内癌的阳性率显著高于肠型交界性黏液性肿瘤(P<0.01);随着FIGO分期增高,OTUB1的阳性率及阳性程度增加(P<0.05);OTUB1在输卵管有累及的患者中表达率及阳性程度均高于未累及者(P<0.05);OTUB1在子宫和大网膜累及的患者中阳性率及阳性程度均高于未累及者(P<0.05);OTUB1在有淋巴结转移的患者中表达率及阳性程度均高于无转移者(P<0.05)。结论:OTUB1在卵巢原发性黏液性癌中的蛋白表达水平明显高于卵巢良性黏液性囊腺瘤,并且和FIGO分期及肿瘤侵袭转移性高度相关。在临床病理中可将OTUB1作为卵巢黏液性肿瘤恶变与否的评价指标及肿瘤进展的辅助判别指标。
泛素醛结合物1;卵巢;黏液性肿瘤
卵巢上皮性肿瘤是女性生殖系统最主要的恶性肿瘤之一,以高侵袭性及转移性为主要表现,有研究显示,其5年生存率约37%[1-2]。卵巢原发性黏液性癌是卵巢上皮性恶性肿瘤的主要亚型之一,目前认为其可通过卵巢良性黏液性囊腺瘤逐步演变为卵巢交界性黏液性肿瘤到卵巢原发性黏液性癌[3]。因而如何早期鉴别黏液性肿瘤有无恶性转变具有临床预防意义。
泛素化是人体重要的蛋白翻译后修饰机制之一,并且已被证明参与肿瘤的发生、发展;去泛素化酶是人体内广泛存在的一类蛋白,可通过逆转泛素化过程来影响肿瘤的发生、发展[4]。泛素醛结合物1(OTU deubiquitinase,ubiquitin aldehyde binding 1,OTUB1)蛋白是人体最常见并且丰度最高的去泛素化酶,在肠癌[4]、前列腺癌[5]、乳腺癌[6]及卵巢癌[7]中均异常高表达。OTUB1主要是通过增强肿瘤细胞的侵袭性来发挥促癌功能[8],其是否能用于临床诊断与鉴别诊断卵巢良恶性黏液性肿瘤尚未见报道。
该研究旨在用免疫组织化学手段研究OTUB1在90例卵巢黏液性肿瘤中的蛋白表达率及阳性程度,来观察监控OTUB1是否可用于临床鉴别诊断及肿瘤进展的监测。
1 资料和方法
1.1 入组标准
共挑选2010—2015年于复旦大学附属妇产科医院进行手术治疗及术后病理诊断的90例患者,14例为卵巢良性黏液性囊腺癌,17例为卵巢交界性黏液性肿瘤,59例为卵巢原发性黏液性癌。所有病例均挑选肿瘤病灶最显著的蜡块包埋,并完成切片。
1.2 患者资料
90例患者的临床病史收集内容包括姓名、年龄、发病部位、肿瘤性质、肿瘤大小、累及范围(子宫、大网膜等)及有无淋巴结转移等。
1.3 方法
1.3.1 实验方法
90例患者的切片均在68.5 ℃烤箱内烘烤2 h,随后经过二甲苯、无水乙醇、95%乙醇、85%乙醇及75%乙醇脱蜡,经漂洗后用高压锅进行抗原修复(均匀喷气3 min),随后用兔抗人OTUB1抗体(购自美国Abcam公司)以稀释浓度1∶100温育过夜。第2天经PBS漂洗后采用EnVision二抗系统(购自美国Dako公司)温育30 min后显色,随后经75%乙醇、95%乙醇、无水乙醇及二甲苯透明并完成树胶封片。
1.3.2 观察指标
OTUB1以细胞质着色为阳性标准,仅有细胞膜阳性视为阴性。
1.3.3 诊断及判读标准
阳性评分为0分(无细胞质着色,无论细胞膜或细胞核是否着色,或极其微弱细胞质着色,其阳性区域小于20%)、1分(细胞质微弱着色,阳性区域20%~50%)、2分(细胞质着色温和到中等,阳性区域50%~75%)和3分(细胞质着色强,阳性区域大于75%)。所有免疫组织化学检测均有两名与本研究无关的病理医师评分。
1.4 统计学处理
采用SPSS 20.0软件完成对数据进行统计分析。数值变量以形式表示。分类变量统计采用χ2检验,有序分类变量检验采用Spearman相关性分析。P<0.05为差异有统计学意义。
2 结 果
2.1 OTUB1在不同性质的卵巢黏液性肿瘤中的蛋白表达率及表达特征
在14例卵巢良性黏液性囊腺瘤中,OTUB1表达0分有13例(92.9%),1分有1例(7.1%),以阴性为主要表现(图1A)。在17例卵巢交界性黏液性肿瘤中,12例为肠型,5例为上皮内癌,肠型交界性黏液性肿瘤OTUB1表达0分有2例(16.7%),1分有7例(58.3%),2分有3例(25.0%),以微弱的细胞质着色为主要表现,表达区域一般呈灶性,阳性比例一般低于20%(图1B);上皮内癌OTUB1表达2分有3例(60.0%),3分有2例(40.0%),以中等的细胞质着色为主要表现,表达区域一般呈50%至弥漫性(图1C)。在59例卵巢原发性黏液性癌中,OTUB1表达0分有6例(10.2%),1分有10例(17.0%),2分有29例(49.1%),3分有14例(23.7%),以中到强的细胞质着色为主要表现,表达区域一般呈弥漫性(图1D)。OTUB1在卵巢良性黏液性囊腺瘤、肠型交界性黏液性肿瘤、黏液性上皮内癌及卵巢原发性黏液性癌中的表达差异有统计学意义(P<0.01,图2)。
2.2 OTUB1表达与临床病理参数的关系
采用Spearman相关性分析发现,OTUB1表达与年龄、肿瘤大小无相关性,与FIGO分期显著相关(P<0.05)。详细分析发现,OTUB1表达与输卵管是否累及显著相关(P<0.05),与是否累及子宫、大网膜及是否有淋巴结转移显著相关(P<0.01,表1)。

图1 OTUB1表达评分标准:0分(A)、1分(B)、2分(C)、3分(D)Fig. 1 Scoring standard in expression of OTUB1: score 0 (A), score 1 (B), score 2 (C), score 3 (D)
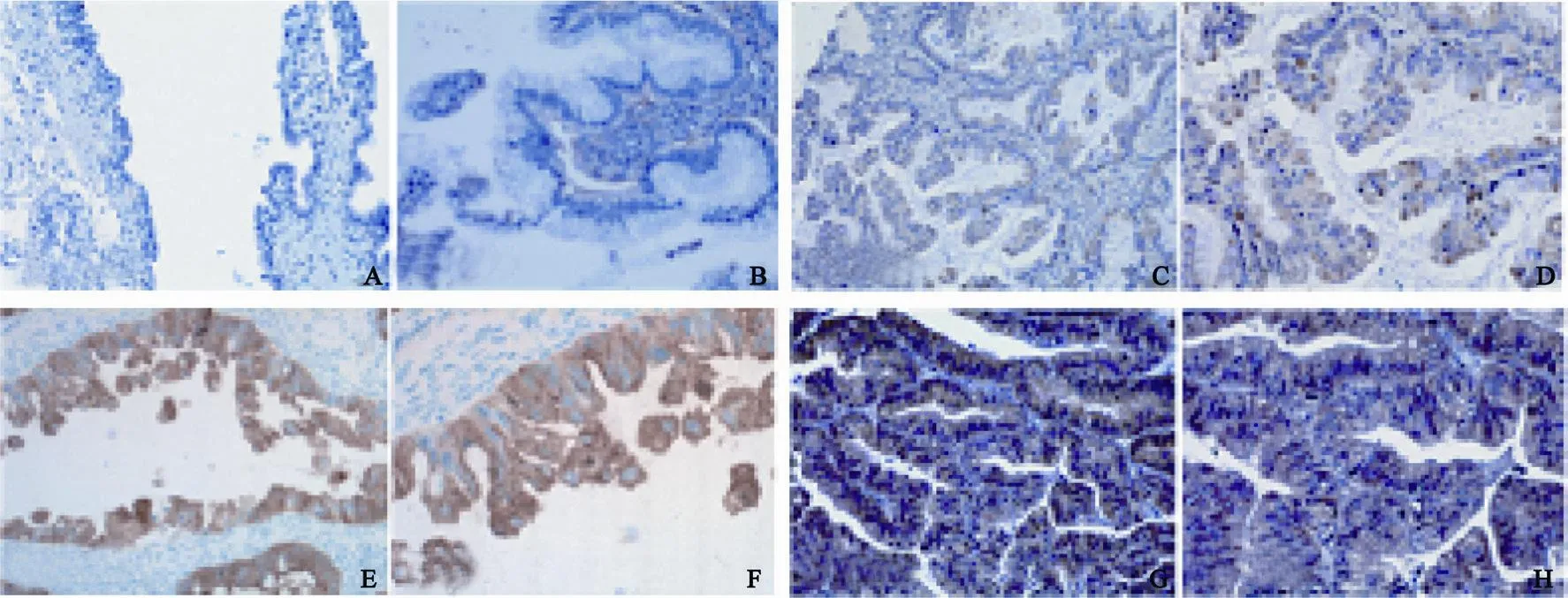
图2 OTUB1在卵巢不同的黏液性肿瘤中的表达Fig. 2 OTUB1 expresses in the di ff erent mucinous tumors of ovary
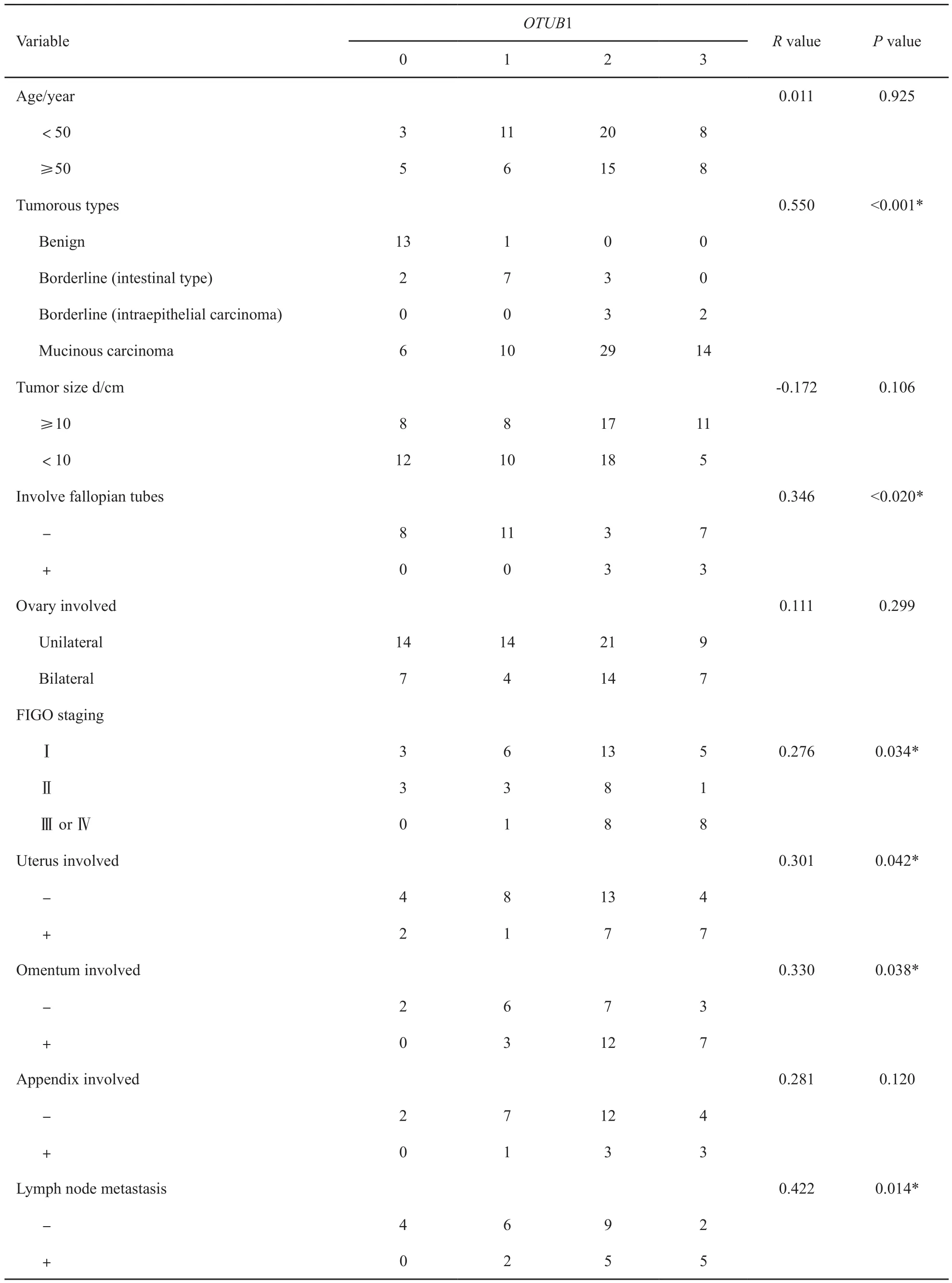
表1 OTUB1表达与卵巢原发性黏液性癌患者临床病理参数关联性分析Tab. 1 Correlation analysis between the expression of OTUB1 and clinicopathological parameters
3 讨 论
卵巢原发性黏液性癌是卵巢上皮性肿瘤的主要亚型之一[9-10]。监测肿瘤的发展对临床治疗具有重要意义,原因在于高期别黏液性癌侵袭性更强,其预后也差于早期黏液性癌[9]。本研究发现,OTUB1不仅在卵巢良性黏液性囊腺瘤和卵巢原发性黏液性癌中的表达差异有统计学意义,在肠型交界性黏液性肿瘤和上皮内癌中的表达差异也有统计学意义,提示其很可能是鉴别良、恶性卵巢黏液性肿瘤的良好指标,并可用于监测高危病例的疾病进展。从机制角度来说,由于在本研究中OTUB1与肿块大小的关系并不显著,但与侵袭相关的系列参数均显著相关,由此推测OTUB1对良、恶性黏液性肿瘤的表达差异是由于其编码基因OTUB1与肿瘤侵袭性的高度关联性。
OTUB1可通过去泛素化凋亡蛋白细胞内抑制物2[11]及信号转导蛋白SMAD3/4[12]激活肿瘤生长因子通路来参与肿瘤细胞的上皮-间质转化通路,提示OTUB1与肿瘤的侵袭转移相关。本研究显示,OTUB1的表达与肿瘤大小无关,但与肿瘤侵袭转移的各项临床参数包括双侧卵巢、输卵管、子宫、大网膜、阑尾累及以及淋巴结转移均显著相关。考虑到FIGO分期本身是基于卵巢肿瘤的侵袭累及范围和淋巴结转移状态[13],可以推测出OTUB1与卵巢原发性黏液性癌FIGO分期具有的密切相关性。我们的前期研究已经证实癌基因OTUB1是卵巢癌的重要预后指标[7]。同时,本研究也发现,OTUB1在卵巢原发性黏液性癌的病例中在累及输卵管、子宫或大网膜的侵袭性病灶处,OTUB1的表达往往呈中到强阳性,且表达面积更弥漫。统计分析也提示,OTUB1与侵袭相关的临床参数均显著相关,由此提示OTUB1在侵袭性更强的病灶处可能具有更强的表达且与肿瘤的侵袭转移性高度相关。
综上所述,OTUB1在卵巢黏液性肿瘤的诊断中具有临床应用价值。
[1] ANURADHA S, WEBB P M, BLOMFIELD P, et al. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study[J]. Med J Aust, 2014, 201(5): 283-288.
[2] CHEN W. Cancer statistics: updated cancer burden in China[J]. Chin J Cancer Res, 2015, 27(1): 1.
[3] WANG J, EL-BAHRAWY M A. Expression profile of mucins in ovarian mucinous tumors: distinguishing primary ovarian from metastatic tumors[J]. Int J Gynecol Pathol, 2014, 33(2): 166-175.
[4] LIU X, JIANG W N, WANG J G, et al. Colon cancer bears overexpression of OTUB1[J]. Pathol Res Pract, 2014, 210(11): 770-773.
[5] IGLESIAS-GATO D, CHUAN Y C, JIANG N, et al. OTUB1 de-ubiquitinating enzyme promotes prostate cancer cell invasion in vitro and tumorigenesis in vivo[J]. Mol Cancer, 2015, 14: 8.
[6] KARUNARATHNA U, KONGSEMA M, ZONA S, et al. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance[J]. Oncogene, 2016, 35(11): 1433-1444.
[7] WANG Y, ZHOU X, XU M, et al. OTUB1-catalyzed deubiquitination of FOXM1 facilitates tumor progression and predicts a poor prognosis in ovarian cancer[J]. Oncotarget, 2016, 7(24): 36681-36697.
[8] WENG W, ZHANG Q, XU M, et al. OTUB1 promotes tumor invasion and predicts a poor prognosis in gastric adenocarcinoma[J]. Am J Transl Res, 2016, 8(5): 2234-2244.
[9] LEDERMANN J A, LUVERO D, SHAFER A, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for mucinous ovarian carcinoma[J]. Int J Gynecol Cancer, 2014, 24(9 Suppl 3): 14-19.
[10] CHIANG Y C, CHEN C A, CHIANG C J, et al. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan[J]. J Gynecol Oncol, 2013, 24(4): 342-351.
[11] GONCHAROV T, NIESSEN K, DE ALMAGRO M C, et al. OTUB1 modulates c-IAP1 stability to regulate signalling pathways[J]. EMBO J, 2013, 32(8): 1103-1114.
[12] HERHAUS L, AL-SALIHI M, MACARTNEY T, et al. OTUB1 enhances TGFβ signalling by inhibiting the ubiquitylation and degradation of active SMAD2/3[J]. Nat Commun, 2013, 4: 2519.
[13] ZEPPERNICK F, MEINHOLD-HEERLEIN I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer[J]. Arch Gynecol Obstet, 2014, 290(5): 839-842.
Immunohistochemical analysis of OTUB1 in 90 cases of ovarian mucinous tumors
ZHU Qin, LU Linghui, WANG Yiqin (Department of Pathology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai 200011, China)
WANG Yiqin E-mail: yiqinwang11@icloud.com
Background and purpose: The ovarian mucinous tumor is one of the major subtypes of the ovarian epithelial cancer. Ubiquitination is one of the main post-translational modifications, which has proven to be involved in tumorigenicity. Deubiquitinase is the protein enzyme that could reverse the process of ubiquitination to affect the initiation and progression of malignancies. This study aimed to analyze the expression and clinical application of deubiquitinase OTUB1 (OTU deubiquitinase, ubiquitin aldehyde binding 1) in ovarian primary mucinous tumors. Methods: This study collected 90 cases of ovarian primary mucinous tumors during 2010-2015 in Obstetrics and Gynecology Hospital of Fudan University, and then collected the clinicopathological information and performed the immunochemistry. Results: Fourteen out of 90 cases were ovarian primary mucinous cystadenoma, 17 were borderline mucinous tumor (intestinal type and intraepithelial carcinoma), and 59 were ovarian primary mucinous carcinoma. The expression rate and intensity of OTUB1 were much higher in malignant cases than those in benign ones (P<0.05). The expression rate and intensity of OTUB1 were much higher in cases with mucinous intraepithelial carcinoma than those in cases with intestinal type borderline mucinous tumor (P<0.05). The expression rate and intensity of OTUB1increased with the advance of FIGO staging (P<0.05). The expression rate and intensity of OTUB1 were much higher in cases with involved fallopian tubes than those in cases without involved fallopian tubes (P<0.05). The expression rate and intensity of OTUB1 were much higher in cases with involved uterus and omentum than those in cases without involvement (P<0.05). The expression rate and intensity of OTUB1 were much higher in cases with lymph node metastasis than those in cases without involvement (P<0.05). Conclusion: There is significant difference in OTUB1 expression between ovarian primary mucinous carcinoma and benign mucinous cystadenoma. It is highly correlated to FIGO staging and invasion and metastasis of tumor. OTUB1 could be used in differential diagnosis and in monitoring the tumor initiation and progression in ovarian mucinous carcinoma.
OTU deubiquitinase, ubiquitin aldehyde binding 1; Ovary; Mucinous tumors
10.19401/j.cnki.1007-3639.2017.06.014
R737.31
A
1007-3639(2017)06-0482-05
2017-01-05
2017-03-20)
国家自然科学基金会青年项目(81602269)。
王懿琴 E-mail: yiqinwang11@icloud.com

