超声造影与增强螺旋CT诊断肝细胞癌的对比研究
时静祥,王毅军,经翔,王凤梅,丁建民,张翔,张勤
超声造影与增强螺旋CT诊断肝细胞癌的对比研究
时静祥,王毅军,经翔,王凤梅,丁建民,张翔,张勤
目的比较超声造影(CEUS)与增强螺旋CT(CECT)对肝硬化背景下肝细胞癌(HCC)的诊断效能。方法对207例241个乙肝肝硬化背景下肝脏局灶性病变进行CEUS和CECT检查,以病理结果为“金标准”,将两种检查方法的诊断结果与病理结果进行对比,诊断差异性评价采用McNemar检验,一致性评价采用Kappa检验。结果(1)病理结果显示,113个≤2 cm的病灶中,HCC病灶63个,良性病变50个。CEUS、CECT对比“金标准”McNemar检验的差别均无统计学意义(P分别为0.824、0.082),Kappa检验CEUS、CECT与“金标准”结果一致性一般(Kappa值分别为0.643、0.421);CEUS诊断HCC的敏感度、特异度、阳性预测值、阴性预测值和准确度均高于CECT。在显示动脉期血供方面,CEUS 增强显示率高于 CECT[87.30%(55/63)vs.69.84%(44/63),χ2=5.704,P=0.017]。(2)病理结果显示,128个>2 cm的病灶中,HCC病灶77个,良性病变51个。CEUS、CECT对比“金标准”McNemar检验的差别均无统计学意义(P分别为0.481、0.167),Kappa检验CEUS、CECT与“金标准”结果一致性一般(Kappa值分别为0.710、0.697);两者诊断HCC的敏感度、特异度、阳性预测值、阴性预测值和准确度差异不大。在显示动脉期血供方面,CEUS增强显示率与CECT差异无统计学意义[89.61%(69/77)vs.85.71%(66/77),χ2=0.540,P=0.462]。结论对于直径≤2 cm的HCC,CEUS诊断的效果优于CECT;对于直径>2 cm的HCC而言,两者的诊断能力是相似的。
癌,肝细胞;肝硬化;超声造影;增强螺旋CT
原发性肝细胞癌(hepatocellular carcinoma,HCC)是一种常见的恶性肿瘤,其特征是进展迅速、预后较差[1]。然而,许多HCC患者直到进展期才被成功诊断,而此时却丧失了根治性治疗的机会,因此,早期诊断对于HCC的成功治疗极为关键。
近年来,随着影像学技术的发展,许多具有HCC风险的患者得到规律的监测。超声造影(contrast-enhanced ultrasound,CEUS)是超声诊断领域的重要突破,在肝脏局灶性病变定性诊断中起着越来越重要的作用,其原理是通过使用微泡造影剂及相关的影像学技术来显示病灶血流及周围组织灌注信息,以此来区分病变的良恶性[2-3]。增强螺旋CT(contrast-enhanced helical computed tomography,CECT)被美国肝病协会推荐为HCC首选的无创性检查方法[4]。有研究显示,CEUS对于肝癌的诊断准确率高于CECT[5]。另有研究表明,两者对于肝癌的诊断能力是相似的[6]。本文对比分析了CEUS与CECT在HCC诊断中的应用价值。
1 对象与方法
1.1 研究对象 选取2013年1月—2015年9月我院常规B超检查不能完全明确诊断的肝占位病变患者207例,其中男 159例,女 48例,年龄 27~79岁,平均(56±10)岁。所有患者均确诊为乙肝肝硬化,在超声检查后均在我院接受了CEUS和CECT检查(两者间隔时间≤2周);207例共241个病灶均有病理学支持,53个病灶经手术病理确诊,188个病灶经穿刺病理证实(在B超引导下,采用18 G粗针,每个病灶穿刺2~3针),所有经穿刺病理证实的良性病变经过12个月随访排除恶性可能(随访每3个月行腹部超声检查,每6个月行CECT检查)。
1.2 检查方法
1.2.1 CEUS技术和方法 超声检查采用Philips iU22系统,使用多频探头C5-2,频率为2.0~5.0 MHz。先行常规腹部超声扫描,观察肝脏及病灶情况。选择病灶最清晰的切面行CEUS检查,此时团注造影剂SonoVue,使用时加入生理盐水5 mL,每次造影量2.4 mL,检查时使用低机械指数0.04~0.08,实时观察病灶内造影剂灌注情况及回声强度变化。观察不同病灶时间隔10 min行第2次造影。将造影过程分为3个时相:动脉期(为开始注射造影剂30 s内)、门脉期(31~120 s)和延迟期(121 s以后)。依据病灶在3个时相相对于肝实质的回声对比将增强程度分为高增强、等增强和低增强3种。CEUS以病灶动脉期呈高增强,门脉期或延迟期消退为低增强作为判断HCC的标准。
1.2.2 CECT检查技术和方法 CT扫描使用64排螺旋CT,层厚7 mm,造影剂为碘海醇,浓度为350 mgI/mL,总量按1.5 mL/kg体质量计算,经肘正中静脉高压单相注射,注射后25~35 s行动脉期扫描,60~70 s开始门脉期扫描,延迟期扫描时间为120 s以后。依据病灶在动脉期、门脉期及延迟期相对于肝实质的密度差值可将密度分为高密度、等密度和低密度3种。CECT诊断HCC的依据为动脉期病灶呈高密度、门脉期或延迟期呈低密度。
1.2.3 组织学检查 所有标本均使用4%甲醛固定,石蜡包埋,HE染色。最终的诊断由两位具有丰富经验的病理医师做出。诊断标准参照 International Working Party Criteria[7-8],对于HCC与高级别不典型增生结节的区分参考相关指南[9-10]。
1.3 统计学方法 采用SPSS 17.0软件进行统计分析。符合正态分布的计量资料以均数±标准差(±s)表示,计数资料以例(%)或频数表示;以病理结果为“金标准”,两种检查方法与金标准结果的差异性评价采用McNemar检验,一致性评价采用Kappa检验(Kappa≥0.75两者一致性较好,0.4<Kappa<0.75两者一致性一般,Kappa≤0.4两者一致性较差)。P<0.05为差异有统计学意义。
2 结果
2.1 病理结果 本组207例患者共241个病灶,病灶大小 0.9~6.5 cm,平均(2.8±1.3)cm,其中 113(46.89%)个病灶≤2 cm,128(53.11%)个病灶>2 cm。组织病理学检查结果显示,HCC病灶共140(58.09%)个,不典型增生结节12(4.98%)个,肝硬化再生结节78(32.37%)个,局灶性结节性增生(FNH)5(2.07%)个,血管瘤 6(2.49%)个。
2.2 对≤2 cm病灶的两种影像学对比分析 113个≤2 cm的病灶中,HCC病灶63个,良性病变50个。CEUS、CECT及病理结果见表1。CEUS对比“金标准”McNemar检验的差别无统计学意义,Kappa检验CEUS和“金标准”两种方法结果一致性一般;CECT对比“金标准”McNemar检验的差别无统计学意义,Kappa检验CECT和“金标准”两种方法结果一致性一般;CEUS诊断HCC的敏感度、特异度、阳性预测值、阴性预测值和准确度均高于CECT,见表2。在显示动脉期血供方面,63个HCC中CEUS动脉期显示高增强病灶55个(87.30%),CECT动脉期显示高密度病灶44个(69.84%),CEUS 增强显示率高于 CECT(χ2=5.704,P=0.017),见图1。
2.3 对>2 cm病灶的两种影像学对比分析 128个>2 cm的病灶中,HCC病灶77个,良性病变51个。CEUS、CECT及病理结果见表3。CEUS对比“金标准”McNemar检验的差别无统计学意义,Kappa检验CEUS和“金标准”两种方法结果一致性一般;CECT对比“金标准”McNemar检验的差别无统计学意义,Kappa检验CECT和“金标准”两种方法结果一致性一般;两者诊断HCC的敏感度、特异度、阳性预测值、阴性预测值和准确度差异不大,见表4。在显示动脉期血供方面,77个HCC病灶中CEUS动脉期显示高增强病灶69个(89.61%),CECT动脉期显示高密度病灶66个(85.71%),两者差异无统计学意义(χ2=0.540,P=0.462),见图 2。
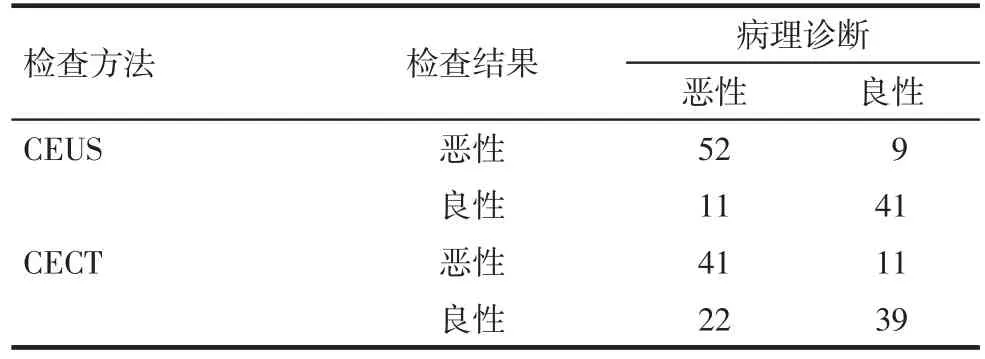
Tab.1 Pathological results of HCC(≤2 cm)detected by CEUS and CECT表1CEUS、CECT与病理结果(病灶≤2 cm)

Tab.2 Statistical results of HCC(≤2 cm)detected by CEUS and CECT compared with that of gold standard表2 CEUS、CECT与金标准的诊断试验评价(病灶≤2 cm)

Fig.1 Images of HCC in right posterior lobe(1.2 cm)detected by CEUS and CECT图1 肝右后叶1.2 cm HCC的CEUS及CECT表现
3 讨论
一般而言,肝硬化结节癌变的过程通常经历再生结节、不典型增生结节、HCC等几个阶段,在这一过程中伴随着新生动脉血管的增多,病灶供血也由以门静脉供血为主变为以肝动脉供血为主,这也是影像学诊断的基础[11-12]。但一部分小肝癌存在以下特点:首先,肿瘤内的动脉血管发育不完全;其次,肿瘤内肝窦血管化不完全;再次,肿瘤内肝窦与周围肝组织相延续,这就使得一部分动脉血流入肿瘤周围肝组织;以上特点使得此部分小肝癌在影像学上难以诊断[13]。本研究中CEUS诊断直径≤2 cm HCC的效果、动脉相增强显示率高于CECT,这与既往的研究结果一致[14-15]。可能原因如下:(1)超声造影为实时成像,可动态观察造影剂进入肝脏病变直至消退的完整过程,更容易捕捉到病灶造影过程中一些稍纵即逝的变化,如造影剂注射后极早期的变化;而增强CT为间歇扫描,在3个时相中分别选取固定时间进行扫描,不同病灶增强开始和持续时间差异很大,因而可能遗漏某些特征性变化;例如,造影剂注射后8~12 s的极早期常被CT遗漏,从而错失重要的诊断信息。(2)超声造影使用血池造影剂,造影剂不进入细胞外间隙,使微泡在血液中维持充足的时间来观察肝脏病灶血供的变化情况,尤其延迟相的变化情况;而增强CT使用非血池造影剂,造影剂通常会弥散到组织间隙,从而导致延迟相的相关信息被忽略[15-16]。

Tab.3 Pathological results of HCC>2 cm detected by CEUS and CECT表3CEUS、CECT与病理结果(病灶>2 cm)

Tab.4 Statistical results of HCC>2 cm detected by CEUS and CECT compared with that of gold standard表4CEUS、CECT与金标准的诊断试验评价(病灶>2 cm)

Fig.2 Images of HCC in right anterior lobe(2.8 cm)detected by CEUS and CECT图2 肝右前叶2.8 cm HCC的CEUS及CECT表现
本研究中CEUS与CECT诊断直径>2 cm HCC的效果及显示动脉期血供方面无明显差异,主要原因在于随着HCC的逐渐进展,肿瘤直径逐渐增加,病变的肝动脉供血也越发明显,影像学的表现也更典型,因此两种检查方法都能取得较好的诊断效果,这与以前的研究结果一致[16]。
当然,本研究为回顾性研究,在患者选择上难免存在偏倚,如本实验入组患者均为乙肝肝硬化,对于其他原因所形成的HCC需要进一步研究。而且CEUS本身也存在一定的局限性,如注射1次造影剂通常只能观察1个病灶,而CECT 1次可以显示肝脏所有病变情况等。
总之,对于乙肝肝硬化背景下直径≤2 cm的HCC,CEUS诊断的效能优于CECT,对于直径>2 cm的HCC而言,两者的诊断能力是相似的,因此CEUS是一种可靠的小肝癌早期诊断方法。
[1]Huang Y,Wang F,Wang Y,et al.Intrahepatic interleukin-17+T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma[J].J Gastroenterol Hepatol,2014,29(4):851-859.doi:10.1111/jgh.12418.
[2]Shin SK,Kim YS,Choi SJ,et al.Contrast-enhanced ultrasound for the differentiation of small atypical hepatocellular carcinomas from dysplastic nodules in cirrhosis[J].Dig Liver Dis,2015,47(9):775-782.doi:10.1016/j.dld.2015.05.001.
[3]刘建勇,周永和,李嘉,等.原发性肝癌病理分化程度与超声造影表现的关系[J].天津医药,2015,43(8):925-928.Liu JY,Zhou YH,Li J,et al.Correlation of histopathologic grading of hepatocellular carcinoma with its contrast-enhanced ultrasound[J].Tianjin Med J,2015,43(8):925-928.doi:10.11958/j.issn.0253-9896.2015.08.026.
[4]Bruix J,Sherman M.Management of hepatocellular carcinoma:an update[J].Hepatology,2011,53(3):1020-1022.doi:10.1002/hep.24199.
[5]李锐,张晓航,张萍,等.低机械指数超声造影与增强螺旋CT诊断≤2 cm肝细胞癌的比较研究[J].中华超声影像学杂志,2007,16(11):963-965.Li R,Zhang XH,Zhang P,et al.Diagnosis of small hepatocellular carcinoma:comparison of low mechanical index real-time contrast-enhanced ultrasonography with contrast-enhanced helical CT[J].Chin J Ultrasonogr,2007,16(11):963-965.
[6]经翔,刘艳丽,张翔,等.超声造影与增强螺旋CT诊断肝硬化背景下≤2 cm结节样病灶的比较研究[J].中华超声影像学杂志,2010,19(1):16-20.Jing X,Liu YL,Zhang X,et al.Diagnosis of small focal nodular lesions in patients with liver cirrhosis:comparison between contrast-enhanced ultrasound and contrastenhanced helical CT[J].Chin J Ultrasonogr,2010,19(1):16-20.doi:10.3760/cma.j.issn.1004-4477.2010.01.005.
[7]Iavarone M,Sangiovanni A,Forzenigo LV,et al.Diagnosis of hepatocellular carcinoma in cirrhosis by dynamic contrast imaging:the importance of tumor cell differentiation[J].Hepatology,2010,52(5):1723-1730.doi:10.1002/hep.23903.
[8]Cong WM,Bu H,Chen J,et al.Practice guidelines for the pathological diagnosis of primary liver cancer:2015 update[J].World J Gastroenterol,2016,22(42):9279-9287.doi:10.3748/wjg.v22.i42.9279.
[9]Di Martino M,Anzidei M,Zaccagna F,et al.Qualitative analysis of small(≤2 cm)regenerative nodules,dysplastic nodules and welldifferentiated HCCs with gadoxetic acid MRI[J].BMC Med Imaging,2016,16(1):62.doi:10.1186/s12880-016-0165-5.
[10]Roncalli M,Borzio M,Di Tommaso L.Hepatocellular dysplastic nodules[J].Ann Ital Chir,2008,79(2):81-89.
[11]Ojima H,Masugi Y,Tsujikawa H,et al.Early hepatocellular carcinoma with high-grade atypia in small vaguely nodular lesions[J].Cancer Sci,2016,107(4):543-550.doi:10.1111/cas.12893.
[12]Sciarra A,Di Tommaso L,Nakano M,et al.Morphophenotypic changes in human multistep hepatocarcinogenesis with translational implications[J].J Hepatol,2016,64(1):87-93.doi:10.1016/j.jhep.2015.08.031.
[13]Hayashi H,Nishigaki Y,Tomita E,et al.Usefulness of early vascular phase images from contrast-enhanced ultrasonography using Sonazoid for the diagnosis of hypovascular hepatocellular carcinoma[J].Hepatol Res,2016,46(6):497-504.doi:10.1111/hepr.12580.
[14]Liu JJ,Li HX,Chen ZB,et al.Consistency analysis of contrastenhanced ultrasound and contrast-enhanced CT in diagnosis of small hepatocellular carcinoma[J].Int J Clin Exp Med,2015,8(11):21466-21471.
[15]Trillaud H,Bruel JM,Valette PJ,et al.Characterization of focal liver lesions with SonoVue-enhanced sonography:international multicenter-study in comparison to CT and MRI[J].World J Gastroenterol,2009,15(30):3748-3756.doi:10.3748/wjg.15.3748.
[16]Li R,Guo Y,Hua X,et al.Characterization of focal liver lesions:comparison of pulse-inversion harmonic contrast-enhanced sonography with contrast-enhanced CT[J].J Clin Ultrasound,2007,35(3):109-117.doi:10.1002/jcu.20310.
(2017-04-05收稿 2017-04-17修回)
(本文编辑 陈丽洁)
Comparison of contrast-enhanced ultrasound and contrast-enhanced helical computed tomography in diagnosis of hepatocellular carcinoma
SHI Jing-xiang,WANG Yi-jun,JING Xiang,WANG Feng-mei,DING Jian-min,ZHANG Xiang,ZHANG Qin
Tianjin Third Central Hospital,Key Laboratory of Artificial Cell,Institute of Hepatobiliary Disease,Artificial Cell Engineering Technology Research Center of Public Health Ministry,Tianjin 300170,China
ObjectiveTo compare the diagnostic value of contrast-enhanced ultrasound(CEUS)and contrastenhanced helical computed tomography(CECT)for hepatocellular carcinoma(HCC)with liver cirrhosis.MethodsTwo hundreds and forty-one focal liver lesions in 207 patients with Hepatitis B virus(HBV)cirrhosis were detected with CEUS and CECT,respectively.Pathological results were used as"gold standard"to compare the two methods.Diagnostic results of the two methods were compared with pathological results.Differences were assessed using the McNemar test,and the Kappa test was used for consistency evaluation.Results(1)For 113 liver lesions that were≤2 cm,the number of HCC lesions was 63,and the number of benign lesions was 50.There were no significant differences in results of CEUS and CECT compared with that of the"gold standard"of McNemar test results(P=0.824,P=0.082).Consistency of the Kappa test results of CEUS and CECT in comparison with the"gold standard"was general(Kappa=0.643,Kappa=0.421).The sensitivity,specificity,positive predictive value,negative predictive value and accuracy of HCC diagnosed by CEUS were higher than those of CECT.The rate of arterial enhancement was better for CEUS[87.30%(55/63)]than that for CECT[69.84%(44/63),χ2=5.704,P=0.017].(2)For 128 liver lesions that were> 2 cm,the number of HCC lesions was 77,and the number of benign lesions was 51.There were no significant differences in the diagnostic results between McNemar test and CEUS and CECT tests(P=0.481,P=0.167).Consistency of the Kappa test results of CEUS and CECT and"gold standard"was general(Kappa=0.710,Kappa=0.697).The sensitivity,specificity,positive predictive value,negative predictive value and accuracy of HCC were not different between two diagnostic methods.The rate of arterial enhancement was 89.61%(69/77)for CEUS and 85.71%(66/77)for CECT,and there was no significant difference between the two groups(χ2=0.540,P=0.462).ConclusionFor HCC ≤ 2 cm,the diagnostic performance of CEUS is better than that of CECT.For HCC>2 cm,the diagnostic performance is similar for the two diagnostic methods.
carcinoma,hepatocellular;liver cirrhosis;contrast-enhanced ultrasound;contrast-enhanced helical CT
R445.1
:A
10.11958/20170410
天津市卫生局科技基金项目(2013KY03,2014KR04)
天津市第三中心医院,天津市人工细胞重点实验室,天津市肝胆疾病研究所,卫生部人工细胞工程技术研究中心(邮编300170)
时静祥(1982),男,主治医师,博士,主要从事肝癌诊断和治疗的研究

