菲并咪唑类有机电致发光材料的研究进展与应用前景
何 丹,刘 斌,佟庆笑
(汕头大学化学系及广东省有序结构材料的制备与应用重点实验室,汕头515063)
菲并咪唑类有机电致发光材料的研究进展与应用前景
何 丹,刘 斌,佟庆笑
(汕头大学化学系及广东省有序结构材料的制备与应用重点实验室,汕头515063)
菲并咪唑作为一类优秀的蓝光材料构筑基元,具有荧光量子产率高、热稳定性好,载流子注入与传输能力相对平衡,易修饰好合成等优点,在目前高效深蓝光荧光材料和磷光主体材料的设计上备受关注.本文综述了近几年来菲并咪唑类有机电致发光材料的发展现状,系统地介绍了菲并咪唑基团的结构特征以及各类衍生物的器件性能,展望了其在未来全彩显示和固态照明领域上的应用前景.
菲并咪唑;有机电致发光材料;蓝色荧光材料;磷光主体材料
有机电致发光器件(OLED)具有驱动电压低、响应速度快、视角宽、发光效率高、重量轻而薄、可柔性折叠等优点,被誉为最具前景的“梦幻显示器”[1-3].此外,OLED还可以用作全固态照明光源.与目前其它照明器件相比,其能效高、易实现平面白光、抗震能力强、使用温度范围广、光色柔和等优点,同时具有高效节能、环保友好、安全无害等优势,是一种非常理想、有前途的照明光源[4-7].发光材料是OLED技术的核心,高效且色纯度好的蓝光材料在实现高质量的白光照明和全彩显示中扮演着重要角色.一方面,蓝光材料可以充当能量转换器的作用,将能量传递给红光、绿光等低能量的发光材料,产生不同颜色的光[8-10].另一方面,蓝光材料可以增加色域,降低显示器件的能耗,尤其是色坐标CIEy<0.1的高效深蓝光材料,效果尤其明显[11-13].
经过二十年的发展,红光和绿光材料的研究已经相对成熟,相比之下,蓝光材料的发光性能和寿命都逊色很多,这是因为蓝光材料本身具有较宽的能隙,电荷注入能垒大,导致器件效率低,启动电压高等问题[14].目前报道的蓝光材料主要是基于蒽[15-19]、芴[20-22]、芳胺[23-24]、喹啉[25-27]、砜[28-29]等为构筑基元的衍生物.近几年来,菲并咪唑由于其高荧光量子产率,相对平衡的载流子传输性能,高的热力学稳定性,加上其合成方法简单、分离提纯容易等特点,在高效蓝色荧光材料和磷光主体材料的研究与应用上极具潜力[30-32].
1 菲并咪唑的结构特点
菲并咪唑在分子结构上主要由咪唑五元氮杂环、菲共轭单元以及N1位置取代的苯环组成.如图1所示,刚性的菲环平面,能够有效增加光吸收截面,提高荧光量子效率[33].研究发现菲并咪唑在四氢呋喃溶液(THF)中强紫光发射(~370 nm),发光效率达到70%,禁带宽度为3.40 eV,为典型的宽禁带材料;而咪唑环的非中心对称结构使其具有双极性.一方面,1号位氮原子的共轭模式为为富电子态,与吡咯中的氮原子类似;另一方面,3号位上的氮原子表现出缺电子态,与吡啶中的氮原子类似.这样特殊的结构,方便供电子和吸电子基团的修饰[34-36],在材料的设计上备受青睐[37-43].至于合成方面,芳香类菲并咪唑表现出很大的优势,它的合成可以通过“一锅煮”反应方便得到,产率高,可实现大规模生产[35,41-42].
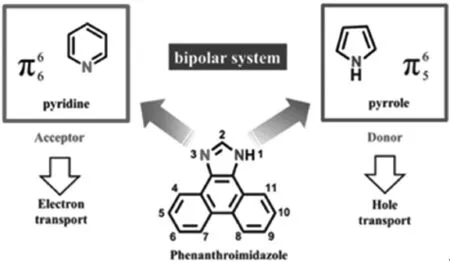
图1 菲并咪唑的结构
2 菲并咪唑类有机电致发光材料的性质与应用
在文献的调研和综述过程中,我们发现菲并咪唑类有机电致发光材料主要在荧光材料和主体材料上有着广泛的应用.其中,根据材料的结构设计特点,我们把菲并咪唑类荧光材料细分成双菲并咪唑类和单菲并咪唑类来逐一介绍.
2.1 双菲并咪唑类荧光材料
基于菲并咪唑本身的高效发光性质,Liu课题组[44]和Ma课题组[11]把两个菲并咪唑基团对位连接分别得到了结构相似的双菲并咪唑衍生物PPIP和BPPI.如图2所示,其中,基于PPIP的器件获得了最大电流效率(CEmax)7.47 cd A-1,最大外量子效率(EQEmax)6.31%和最大功率效率(PEmax)7.3 lm W-1,开启电压(Von)低于3 V,但效率滚降比较严重,色纯度有待提高(CIEy=0.14).Ma课题组发现分子BPPI虽发光高效,但载流子传输性能不够平衡,通过在N1位置的苯环修饰得到了氰基取代的菲并咪唑衍生物CN-BPPI[45],利用分子间的弱作用力,材料的热稳定性和发光量子产率明显提高(固态、液态都约为1),电子和空穴的注入和传输性能更加平衡,光电性能进一步得到完善.为了改善分子BPPI的色纯度,Wang等人[46]把对位连接的联苯变成间位方式,得到一对二聚菲并咪唑的同分异构体Z-BPPI和L-BPPI.分子均在深蓝区发光,CIE色坐标分别为(0.16,0.10)和(0.16,0.11).但由于分子的高度扭曲,导致聚集态结构堆积不紧密,难以形成长程的载流子传输通道,一定程度上降低了器件的性能.

图2 双菲并咪唑衍生物PPIP、TPIP、APIP、BPPI、CN-BPPI、Z-BPPI和L-BPPI
台湾的Cheng课题组[47]在联苯之间引入双键获得了两种n-型客体发光材料PPIE和TPIE.如图3所示,双键的引入使分子扭曲增大,光谱蓝移.其中以PPIE为客体的掺杂器件的CEmax为10.4 cd A-1,EQEmax为7.9%,CIE(0.14,0.15),是目前报道的菲并咪唑类蓝色荧光材料中最高器件效率.我们课题组[38,48]在联苯之间引入苯环和萘环分别得到了两系列深蓝发光分子BBTPI和XBTPI,NBTPI和2NBTPI.BBTPI采取错位平行的堆积方式促进了分子间的载流子交流作用,基于它的非掺杂器件的CEmax和EQEmax分别达到5.48 cd A-1和5.77%,CIE(0.15,0.10).而XBTPI分子的色饱和度更好,CIE(0.16,0.05),但EQEmax只有4.93%,这可能是聚集态下XBTPI分子间未能形成有效的载流子交流通道,致使载流子迁移性能下降.而2NBTPI分子由于空间位阻大的萘环使分子的扭曲程度加重,光谱蓝移到(0.15,0.09),EQEmax达到了5.95%,而且效率滚降非常小,在1000 cd m-2的亮度下,外量子效率依然保持着5.6%.
间位连接模型是提高分子色纯度的有效策略,我们以苯环和吡啶环为桥连分别得到两个间位相连的双菲并咪唑衍生物MBBTPI和2,6-BTPIPy[49-50].间位方式成功地限制了分子的共轭长度,光谱蓝移到深蓝区,基于MBBTPI的器件的CIE色坐标为(0.16,0.05),CEmax达到了1.99 cd A-1.而2,6-BTPIPy分子不仅深蓝发光,吡啶环的拉电子作用使分子具有优秀的电子传输能力,以它为发光层和电子传输层的非掺杂双层器件的EQEmax达到了4.26%,CIE色坐标为(0.15,0.10);可以媲美大多数对位连接的高效蓝光材料. Wang等人[51]为进一步提高分子的色纯度,采取双间位的连接方式合成了分子MM-BPPI 和MM-CNBPPI,分子均在蓝紫光区发光,相应的CIE色坐标分别为(0.158,0.060)和(0.156,0.050),但大幅度的扭曲造成器件性能不够理想.
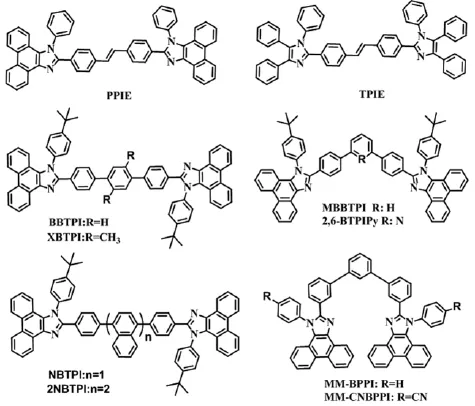
图3 双菲并咪唑衍生物PPIE和TPIE、BBTPI、XBTPI、NBTPI、2NBTPI、MBBTPI、2,6-BTPIPy、MM-BPPI和MM-CNBPPI
菲并咪唑的N1和C2号位置容易修饰,且有着不同的发光特性,Wang等人[52]结合实验和理论计算对比BPPI和N-BPPI分子的发光性质(图4),发现BPPI相邻的高能级激子能垒较大,难以转化成单线态激子辐射发光;而N-BPPI虽发光效率低,但由于较强的轨道耦合作用,激子利用率高.我们课题组巧妙结合两个位置的特点,合成了两个非对称型双菲并咪唑衍生物4NPI-BP-4PI和3NPI-BP-4P[53].它们的非掺杂器件的EQEmax分别为5.56%和4.95%,CIE色坐标为(0.15,0.08)和(0.15,0.06),激子生成率高达31%,突破了传统荧光材料单线态激子生成率25%的理论极限.Ma课题组以砜为受体,菲并咪唑为供体得到了一个局域态(LE)和电荷转移态(CT)杂化(HLCT)的蓝光分子PMSO[54],利用CT激子的弱束缚能力,发生CT激子的返转,使得基于它的掺杂器件高效深蓝发光,CIE色坐标为(0.152,0.077)且EQEmax高达6.8%.
2.2 单菲并咪唑类荧光材料
在菲并咪唑基团的C2和N1位置引入不同的功能基团是构筑菲并咪唑衍生物的常用方法.我们课题组合成了一系列以芳胺为给体的菲并咪唑衍生物TPA-BPI、TPA-TPI 和PATPA[30].如图5所示,在N1位置引入的三苯胺对分子的能级几乎没有影响,且器件的启动电压高,电致发光效率低劣,而噻吩桥连虽有利于空穴的传输,但光谱偏离蓝光区.而在C2位置引入苯胺的TPA-BPI发光高效,器件性能突出,CEmax、PEmax和EQEmax分别为2.63 cd A-1,2.53 lm W-1和3.08%,CIE色坐标为(0.15,0.09).为了抑制分子的堆积作用和整体的共轭性,我们在菲并咪唑的C2位置引入树枝状的Müllen基团得到了一系列蓝紫光到天蓝光发射的分子TTP-TPI、DPT-TPI和DPF-TPI[37].基于它们的器件的启动电压低(Von≤3V),效率高效、稳定.其中基于TTP-TPI的器件的CEmax和EQEmax分别为2.1 cd A-1和5.02%,CIE(0.16,0.05).

图4 双菲并咪唑衍生物NBPPI、4NPI-BP-4PI、3NPI-BP-4P、2,6-BTPIPy和PMSO

图5 菲并咪唑衍生物TPA-BPI、TPA-TPI、PATPA、TTP-TPI、DPT-TPI和DPF-TPI
利用CT态是构建高效发光材料的有效途径,Ma课题组对菲并咪唑-给体基团类材料的CT态性质及其对电致发光性能的影响进行了深入的研究.如图6所示,结合理论计算和实验设计发现TPA-PPI分子中的高能态CT激子通过“热激子”的过程,转变成单线态激子辐射跃迁,从而成功实现效率的突破,利用该材料作为发光层的非掺杂器件的EQEmax、和CEmax分别为5.02%和5.66 cd A-1,CIE(015,0.11),材料的激子利用率超过荧光材料自旋统计25%的限制[32].随后,为了改善器件的色纯度,他们进一步成功得到了扭曲型的HLCT分子TPA-PIM和mTPA-PPI[55-56].材料均在蓝紫区发光,器件性能出色.其中TPA-PIM的电致发光光谱的半峰宽只有35 nm,色坐标为(0.161,0.046),EQEmax为3.0%;而mTPA-PPI分子的EQEmax达到了3.33%,色坐标为(0.161,0.049).通过在N1位置引入强吸电子基团氰基后,他们发现TBPMCN分子是一个CT和LE杂化准等价的黄金HLCT分子,即光致发光效率和电致发光效率兼优,基于它的非掺杂器件的EQEmax高达7.8%,CEmax为10.5 cd A-1,CIE色坐标为(0.16,0.16)[57-58].

图6 D-A型菲并咪唑衍生物TPA-PPI、TPA-PIM、mTPA-PPI、TPM、TPMCN和TBPMCN
华中科技大学的Wang课题组[59-60]相继在菲并咪唑的C2和N1位引入芳基蒽得到了一对同分异构体2-NaCPI和2-NaNPI.如图7所示,研究发现空间位阻较大的蒽能够有效打断分子的共轭,使分子在深蓝区发光.Li等人[61]同时在C2和N1位连接芳基蒽合成了高扭曲结构的分子DPA-PPI,分子的HOMO和LUMO轨道完全分离,具有双极性,以它为发光层的掺杂器件的色坐标蓝移为(0.15,0.06),EQEmax为5.0%.最近,吉林大学的Wang课题组[62]将芳胺基、蒽基和菲并咪唑的优点结合在一起合成了两个高固体发光量子产率的绿光材料DPPA-PPI和tBuDPPA-PPI.叔丁基有效地抑制了分子的堆积效,tBuDPPA-PPI分子的发光位置蓝移,而基于DPPA-PPI的非掺杂绿光器件性能突出,最大发光亮度高达100 290 cd m-2,CEmax、PEmax和EQEmax分别为14.3 cd A-1、13.9 lm W-1和5.02%.
Lu课题组[63]利用芳基硅的四面体结构来限制了分子的共轭和ICT作用合成了一个高效的蓝紫光分子SiPIM.基于它的蒸镀薄膜器件的色坐标为(0.163,0.040),CEmax和EQEmax分别为1.94 cd A-1和6.29%,是目前报道的效率最高的蓝紫光器件;同时,SiPIM具有良好的溶解性和成膜性,基于它的溶液加工型器件的色坐标依然保持在(0.157,0.041).后来,他们以芳基硅为桥连合成了光学和电学能隙相分离的宽带隙发光材料DCzSiPI[64].研究发现,材料具有优秀的载流子传输能力,基于它的非掺杂器件的EQEmax为3.5%,激子利用效率高达61%.
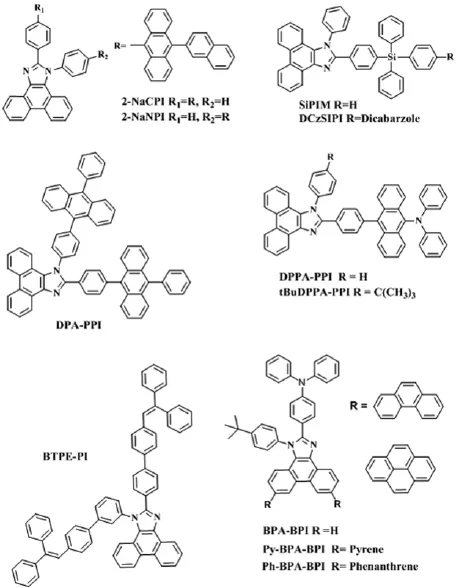
图7 菲并咪唑衍生物SiPIM、DCzSiPI、1-NaCPI、BTPE-PI、DPA-PPI、DPPA-PPI、BuDPPA-PPI、Ph-BPA-BPI和PyBPA-BPI
聚集诱导荧光猝灭现象(ACQ)是造成材料光谱红移和发光效率低的重要原因.Tang课题组[14]把具有聚集诱导荧光增强(AIE)的作用三苯基乙烯基团同时引入到菲并咪唑的N1和C2位置,成功得到了AIE活化的深蓝光材料BTPE-PI,以其为发光层的非掺杂器件的效率分别为4.9 cd A-1,4.4 lm W-1和4.0%,色坐标为(0.15,0.12);作为掺杂器件的发光层也实现了正白光发射,CEmax和EQEmax分别为10.7 cd A-1和6.4%,色坐标为(0.33,0.33).最近,我们课题组首次在菲并咪唑的6号和9号位引入刚性的菲和芘基团,分别得到了深蓝光发射的Ph-BPA-BPI分子和天蓝光发射的Py-BPA-BPI分子[65].分子表现出HLCT特性,基于它们的非掺杂蓝光器件的激子利用率分别为36.2%和39.2%.其中,基于Py-BPA-BPI的非掺杂器件的CEmax、PEmax和EQEmax分别为10.9 cd A-1、10.5 lm W-1和5.64%,CIE色坐标为(0.17,0.29).
2.3 菲并咪唑类磷光主体材料
菲并咪唑是典型的宽带隙材料(~3.4eV),有着较高的三线态能级(ET),因此在主体材料的设计上引起了广泛的关注.Yang等人将高ET的咔唑基团与菲并咪唑相连,改变它们的连接方式合成了一系列双极性主体材料pPhBICP、mPhBICP、pPhBINCP和mPhBINCP[41].如图8所示,材料具有较高的ET(>2.5 eV).其中,以mPhBINCP为主体材料的绿光器件的CEmax为77.6 cd A-1,PEmax为80.3 lm W-1,EQEmax高达21.0%.随后,他们课题组在N1和C2位置同时引入咔唑并改变它们之间的连接方式,得到了一系列热稳性和成膜性能更好的双极性绿光主体材料PhBIDpCP、PhBIDpmCP、PhBIDmpCP 和PhBIDmCP[42].其中PhBIDmpCP分子的载流子平衡,以它为主体的绿光器件效率也达到了74.3 cd A-1和74.4 lm W-1.

图8 基于菲并咪唑的主体材料pPhBICP、mPhBICP、pPhBINCP、mPhBINCP、PhBIDpCP、PhBIDpmCP、PhBIDmpCP和PhBIDmCP.
吉林大学的Wang等人利用咪唑环氮原子的配位作用合成了两个新颖的双功能菲并咪唑金属衍生物Be(PPI)2和Zn(PPI)2[66].如图9所示,两个金属配合物具有刚性的扭曲结构,材料高效深蓝发光且具有较高的ET.以Zn(PPI)2为发光层的非掺杂器件的CEmax为2.52 cd A-1,EQEmax为2.82%,色坐标为(0.15,0.09);作为主体材料的绿光器件的CEmax和PEmax也达到了58.0 cd A-1和67.5 lm W-1.后来,他们把膦氧基团和高扭曲的芳基硅引入菲并咪唑当中,分别得到了一系列的双功能材料DPO-PPI、DPO-2PPI和Si (PPI)2[40,67].膦氧基团的极化作用可以降低载流子的注入势垒,促进载流子的注入和传输,基于DPO-2PPI的红、绿光器件的PEmax分别达到了21.3 lmW-1和73.3 lmW-1;而四面体结构的芳基硅有效地抑制了分子的ICT作用和分子的整体共轭,材料具有双极性和较高的ET,基于Si(PPI)2的绿光器件的PEmax为51.1 lmW-1,EQEmax为19.2%.
最近,他们在N1位置引入菲并咪唑基团,在C2位置引入芳胺基团合成了两个较小的单、三线态能级差的双功能材料PPI-PPITPA和PPI-PPIPCZ[68],分子的前沿轨道分离,形成双载流子通道且具有较高的ET.基于PPI-PPITPA分子的非掺杂器件的CEmax、PEmax和EQEmax分别为5.6 cd A-1,5.5 lmW-1和7.7%,CIE色坐标为(0.15,0.08);而PPI-PPIPCZ分子的器件性能更出色,EQEmax达到了8.1%,CIE(0.15,0.07),是目前非掺杂深蓝光器件中表现最突出的.作为主体材料,器件的性能同样突出.其中,最好的黄光器件的CEmax、PEmax和EQEmax分别为61.8 cd A-1,65.5 lm W-1和19.1%,红光和绿光器件的EQEmax分别为18.0%和17.8%;充分展现了材料的多功能性和实用性.
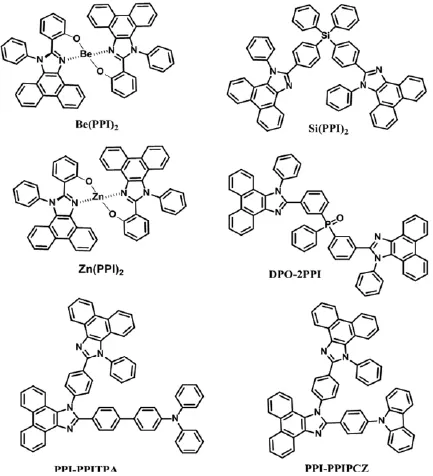
图9 基于菲并咪唑的主体材料Be(PPI)2、Zn(PPI)2、Si(PPI)2、DPO-2PPI、PPI-PPITPA、PPI-PPIPCZ和PhBPI.
Su课题组报道了两个硫杂化三苯胺基团为供体的菲并咪唑衍生物PPI-TPA-SO2-1和PPI-TPA-SO2-2[69].如图10所示,与分子PPI-TPA相比,它们拥有更高的ET(>2.45 eV).其中,基于PPI-TPA-SO2-2分子的F/P型白光器件的CEmax、PEmax和EQEmax分别为47.6 cd A-1、53.4 lm W-1和15.6%.随后,他们在TPA-PPI的桥连上引入苯环得到同样具有HLCT特性和高ET的PhBPI分子[70].研究发现:高能级的三线态激子可以通过激子逆转的过程一方面增加材料的单线态激子生成率,另一方面巧妙地解决了三线态激子湮灭的难题,基于分子的单发光层F/P型白光器件的CEmax为51 cd A-1,PEmax为56.6 lm W-1,EQEmax为21.9%,是目前报道的最好的单发光层F/P型白光器件.最近,我们课题组利用芴基9号位的sp3杂化碳原子的间隔作用,成功合成了载流子传输性能平衡和ET较高的双极性分子PPI-F-TPA[71].以它为发光层的非掺杂蓝光器的CIE色坐标为(0.16,0.05),EQEmax高达3.11%.同时作为橘红光的主体材料的器件效率分别为27 cd A-1,28.3 lm W-1和12.5%,而且在高亮度10 000 cd m-2下,器件的效率衰减率仅为13.6%,是目前报道的橘红光或红光器件中衰减率最小之一.

图10 基于菲并咪唑的主体材料PPI-TPA-SO2-1、PPI-TPA-SO2-2、PhBPI和PPI-F-TPA
3 结语
综上可知,发展高效的有机电致发光材料具有重要的理论意义和实用价值,菲并咪唑因其独有的特点在目前高效深蓝光荧光材料和磷光主体材料的设计上具有明显的优势.随着OLED技术的进步和材料的发展,相信菲并咪唑类材料在未来的全彩显示和白光照明领域上有着更大的发展潜力和应用前景.
[1]黄剑,曹镛.有机电致发光材料研究进展[J].化工新型材料,2001,29(9):10-15.
[2]陈金鑫,黄孝文.OLED梦幻显示器——材料与器件[J].北京:人民邮电出版社,2011:201.
[3]黄春辉,李富友,黄维.有机电致发光材料与器件导论[M].上海:复旦大学出版社,2005.
[4]MA S,FU Y,NI D,et al.Spiro-fluorene based 3D donor towards efficient organic photovoltaics[J]. Chemical Communications,2012,48(97):11847-11849.
[5]WANG N,YU J,ZHENG Y,et al.Organic photovoltaic cells based on a medium-bandgap phosphorescent material and C60[J].The Journal of Physical Chemistry C,2012,116(9):5887-5891.
[6]MURAWSKI C,LEO K,GATHER M C.Efficiency roll-off in organic light-emitting diodes[J]. Advanced Materials,2013,25(47):6801-6827.
[7]FLEETHAM T,ECTON J,WANG Z,et al.Single-doped white organic light-emitting device with an external quantum efficiency over 20%[J].Advanced Materials,2013,25(18):2573-2576.
[8]BARTELS L.Tailoring molecular layers at metal surfaces[J].Nature Chemistry,2010,2(2):87-95.
[9]YOKOYAMA T,YOKOYAMA S,KAMIKADO T,et al.Selective assembly on a surface of supramolecular aggregates with controlled size and shape[J].Nature,2001,413(6856):619-621.
[10]PAWIN G,WONG K L,KWON K Y,et al.A homomolecular porous network at a Cu(Ⅲ)surface [J].Science,2006,313(5789):961-962.
[11]WANG Z,LU P,CHEN S,et al.Phenanthro[9,10-d]imidazole as a new building block for blue light emitting materials[J].Journal of Materials Chemistry,2011,21(14):5451-5456.
[12]LAMPERT M A,MARK P.Current injection in solids[M].New York:Academic Press,1970.
[13]CHU T Y,SONG O K.Hole mobility of N,N′-bis(naphthalen-1-yl)-N,N′-bis(phenyl)benzidine investigated by using space-charge-limited currents[J].Applied Physics Letters,2007,90(20):203512-203512.
[14]QIN W,YANG Z,JIANG Y,et al.Construction of efficient deep blue aggregation-induced emission luminogen from triphenylethene for nondoped organic light-emitting diodes[J].Chemistry of Materials,2015,27(11):3892-3901.
[15]SHIH P I,CHUANG C Y,CHIEN C H,et al.Highly efficient non-doped blue-light-emitting diodes based on an anthrancene derivative end-capped with tetraphenylethylene groups[J].Advanced Functional Materials,2007,17(16):3141-3146.
[16]CHIEN C H,CHEN C K,HSU F M,et al.Multifunctional deep-blue emitter comprising an anthracene core and terminal triphenylphosphine oxide groups[J].Advanced Functional Materials,2009,19(4):560-566.
[17]HU J Y,PU Y J,SATOH F,et al.Bisanthracene-based donor-acceptor-type light-emitting dopants:highly efficient deep-blue emission in organic light-emitting devices[J].Advanced Functional Materials,2014,24(14):2064-2071.
[18]KIM R,LEE S,KIM K H,et al.Extremely deep blue and highly efficient non-doped organic light emitting diodes using an asymmetric anthracene derivative with a xylene unit[J].Chemical Communications,2013,49(41):4664-4666.
[19]ZHANG T,LIU D,WANG Q,et al.Deep-blue and white organic light-emitting diodes based on novel fluorene-cored derivatives with naphthylanthracene endcaps[J].Journal of Materials Chemistry,2011,21(34):12969-12976.
[20]WONG K T,CHIEN Y Y,CHEN R T,et al.Ter(9,9-diarylfluorene)s:highly efficient blue emitter with promising electrochemical and thermal stability[J].Journal of the American Chemical Society,2002,124(39):11576-11577.
[21]TAO S L,PENG Z K,ZHANG X H,et al.Highly efficient non-doped blue organic light-emitting diodes based on fluorene derivatives with high thermal stability[J].Advanced Functional Materials,2005,15(10):1716-1721.
[22]ZOUY,ZOUJ,YET,et al.Unexpected propeller-like hexakis(fluoren-2-yl)benzene cores forsix-arm star-shaped oligofluorenes:highly efficient deep-blue fluorescent emitters and good hole-transporting materials[J].Advanced Functional Materials,2013,23(14):1781-1788.
[23]TONG Q X,LAI S L,CHAN M Y,et al.Highly efficient blue organic light-emitting device based on a nondoped electroluminescent material[J].Chemistry of Materials,2008,20(20):6310-6312.
[24]LIN S L,CHAN L H,LEE R H,et al.Highly efficient carbazole- -dimesitylborane bipolar fluorophores for nondoped blue organic light-emitting diodes[J].Advanced Materials,2008,20(20):3947-3952.
[25]HANCOCK J M,GIFFORD A P,TONZOLA C J,et al.High-efficiency electroluminescence from new blue-emitting oligoquinolines bearing pyrenyl or triphenyl endgroups[J].The Journal of Physical Chemistry C,2007,111(18):6875-6882.
[26]TONZOLACJ,KULKARNIAP,GIFFORDAP,etal.Blue-light-emitting oligoquinolines:synthesis,properties and high-efficiency blue-light-emitting diodes[J].Advanced Functional Materials,2007,17 (6):863-874.
[27]LEE S J,PARK J S,YOON K J,et al.High-efficiency deep-blue light-emitting diodes based on phenylquinoline/carbazole-based compounds[J].Advanced Functional Materials,2008,18(24):3922-3930.
[28]ZHANG Q,LI J,SHIZU K,et al.Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes[J].Journal of the American Chemical Society,2012,134(36):14706-14709.
[29]HIRATA S,SAKAI Y,MASUI K,et al.Highly efficient blue electroluminescence based on thermally activated delayed fluorescence[J].Nature Materials,2015,14(3):330-336.
[30]ZHANG Y,LAI S L,TONG Q X,et al.High efficiency nondoped deep-blue organic light emitting devices based on imidazole--triphenylamine derivatives[J].Chemistry of Materials,2011,24(1):61-70.
[31]ZHANG Y,LAI S L,TONG Q X,et al.Synthesis and characterization of phenanthroimidazole derivatives for applications in organic electroluminescent devices[J].Journal of Materials Chemistry,2011,21(22):8206-8214.
[32]LI W,LIU D,SHEN F,et al.A twisting donor-acceptor molecule with an intercrossed excited state for highly efficient,deep-blue electroluminescence[J].Advanced Functional Materials,2012,22(13):2797-2803.
[33]RICHAUD A,BARBA-BEHRENSN,MÉNDEZF.Chemical reactivity of the imidazole:a semblance of pyridine and pyrrole?[J].Organic Letters,2011,13(5):972-975.
[34]张莹.菲并咪唑衍生物的设计,合成,表征及其在有机电致发光器件中的应用[D].汕头:汕头大学,2011.
[35]袁熠.菲并咪唑衍生物的设计、合成、表征及其在非掺杂有机电致发光器件中的应用[D].汕头:汕头大学,2013.
[36]陈文铖.基于双菲并咪唑衍生物的高效深蓝有机电致发光材料的设计、合成及其应用[D].汕头:汕头大学,2014.
[37]YUAN Y,CHEN J X,LU F,et al.Bipolar phenanthroimidazole derivatives containing bulky polyaromatic hydrocarbons for nondoped blue electroluminescence devices with high efficiency and lowefficiency roll-off[J].Chemistry of Materials,2013,25(24):4957-4965.
[38]CHEN W C,YUAN Y,WU G F,et al.Staggered face-to-face molecular stacking as a strategy for designing deep-blue electroluminescent materials with high carrier mobility[J].Advanced OpticalMaterials,2014,2(7):626-631.
[39]YUAN Y,LI D,ZHANG X,et al.Phenanthroimidazole-derivative semiconductors as functional layer in high performance OLEDs[J].New Journal of Chemistry,2011,35(7):1534-1540.
[40]WANG K,WANG S,WEI J,et al.New multifunctional phenanthroimidazole-phosphine oxide hybrids for high-performance red,green and blue electroluminescent devices[J].Journal of Materials Chemistry C,2014,2(33):6817-6826.
[41]HUANG H,WANG Y,ZHUANG S,et al.Simple phenanthroimidazole/carbazole hybrid bipolar hostmaterialsforhighlyefficient green and yellow phosphorescent organic light-emitting diodes[J].The Journal of Physical Chemistry C,2012,116(36):19458-19466.
[42]HUANG H,WANG Y,WANG B,et al.Controllably tunable phenanthroimidazole-carbazole hybrid bipolar host materials for efficient green electrophosphorescent devices[J].Journal of Materials Chemistry C,2013,1(37):5899-5908.
[43]WANG K,WANG S,WEI J,et al.Novel diarylborane-phenanthroimidazole hybrid bipolar host materialsforhigh-performancered,yellow and green electrophosphorescent devices[J].Organic Electronics,2014,15(11):3211-3220.
[44]KUO C J,LI T Y,LIEN C C,et al.Bis(phenanthroimidazolyl)biphenyl derivatives as saturated blue emitters for electroluminescent devices[J].Journal of Materials Chemistry,2009,19(13):1865-1871.
[45]WANG Z M,SONG X H,GAO Z,et al.Tuning of the electronic and optical properties of 4,4′-bis (1-phenyl-phenanthro[9,10-d]imidazol-2-yl)biphenylvia cyano substitution in un-conjugated phenyl[J]. RSC Advances,2012,2(25):9635-9642.
[46]WANG Z,FENG Y,LI H,et al.Dimeric phenanthroimidazole for blue electroluminescent materials:the effect of substituted position attached to biphenyl center[J].Physical Chemistry Chemical Physics,2014,16(22):10837-10843.
[47]CHOU H H,CHEN Y H,HSU H P,et al.Synthesis of diimidazolylstilbenes as n-type blue fluorophores:alternative dopant materials for highly efficient electroluminescent devices[J].Advanced Materials,2012,24(43):5867-5871.
[48]CHEN W C,YUAN Y,WU G F,et al.Molecular modification on bisphenanthroimidazole derivative for deep-blue organic electroluminescent material with ambipolar property and high performance[J].Organic Electronics,2015,17:159-166.
[49]CHEN W C,WU G F,YUAN Y,et al.A meta-molecular tailoring strategy towards an efficient violet-blue organic electroluminescent material[J].RSC Advances,2015,5(23):18067-18074.
[50]ZHU Z L,CHEN W,ZHANG L D,et al.Pyridine based meta-linking deep-blue emitter with high conjugation extent and electroluminescent efficiencies[J].Journal of Materials Chemistry C,2016,4,6249-6255.
[51]WANG Z,LI X,XUE K,et al.Towards stable deep-blue emission and low efficiency roll-off in OLEDs based on phenanthroimidazole dimers[J].Journal of Materials Chemistry C,2016,4(9):1886-1894.
[52]WANG Z,FENG Y,ZHANG S,et al.Construction of high efficiency non-doped deep blue emitters based on phenanthroimidazole:remarkable substitution effectson the excited state properties and deviceperformance[J].Physical Chemistry Chemical Physics,2014,16(38):20772-20779.
[53]CHEN M,YUAN Y,ZHENG J,et al.Novel bipolar phenanthroimidazole derivative design for a nondoped deep-blue emitter with high singlet exciton yields[J].Advanced Optical Materials,2015,3 (9):1215-1219.
[54]TANG X,BAI Q,PENG Q,et al.Efficient deep blue electroluminescence with an external quantum efficiencyof6.8%and cie y<0.08 based on a phenanthroimidazole sulfone hybrid donor acceptor molecule [J].Chemistry of Materials,2015,27(20):7050-7057.
[55]LI W,YAO L,LIU H,et al.Highly efficient deep-blue OLED with an extraordinarily narrow FHWM of35nmandaycoordinate<0.05 based on a fully twisting donor acceptor molecule[J].Journal of Materials Chemistry C,2014,2(24):4733-4736.
[56]LIU H,BAI Q,YAO L,et al.Highly efficient near ultraviolet organic light-emitting diode based on a meta-linked donor-acceptor molecule[J].Chemical Science,2015,6(7):3797-3804.
[57]ZHANG S,LI W,YAO L,et al.Enhanced proportion of radiative excitons in non-doped electrofluorescencegeneratedfromanimidazolederivative with an orthogonal donor acceptor structure[J].Chemical Communications,2013,49(96):11302-11304.
[58]ZHANG S,YAO L,PENG Q,et al.Achieving a significantly increased efficiency in nondoped pure blue fluorescent OLED:A quasi-equivalent hybridized excited state[J].Advanced Functional Materials,2015,25(11):1755-1762.
[59]ZHUANG S,SHANGGUAN R,JIN J,et al.Efficient nondoped blue organic light-emitting diodes based on phenanthroimidazole-substituted anthracene derivatives[J].Organic Electronics,2012,13 (12):3050-3059.
[60]ZHUANG S,SHANGGUAN R,HUANG H,et al.Synthesis,characterization,physical properties,and blue electroluminescent device applications of phenanthroimidazole derivatives containing anthracene or pyrene moiety[J].Dyes and Pigments,2014,101:93-102.
[61]HE C,GUO H,PENG Q,et al.Asymmetrically twisted anthracene derivatives as highly efficient deep-blue emitters for organic light-emitting diodes[J].Journal of Materials Chemistry C,2015,3 (38):9942-9947.
[62]GAO Z,CHENG G,SHEN F,et al.Highly efficient deep blue light emitting devices based on triphenylsilane modified phenanthro[9,10-d]imidazole[J].Laser&Photonics Reviews,2014,8(1):L6-L10.
[63]LIU H,CHEN P,HU D,et al.Separation of electrical and optical energy gaps:selectively adjusting the electrical and optical properties for a highly efficient blue emitter[J].Chemistry-A European Journal,2014,20(8):2149-2153.
[64]LI C,WEI J,SONG X,et al.Non-doped luminescent material based organic light-emitting devices displaying high brightness under very low driving voltage[J].Journal of Materials Chemistry C,2016,4 (29):7013-7019.
[65]HUANG S,QI X,LIU T,et al.Towards safer rocket fuels:hypergolic imidazolylidene-borane compounds as replacements for hydrazine derivatives[J].Chemistry-A European Journal,2016,22 (29):10187-10193.
[66]WANG K,ZHAO F,WANG C,et al.High-performance red,green,and blue electroluminescent devices based on blue emitters with small singlet-triplet splitting and ambipolar transport property[J]. Advanced Functional Materials,2013,23(21):2672-2680.
[67]LIU D,DU M,CHEN D,et al.A novel tetraphenylsilane-phenanthroimidazole hybrid host material for highly efficient blue fluorescent,green and red phosphorescent OLEDs[J].Journal of Materials Chemistry C,2015,3(17):4394-4401.
[68]LI C,WANG S,CHEN W,et al.High performance full color OLEDs based on a class of molecules with dual carrier transport channels and small singlet-triplet splitting[J].Chemical Communications,2015,51(53):10632-10635.
[69]LI Y,LI X L,CAI X,et al.Deep blue fluorophores incorporating sulfone-locked triphenylamine:the key for highly efficient fluorescence-phosphorescence hybrid white OLEDs with simplified structure [J].Journal of Materials Chemistry C,2015,3(27):6986-6996.
[70]OUYANG X,LI X L,AI L,et al.Novel“hot exciton”blue fluorophores for high performance fluorescent/phosphorescent hybrid white organic light-emitting diodes with superhigh phosphorescent dopant concentration and improved efficiency roll-off[J].ACS Applied Materials&Interfaces,2015,7 (15):7869-7877.
[71]LIU B,ZHAO J,LUO C,et al.A novel bipolar phenanthroimidazole derivative host material for highlyefficientgreenandorange-redphosphorescentOLEDswithlowefficiencyroll-offathighbrightness[J]. Journal of Materials Chemistry C,2016,4(10):2003-2010.
Development and Application Perspective of Organic Lightemitting Materials Based on Phenanthroimidazole Derivatives
HE Dan,LIU Bin,TONG Qingxiao
(Department of Chemistryand KeyLaboratoryfor Preparation and Application of Ordered Structural Materials of GuangdongProvince,Shantou University,Shantou 515063,Guangdong,China)
As a kind of excellent blue material building block,Phenanthro[9,10-d]dimidazole (PI)has attracted extensive attention on the design of efficient deep blue fluorescent and phosphor host materials,due to theirs high photoluminescence(PL)efficiency,good thermal stability,relatively balanced carrier injection and transport capacity,easy modification and facile synthesis.In this paper,research and development of organic electroluminescent materials based on PI are reviewed.The structure characteristics of PI group and the device performances of all kinds of derivatives systematically are introduced.The applications of PI derivatives in the full-color display and solid-state lighting in the future are prospected.
phenanthroimidazole derivatives;organic light-emitting materials;blue fluorescence materials;phosphorescent host materials
O622.6
A
2016-09-05
佟庆笑(1974—),男(汉),山东梁山人,教授,博士生导师.研究方向:有机光功能材料,有机及超分子光化学,精细化学品.E-mail:qxtong@stu.edu.cn.
国家自然科学基金面上项目(51673113),国家基础研究计划973项目(2013CB834803)
1001-4217(2017)02-0026-15

