miR-34a通过Snail诱导肺癌EMT及促进其转移的分子机制①
刘行仁 白义凤 梁 良 冯 静 邓 菲
(四川省医学科学院·四川省人民医院呼吸科,成都610072)
·基础免疫学·
miR-34a通过Snail诱导肺癌EMT及促进其转移的分子机制①
刘行仁 白义凤②梁 良②冯 静③邓 菲④
(四川省医学科学院·四川省人民医院呼吸科,成都610072)
目的:探讨miR-34a在肺癌组织中的表达情况以及miR-34a在肺癌细胞侵袭和迁移过程中的作用及其机制。方法:qPCR检测肺癌和正常肺组织中miR-34a的表达情况;使用miR-34a-mimic和miR-34a-inhibitor过表达和沉默miR-34a,qPCR检测沉默和过表达效果;Western blot检测沉默和过表达miR-34a后Snail蛋白的表达情况;荧光素酶报告基因检测miR-34a与Snail的相互作用;Transwell侵袭实验检测miR-34a的表达对肺癌细胞侵袭能力的影响;划痕实验检测miR-34a的表达对肺癌细胞迁移能力的影响;Western blot检测E-Cadherin、Vimentin和Twist蛋白的表达情况。结果:与正常肺组织相比,肺癌组织中miR-34a表达明显降低;且晚期、低分化和有淋巴结转移的肺癌组织miR-34a表达明显较早期、高分化和无淋巴结转移的肺癌组织低;miR-34a-mimic和miR-34a-inhibitor可以有效抑制和过表达miR-34a的表达;miR-34a能与Snail的3′ UTR特异性结合;miR-34a可以调控肺癌H1650细胞的侵袭迁移能力;过表达miR-34a上调E-Cadherin,同时下调Vimentin和Twist蛋白的表达,沉默miR-34a则相反。结论:miR-34a在肺癌中表达明显降低,且跟肺癌分期分级以及淋巴结转移与否密切相关,同时miR-34a可以通过上皮间质转化调节肺癌细胞侵袭和迁移能力。
miR-34a;肺癌;上皮间质转化;E-Cadherin;Transwell;Snail
肺癌是世界上发病率最高的恶性肿瘤。肺癌也是我国发病率和死亡率最高的恶性肿瘤[1]。全球每年有超过160万新发病例,占所有新发肿瘤的12.7%。同时肺癌也是死亡率最高的恶性肿瘤,每年有超过140万肺癌患者死亡,占所有肿瘤死亡人数的19%[2]。虽然近年来肺癌治疗手段不断进步,但是肺癌患者尤其是晚期肺癌患者的五年生存率仍然很低。局部复发、淋巴结转移以及远处转移是导致患者死亡的主要原因[3]。
肿瘤转移是个多步骤,多阶段的复杂过程,主要包括局部浸润、浸入血管、随血液循环系统转移并在其中存活、移出血管和在新的部位定居并增殖,而肿瘤转移的第一步是细胞与细胞间黏附的破裂[4,5]。Snail是近年发现的锌指转录因子,是肿瘤进展过程中一个重要的调节子,可以促进肿瘤浸润和转移的因子[6]。Burton等[7]研究表明,Snail与乳腺癌组织分期和分级、淋巴结转移的情况密切相关。Palma等[8]发现,Snail的高表达可以促进上皮间质转化的发生,从而促进肿瘤细胞的侵袭和迁移。
miRNA是高度保守的非编码RNA,通过调控相应基因的表达,参与细胞的增殖、凋亡、细胞分化等生物学行为,同时也可参与恶性肿瘤的侵袭、迁移、肿瘤微环境的调节以及肿瘤干细胞的调控等[9,10]。许多研究表明,miR-34a的缺失可能与肝癌、乳腺癌和结肠癌等肿瘤发生和发展密切相关[11-13]。但是miR-34a在肺癌发生发展过程中机制不甚明确。故本研究拟探讨miR-34a在肺癌中的表达情况,以及在肺癌转移过程中的作用及机制。
1 材料与方法
1.1 材料
1.1.1 临床标本采集及处理 收集2015年1月-2016年3月在我院收治的100例肺癌患者,其中男性65例,女性35例;年龄(61.24±3.61)岁。所有患者术前无化疗或放疗,全部患者术后病理分期均经两名副高以上病理科医师共同阅片确定,根据肺癌TNM分期标准,Ⅰ级39例、Ⅱ级18例、Ⅲ级37例、Ⅳ级6例。低分化28例,中分化32例,高分化40例。45例发生淋巴结转移,55例未发生淋巴结转移。同时取癌旁正常组织100例。肿瘤组织离体后分两块,一块迅速投入RNA保存液中,另一块用经焦碳酸二乙酯处理的冷磷酸缓冲液冲洗,去除血迹,迅速投入液氮冻存。
1.1.2 细胞株与主要试剂 人肺癌细胞株H1650购自ATCC。细胞培养条件:含10%胎牛血清的RPMI DMEM,37℃,5%CO2条件下培养。胎牛血清,RPMI DMEM培养基均购自Gibco公司。Snail、E-cadherin、Vimentin、Twist单克隆抗体购自CST。Transwell小室购自美国Millipore公司。Matrigel购自美国BD公司。脂质体LipofectamineTM2000、miR-34a mimic、miR-34a-inhibitor购自上海吉凯基因。Trizol购自美国Ambion公司。逆转录试剂盒(FSQ-101)购自日本TOYOBO公司。PCR试剂盒购自美国Kapa公司。荧光素酶活性检测试剂盒购自Promega公司。荧光素酶报告载体由Promega公司合成。
1.2 方法
1.2.1 qPCR检测miR-34a的表达 按照Trizol说明书提取组织中总RNA,超微量分光光度计测定RNA浓度,复能基因有限公司设计miR-34a引物,上游引物:5′-GTGCAGGGTCCGAGGT-3′;下游引物:5′-GCCGCTGGCAGTGTCTTAGCTG-3′。以100 ng总RNA为模板,逆转录cDNA,反应条件为:37℃ 15 min,98℃ 5 min。后根据Kapa PCR试剂盒说明书进行PCR反应。获得数据以RQ=2-ΔΔCt计算mRNA表达量。实验重复3次。
1.2.2 细胞转染 取对数生长期的肺癌细胞H1650细胞,按照LipofectamineTM2000转染试剂盒说明书将miR-34a-mimic、miR-34a-inhibitor和阴性对照转染细胞。转染后qPCR检测miR-34a的表达情况。
1.2.3 Western blot检测细胞转染后中Snail、E-cadherin、Vimentin、Twist的表达 取转染miR-34a-mimic和miR-34a-inhibitor 48 h的细胞蛋白,BCA法测定蛋白浓度,加入Loading buffer后变性蛋白。配制10%SDS-PAGE,每孔加入20 μg蛋白样品。使用湿转法电转至PVDF膜,5%脱脂奶粉封闭2 h,1∶1 000 TBST稀释一抗,4℃过夜;加入羊抗兔二抗1∶5 000 稀释,室温孵育2 h,ECL发光。重复3次。
1.2.4 Transwell侵袭实验检测miR-34a对肺癌细胞侵袭能力的影响 所有试剂及器材均于冰上预冷,将Transwell小室置于24孔板内,将Transwell小室内膜均匀涂抹Matrigel胶 50 μl (0.2 μg/μl),37℃孵育15 min,使胶凝固;消化、离心、计数细胞后,按照2.5×104个/ml用无血清培养基稀释细胞,制成细胞悬液;按照每孔200 μl,将细胞悬液加入Transwell上室,同时在Transwell下室加入10%FBS+培养基500 μl,放入37℃孵箱培养;甲醛固定,结晶紫染色15 min,然后用棉签轻轻擦拭内膜上的细胞。显微镜下技术,计数4个高倍视野(×40)下穿过滤膜的细胞数。实验重复3次。
1.2.5 划痕实验检测miR-34a对肺癌细胞迁移能力的影响 划痕实验:将H1650细胞接种于6孔板,待细胞融合度生长在90%时,用200 μl消毒枪头从上而下划线,并在显微镜下观察,测量划痕的初始距离(0 time);在24、48、72 h后,分别测量划痕的距离,并拍照,计算细胞的迁移率。实验重复3次。
1.2.6 荧光素酶活性检测 将荧光素酶报告载体与miR-34a-mimic共转染H1650细胞。以转染pRL-TK作为标准内质控。转染36 h后,收获细胞。按Promega公司荧光素酶活性检测试剂盒说明书检测H1650细胞荧光素酶活性。相对荧光素酶活性=萤火虫荧光素酶活性值/海肾荧光素酶活性值。实验重复3次。
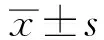
2 结果
2.1 肺癌组织和正常肺组织中miR-34a mRNA的表达 qPCR结果表明:与癌旁正常组织相比,肺癌组织中miR-34a mRNA表达水平明显降低[(2.51±0.31) vs (0.58±0.17),P<0.05],差异有统计学意义(图1)。同时检测miR-34a在肺癌H1650和H889细胞中的表达情况。qRCR结果表明:H1650肺癌细胞中miR-34a表达水平低,H889肺癌细胞中miR-34a表达水平高。
2.2 miR-34a表达与临床病理资料的关系 统计分析表明:miR-34a的表达水平随肺癌病理分期的增加而降低;随分化程度的降低,miR-34a的表达水平逐渐降低;有淋巴结转移的肺癌组织中,miR-34a的表达明显降低;miR-34a的表达水平与年龄和性别无关。结果表明:miR-34a与肺癌病理分期分级以及淋巴结转移与否有关,而与性别、年龄等无关(表1)。

图1 qPCR 检测肺癌组织和细胞株中miR-34a 的表达情况Fig.1 qPCR was used to detect expression of miR-34a in lung cancer tissues and cell linesNote: *.P<0.05.
2.3 miR-34a-mimic和miR-34a-inhibitor转染后显著提高和降低miR-34a mRNA的表达 miR-34a-mimic转染肺癌细胞H1650 48 h后,qPCR结果表明:与NC组相比,miR-34a-mimic组miR-34a mRNA水平明显提高[ (0.22±0.03)vs(0.89±0.13),P<0.01],差异有统计学意义(图2A);与NC组相比,miR-34a-inhibitor组miR-34a mRNA水平明显提高[(0.22±0.03)vs(0.89±0.13),P<0.01],差异有统计学意义(图2B)。结果表明,miR-34a-mimic可以显著提高miR-34a表达水平;miR-34a-inhibitor可以显著降低miR-34a表达水平。
2.4 miR-34a可以调节Snail的表达 Western blot结果显示:与NC组相比,miR-34a-mimic组中Snail蛋白表达水平明显下降(51.4±2.8) vs (13.7±0.8),P<0.05,而miR-34a-inhibitor组中Snail蛋白表达水平明显提高[(72.4±3.1) vs (13.7±0.8),P<0.05],表明miR-34a可以调节Snail的表达(图3)。
2.5 荧光素酶报告基因检测Snail和miR-34a的作用关系 为明确miR-34a能否与Snail 3′UTR结合,将miR-34a-inhibitor与Snail-Wt、Snail-Mut共转染到肺癌H1650细胞中。荧光素酶报告基因结果显示:miR-34a-inhibitor可以明显抑制Snail-Wt的荧光素酶活性;miR-34a-mimic对Snail-Mut的荧光素酶无明显抑制作用。结果表明,miR-34a能与Snail的3′ UTR特异性结合(图4)。
表1 miR-34a表达与肺癌临床病理特征的关系
Tab.1 Relationship between miR-34a expression and clinicopathological features of lung cancer

Clinicopatho-logicaldataQuantityLowlevelofmiR-34aHighlevelofmiR-34aPGender0.612Male653233Female351817Age(year)0.821≤60532627>60472324Differentiation0.004High402416Medium321616Low28622Pathologicalstages0.003Ⅰ392019Ⅱ18513Ⅲ37532Ⅳ606Lymphnodemetastasis0.01No553718Yes451233
2.6 miR-34a可以抑制人肺癌细胞H1650的侵袭能力 细胞侵袭穿过Matrigel的能力可以反映细胞的侵袭能力。Transwell结果显示:NC组通过Matrigel基质胶的细胞数量为(346.57±28.70),明显多于miR-34a-mimic组(131.92±13.80),差异有统计学意义(P<0.01)(图5A);NC组通过Matrigel基质胶的细胞数量为(72.57±3.20),明显少于miR-34a-mimic组(406.57±31.90),差异有统计学意义(P<0.01)(图5B),表明miR-34a可以抑制人肺癌细胞H1650的侵袭能力。
2.7 miR-34a可以抑制人肺癌细胞H1650的迁移能力 在显微镜下分别测量0、24、48、72 h时,各组细胞任意三个部位的划痕的宽度,迁移率=[D(t=24,48,72 h)-D(t=0 h)]/D(t=0 h)。划痕实验结果表明:与NC组相比,在24、48、72 h时,miR-34a-mimic组迁移率明显降低[24 h (54.51±3.15)% vs (13.07±1.12)%,P<0.05;48 h (77.51±5.35)% vs (33.24±2.23)%,P<0.05;72 h (84.22±7.12)% vs (48.21±4.27)%,P<0.01],差异有统计学意义(图6A);与NC组相比,在24、48、72 h时,miR-34a-inhibitor组迁移率明显增高[24 h (55.83±6.25)% vs (14.02±1.29)%,P<0.05;48 h (78.52±5.18)% vs (32.55±2.18)%,P<0.05;72 h (86.52±8.49)% vs (38.23±4.42)%,P<0.01],差异有统计学意义(图6B),表明miR-34a可以抑制人肺癌细胞H1650的迁移能力。

图2 qPCR 检测过表达和沉默miR-34a后的mRNA表达水平Fig.2 qPCR was used to detect mRNA expression levels after overexpression and silencing miR-34aNote: *.P<0.05.

图3 Western blot检测细胞转染后Snail蛋白的表达Fig.3 Expression of Snail were detected by Western blot after transfected with miR-34a-mimic and miR-34a-inhibitorNote: Error bars represent standard error.*.P<0.05.
2.8 miR-34a可以调节E-cadherin、Vimentin和Twist的表达 许多研究表明,上皮间质转化在上皮性肿瘤的侵袭和迁移中激活,是上皮性肿瘤细胞获得侵袭性的关键分子事件,在恶性肿瘤的侵袭和迁移中发挥重要的作用[14]。而E-Cadherin蛋白是上皮间质转化过程中上皮性标志物,而Vimentin和Twist蛋白为上皮间质转化过程中间质性标志物。

图4 荧光素酶报告基因检测Snail是miR-34a的直接靶点Fig.4 Luciferase report gene detects Snail direct target of miR-34aNote: *.P<0.05.

图5 Transwell侵袭实验检测miR-34a对人肺癌细胞H1650侵袭能力的影响Fig.5 Effect of miR-34a on invasion ability of H1650 cells were detected by Transwell matrigel invasion assaysNote: Error bars represent standard error.*.P<0.05.

图6 划痕实验检测miR-34a-mimic和miR-34a-inhibitor对人肺癌细胞H1650迁移能力的影响Fig.6 Effeet of miR-34a on H1650 cell migration ability by wound healing a assaysNote: A.Effect of miR-34a-mimic on H1650 cells migration ability were detected by wound healing assays;B.Effect of miR-34a-inhibitor on H1650 cells migration ability were detected by wound healing assays.Error bars represent standard error.*.P<0.05.

图7 Western blot检测过表达和沉默miR-34a后Ecadherin、Vimentin 和Twist 蛋白的表达水平Fig.7 Western blot was used to detect Ecadherin,Vimentin and Twist protein expression levels after overexpression and silencing miR-34a Note: Error bars represent standard error.*.P<0.05.
Western blot结果显示:与NC组相比,miR-34a-mimic组中Vimentin、Twist蛋白表达水平明显下降[Vimentin (0.12±0.01)% vs (0.82±0.06)%,Twist (0.11±0.02)% vs (0.72±0.06)%,P<0.05],但E-Cadherin表达水平明显增高[(0.76±0.05)% vs (0.22±0.02)% ,P<0.05]。与NC组相比,miR-34a-inhibitor组中Vimentin、Twist蛋白表达水平明显增高[Vimentin (0.13±0.01)% vs (0.92±0.08)%;Twist (0.12±0.02)% vs (0.68±0.06)%,P<0.05],但E-Cadherin表达水平明显降低[(0.83±0.06)% vs (0.21±0.02)% ,P<0.05]。表明miR-34a可以调节E-cadherin、Vimentin和Twist的表达,表明miR-34a可以促进肺癌细胞的上皮间质转化,见图7。
3 讨论
miRNA是一类内源性的、高度保守的非编码单链RNA,广泛存在于植物和多细胞动物的基因组中[15]。成熟的miRNA经过Drosha和Dicer酶剪切,可以与靶基因的3-UTR区结合降解或者抑制靶基因的翻译[16]。miR-34a的编码基因位于染色体1p36,研究表明,miR-34a受p53的直接调控,p54的低表达或者功能异常可以降低对miR-34a转录的刺激信号,从而降低miR-34a的表达,从而影响下游靶基因发挥生物学功能[17,18]。Garofalo等[19]使用miR-34a-mimic转染非小细胞肺癌细胞系进行转染,发现肺癌细胞的增殖能力明显减弱。Gougelet等[20]对肝癌组织中miR-34a的表达分析发现,肝癌组织中miR-34a的表达水平明显降低,且跟肝癌分期和患者不良预后密切相关。
本研究通过检测肺癌和正常肺组织中miR-34a的表达发现,肺癌组织中miR-34a的表达明显降低,miR-34a的表达水平随肺癌病理分期的增加而降低;随分化程度的降低,miR-34a的表达水平逐渐降低;有淋巴结转移的肺癌组织中,miR-34a的表达明显降低,表明miR-34a在肺癌组织中起抑癌作用。
上皮间质转化发生于多种生理和病理过程中,表现为上皮标记基因的表达与细胞间黏附力的丧失,间质标记基因、细胞迁移与运动力的获得和细胞形态的改变[21]。E-cadherin是维持上皮细胞结构极性和完整性的糖蛋白,对于维持细胞与细胞间的黏附能力非常重要。许多研究表明,E-cadherin在许多恶性肿瘤中表达低下,且E-cadherin的低表达与肿瘤侵袭、迁移和不良预后密切相关。而Snail是E-cadherin的抑制子,可以负性调节E-cadherin的表达[22]。Chen等[23]在肝癌中的研究发现,Snail高表达的肿瘤组织中,E-cadherin表达常常下降,同时与肿瘤转移密切相关。
本研究通过使用miR-34a-mimic和miR-34a-inhibitor后,Snail表达明显降低和提高,使用荧光素酶报告基因检测miR-34a和Snail相互作用发现,miR-34a能与Snail的3′ UTR特异性结合。Transwell和划痕实验表明,miR-34a可以调控肺癌细胞的侵袭和迁移能力。Western blot检测E-cadherin、Vimentin和Twist蛋白的表达水平发现,E-cadherin表达水平与Snail相反,但Vimentin和Twist蛋白的表达水平与Snail表达趋势一致。
本研究表明miR-34a在肺癌组织中表达明显降低,而且与肿瘤分期分级以及淋巴结转移密切相关,同时发现miR-34a可以通过诱导上皮间质转化来促进肺癌细胞的侵袭和迁移。提示miR-34a可能参与肺癌的发展过程,有可能成为预测肺癌进展和预后,以至可能成为肺癌治疗的靶标。
[1] Vargas AJ,Harris CC.Biomarker development in the precision medicine era:lung cancer as a case study[J].Nat Rev Cancer,2016,16(8):525-537.
[2] Siegel RL,Miller KD,Jemal A.Cancer statistics,2015[J].CA,2015,65(1):5-29.
[3] Silva GT,Bergmann A,Thuler LCS.Incidence,associated factors,and survival in metastatic spinal cord compression secondary to lung cancer[J].Spine J,2015,15(6):1263-1269.
[4] Condamine T,Ramachandran I.Regulation of tumor metastasis by myeloid-derived suppressor cells[J].Annual Rev Med,2015,66:97.
[5] Zhao D,Besser AH,Wander SA,etal.Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation[J].Oncogene,2015,34(43):5447-5459.
[6] Heerboth S,Housman G,Leary M,etal.EMT and tumor metastasis[J].Clin Translat Med,2015,4(1):1.
[7] Burton LJ,Smith BA,Smith BN,etal.Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion,migration and osteoclastogenesis in prostate and breast cancer cells[J].Carcinogenesis,2015,36(9):1019-1027.
[8] Palma C,Grassi ML,Thomé CH,etal.Proteomic analysis of epithelial to mesenchymal transition (EMT) reveals cross-talk between SNAIL and HDAC1 proteins in breast cancer cells[J].Mole Cell Proteomics,2016,15(3):906-917.
[9] Pipan V,Zorc M,Kunej T.MicroRNA polymorphisms in cancer:a literature analysis[J].Cancers,2015,7(3):1806-1814.
[10] Wong HKA,El Fatimy R,Onodera C,etal.The cancer genome atlas analysis predicts MicroRNA for targeting cancer growth and vascularization in glioblastoma[J].Mole Ther,2015,23(7):1234-1247.
[11] Rokavec M,Öner MG,Li H,etal.IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis[J].J Clin Investiga,2015,125(3):1362-1362.
[12] Xia C,Liu Y,Lou G,etal.DNA-damaging compound 0404 effectively inhibits hepatocellular carcinoma by upregulation of miR-34a and miR-200c expression[J].Cancer Res,2015,75(15 Supplement):1645-1645.
[13] Adams BD,Wali VB,Cheng CJ,etal.MiR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer[J].Cancer Res,2016,76(4):927-939.
[14] Katsuno Y,Lamouille S,Derynck R.TGF-β signaling and epithelial-mesenchymal transition in cancer progression[J].Curr Opin Oncol,2013,25(1):76-84.
[15] Hausser J,Zavolan M.Identification and consequences of miRNA-target interactions [mdash] beyond repression of gene expression[J].Nat Rev Genet,2014,15(9):599-612.
[16] Hausser J,Syed AP.Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation[J].Genome Res,2013,23(4):604-615.
[17] Kim HR,Roe JS,Lee JE,etal.p53 regulates glucose metabolism by miR-34a[J].Biochem Biophysical Res Communicat,2013,437(2):225-231.
[18] Chang TC,Wentzel EA,Kent OA,etal.Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis[J].Molecular Cell,2007,26(5):745-752.
[19] Garofalo M,Jeon YJ,Nuovo GJ,etal.MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer[J].PLoS One,2013,8(6):e67581.
[20] Gougelet A,Sartor C,Bachelot L,etal.Antitumour activity of an inhibitor of miR-34a in liver cancer with β-catenin-mutations[J].Gut,2015:gutjnl-2014-308969.
[21] Biddle A,Mackenzie IC.Cancer stem cells and EMT in carcinoma[J].Cancer Metastasis Rev,2012,31(1-2):285-293.
[22] De Craene B,Berx G.Regulatory networks defining EMT during cancer initiation and progression[J].Nat Rev Cancer,2013,13(2):97-110.
[23] Chen J,Chan AWH.SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling[J].Hepatology,2013,57(6):2287-2298.
[收稿2016-09-28 修回2017-04-20]
(编辑 许四平)
Mechanism of miR-34a on invasion and migration ability of human lung carcinoma by Snail induced EMT
LIUXing-Ren,BAIYI-Feng,LIANGLiang,FENGJing,DENGFei.
DepartmentofRespiration,SichuanProvincialPeople′sHospital,SichuanAcademyofMedicalSciences,Chengdu610072,China
Objective:To investigate the exression of miR-34a on lung cancer and normal lung tissues,and the effect and mechanism of miR-34a in lung cancer cell invasion and migration.Methods: qPCR was used to detect the expression of miR-34a on lung cancer.miR-34a-mimic and miR-34a-inhibitor were used to overexpress and knockdown miR-34a.qPCR was used to detect the effectiveness.Western blot was used to detect the expression of Snail after induced with miR-34a-mimic and miR-34a-inhibitor.Luciferase reporter gene was used to detect interaction between miR-34a and Snail.Transwell invasion assay was used to detect invasion ability after induced with miR-34a-mimic and miR-34a-inhibitor.Scratch assay was used to detect migration ability after induced with miR-34a-mimic and miR-34a-inhibitor.The expression of E-cadherin,Vimentin and Twist were detected by Western blot.Results: miR-34a expression was significantly reduced in lung cancer.With the stage of lung cancer progression,the expression of miR-34a reduced.With the differentiation of lung cancer progression,the expression of miR-34a decreased.Decreasing of miR-34a was associated with lung cancer lymph node metastasis.miR-34a-mimic and miR-34a-inhibitor could overexpress and knockdown miR-34a.miR-34a could regulate expression of Snail.Snail was the direct target of miR-34a;miR-34a could regulate the invasion ability of human lung carcinoma H1650 cells;miR-34a could regulate the migration of human lung carcinoma H1650 cells;miR-34a could regulate the expression of E-cadherin,Vimentin and Twist.Conclusion: miR-34a plays the role of tumor suppressor factor in lung cancer.miR-34a can regulate the invasion and migration ability of lung carcinoma H1650 cells by Snail induced EMT.
miR-34a;Lung cancer;EMT;E-Cadherin;Transwell;Snail
10.3969/j.issn.1000-484X.2017.05.002
①本文受国家自然科学基金(No.81301910)和四川省卫计委科研课题(No.110137)资助。
刘行仁(1980年-),男,主治医师,主要从事肺部感染及肺部肿瘤治疗方面的研究,E-mail:syyliudoc@sina.com。
R541.6 R332
A
1000-484X(2017)05-0646-06
②四川省医学科学院·四川省人民医院肿瘤科,成都610072。
③四川省医学科学院·四川省人民医院中医科,成都610072。
④通讯作者,四川省医学科学院·四川省人民医院肾内科,成都610072,E-mail:dengfei_here@163.com。

