寨卡病毒引发小头症?关系越确定,防治越迫切!
汪小欢,邹鹏,黎媛,陆路
1. 上海市(复旦大学附属)公共卫生临床中心,上海 201508; 2. 复旦大学基础医学院医学分子病毒学教育部/卫生部重点实验室,上海 200032
·特约专稿·
寨卡病毒引发小头症?关系越确定,防治越迫切!
汪小欢1,2,邹鹏1,黎媛1,陆路1,2
1. 上海市(复旦大学附属)公共卫生临床中心,上海 201508; 2. 复旦大学基础医学院医学分子病毒学教育部/卫生部重点实验室,上海 200032
自2015年初至今,寨卡病毒(Zika virus,ZIKV)以巴西为首先后在数十个国家和地区暴发流行。几乎同时,与日俱增的小头症患儿使全球对此陷入警惕状态。目前,全球正在积极探索ZIKV感染所引发的各种神经系统疾病。在越来越多证据表明在细胞水平和动物模型中ZIKV能直接损伤胚胎脑部发育的同时,ZIKV感染者的防治需求也越来越迫切。本文从ZIKV的流行病学、与小头畸形因果关系的研究进展及其预防疫苗和治疗药物的研究现状等方面进行概述。
寨卡病毒;小头症;流行病学;预防疫苗;治疗药物
近期,寨卡病毒(Zika virus,ZIKV)迅速蔓延。2015年3月,巴西巴伊亚地区(东北地区)首先报道了24例ZIKV感染者,此后越来越多的ZIKV疑似感染者出现。至同年5月,巴西卫生部确证了ZIKV的本土传播[1-2]。2015年10月,研究者在巴西伯南布哥州(东北地区)观察到当地小头畸形的新生儿(小头症患儿)数目异常增多。1个月后,巴西卫生部公告该国东北地区的ZIKV感染与小头症患儿骤增有直接关系[3]。愈演愈烈的ZIKV流行趋势及孕妇感染者的严重不良后果使全球对此陷入警惕状态。因此,在逐步探索ZIKV感染与小头畸形关系的同时,相应的预防措施和治疗方案对缓解甚至避免当前ZIKV流行的严峻形势至关重要。
1 ZIKV流行病学
1947年,ZIKV作为黄病毒科黄病毒属的一员首次被发现,研究人员从发热的恒河猴血清中分离出第1株ZIKV,7年后才有其感染人类的报道[4-5]。进入21世纪以来,ZIKV不断暴发流行,2007年波及了雅浦岛等数个岛屿,2013—2014年感染了法属波利尼西亚的数万居民[6-7]。2014年,复活节岛的本地ZIKV流行(感染者在潜伏期内无ZIKV流行地区旅行史)标志着ZIKV开始波及美洲地区[8]。同时期,亚洲地区也报道了ZIKV散在感染病例[9-10]。
自2015年4月,ZIKV于半年内蔓延了至少巴西14个地区,感染病例高达130万[11]。哥伦比亚也同时报道了ZIKV的本土传播及至少5 000多例疑似感染病例[11-12]。2016年11月18日,世界卫生组织(World Health Organization,WHO)更新了ZIKV相关形势报道,自2015年起累计69个国家和地区出现了ZIKV感染病例,包括泰国、韩国、越南、印度尼西亚、马来西亚和新加坡等亚洲国家[13]。2016年11月21日,新加坡国家环境局公布新加坡ZIKV累计感染病例已达453例[14]。中国也在2016年2月出现了输入性ZIKV感染病例[15]。亚洲诸多国家陆续出现ZIKV感染病例,使得ZIKV感染形势不断升级,引起了国际社会的高度关注。ZIKV是一种虫媒病毒,能不断发生突变而更好地侵袭传播媒介和宿主。伊蚊作为ZIKV的传播媒介,几乎存在于美洲各国家,因此ZIKV很可能在整个美洲传播。巴西首次报道ZIKV感染病例半年后,研究者注意到当地小头症患儿数目与日俱增,仅至2016年2月中旬就已超过4 300例[16]。早在2013—2014年法属波利尼西亚ZIKV暴发流行期间,研究者就观察到流行地区小头症患儿和格林-巴利综合征(Guillain-Barré syndrome,GBS)患者数量显著增加[17-18]。此外,越来越多的证据表明ZIKV感染是引发小头症和GBS的可能原因,从而引起全球范围对ZIKV的广泛关注。2016年2月1日,WHO将ZIKV相关小头症等中枢神经系统病变列为国际突发公共卫生事件[19]。综上所述,ZIKV流行病学的时间轴如图1所示。

图1 ZIKV流行病学时间轴
Fig.1 The timeline of Zika virus epidemiology
2 ZIKV与小头症相关性研究:从相关到因果
在ZIKV感染引发的各种神经系统疾病中,小头症最先引起各国研究者的注意。小头症定义为头围(经额枕测量)小于特定年龄和性别平均值的2个标准差[20],是一种大脑皮质神经祖细胞增殖障碍和死亡所致的神经系统发育异常性疾病,大部分患者会表现出不同程度的智力障碍[21]。
小头症病因包括遗传因素、环境因素和母体因素。在母体因素中,孕妇感染病毒可能导致胎儿发生小头症,其中巨细胞病毒或风疹病毒感染均有导致胎儿小头畸形的可能[22-24]。从病原学角度来看,ZIKV也可能是导致小头症的病原体之一。分析此次ZIKV在巴西的流行情况,从出现大量ZIKV感染者到小头症患儿显现的时间大概是半年[25],这也正是孕妇能通过产前B超检查胎儿颅骨发育是否正常的合适时间。因此,从时间的推移来说,ZIKV感染孕妇很可能引发胎儿小头畸形。此外,巴西ZIKV本土传播和小头症患儿骤增的地区均在东北地区,两者也具有空间一致性。
在理论分析支持的同时,研究者积极进行了ZIKV导致小头症的实验研究。Tang等揭示,ZIKV可通过增加细胞死亡和紊乱细胞周期来直接攻击人类神经祖细胞,进而阻碍大脑正常发育[26]。Lazear团队比较5种ZIKV毒株:MR766(Uganda,1947)、Dakar 41519(Senegal,1984)、Dakar 41667(Senegal,1984)、Dakar 41671(Senegal,1984)和H/PF/2013(French Polynesia,2013)[4,27-29],发现H/PF/2013毒株具有更强的毒力[30]。而Tang等在研究中使用的是MR766毒株,非近年ZIKV流行毒株,不能因此认为小头症患儿增加是由于ZIKV毒力变异的结果。相比一般体外实验,类器官3D模型能模仿人类器官形成过程,在研究ZIKV与小头症关系中受到各国研究者的青睐。2016年5月,巴西Garcez及其同事利用人类诱导多能干细胞(induced pluripotent stem cell,iPSC)培养成神经干细胞(neural stem cell,NSC)、神经球和大脑类器官,以神经球呈现神经形成过程的早期特征,以大脑类器官模拟胎儿第一孕期大脑新皮质包括基因表达和皮质分层细胞及其分子事件。结果证实ZIKV诱导iPSC来源的NSC死亡,阻碍神经球形成和发展,从而减慢大脑类器官成长[31]。美国Cauchemez等将ZIKV作用于不同日龄(14、28和80 d)的类器官以模拟ZIKV对不同胎龄大脑的作用,通过观察类器官的特征变化,发现即使早期阶段短暂接触低剂量的ZIKV,类器官也会产生长期日益严重的后果,这与第一孕期感染ZIKV危险性最高的临床发现一致[32]。
此外,各国研究者还致力于通过建立体内模型来研究ZIKV与小头症的因果关系。Rossi等用α干扰素(interferon α,IFN-α)受体缺陷小鼠建立ZIKV感染模型,小鼠大脑在感染后第3天检测到ZIKV,感染后第6天观察到大脑神经病变[33]。2016年5月,中国科学院许执恒和军事医学科学院秦成峰的研究团队建立了ZIKV感染胎鼠模型,直观呈现了ZIKV感染后胎鼠大脑变薄的皮质层和更加稀疏的脑室及脑室下带[34],但该模型没能实现对ZIKV感染胎鼠出生后连续长时期的观察。随后,Goodfellow等成功建立了ZIKV感染鸡胚模型,并以此呈现了ZIKV的高剂量致死效应和类似小头症的中枢神经系统发育障碍的渐进过程[35]。与小鼠模型相比,鸡胚模型实现了对ZIKV感染后的胚胎更长时间的观察。
也有研究者尝试从自身免疫方向对ZIKV感染与小头症关系进行探索。胎儿的ZIKV暴露能引发机体抗病毒免疫反应,同时会针对体内共同存在的蛋白多肽发生交叉反应,一旦这些机体蛋白被攻击,相关的小头症、眼部异常、脑部钙化及神经发育障碍随之发生。Lucchese及其同事分析了ZIKV与人类小头症相关的共同蛋白多肽,并利用免疫抗原决定簇数据库(Immune Epitope Database,IEDB)了解这些共同蛋白多肽引发机体免疫反应的潜能[36]。这为ZIKV感染通过引发感染者的自身免疫性反应从而损伤大脑发育提供了有力支持,也为进一步探索ZIKV与小头症的关系提供了新思路。
3 ZIKV预防疫苗研究:任重道远
2016年初,WHO召集了一次关于ZIKV的多学科讨论会,对加速研究ZIKV预防性疫苗的紧迫性达成一致[37]。以黄热病病毒(yellow fever virus,YFV)、日本脑炎病毒(Japanese encephalitis virus,JEV)及登革病毒(dengue virus,DENV)等黄病毒疫苗的相关研究为先例[38-40],研发针对ZIKV的保护性疫苗是可行的。目前,ZIKV相关疫苗研发有了很大进展,研究者们分别以不同的抗原呈现方式来引发机体的保护性免疫,包括核酸疫苗、纯化灭活病毒疫苗(purified inactivated virus,PIV)、减毒活病毒疫苗(live attenuated virus,LAV)及其他能表达ZIKV抗原的载体疫苗[41]。Larocca等构建了prM-Env共表达质粒抗ZIKV的DNA疫苗,其对ZIKV感染的抵抗作用在小鼠模型中得到验证[42]。类似的西尼罗病毒(West Nile virus,WNV)和DENV的DNA疫苗已进入Ⅰ期临床试验[43-44],这鼓舞了ZIKV核酸疫苗的研究。基于DENV安全有效的LAV临床前试验结果[45],针对ZIKV的LAV也投入研发。此外,针对黄病毒的PIV在多种病毒中进行了研发,包括DENV、蜱传脑炎病毒(tick borne encephalitis virus,TBEV)和JEV[46-48]。总之,PIV具有很广泛的应用价值,包括较广的接种年龄范围、适用于抵抗力低下的免疫缺陷者及可与其他疫苗共用,其安全性也被普遍认可。因此,ZIKV疫苗研发可充分利用PIV的这些优点。关于ZIKV疫苗的研究进展见表1。其中,Kim等研发了针对E蛋白胞外区的特异性腺病毒载体疫苗,并在乳鼠模型中获得验证。乳鼠由接种疫苗的雌鼠与未接种疫苗的雄鼠交配所得,显示了其潜在应用价值,能有效避免因女性孕前感染ZIKV而导致胎儿小头畸形的风险[49-50]。这些不同种类的ZIKV疫苗均通过动物模型验证了其高效的体内预防作用,应进一步进行临床试验,以加快预防性疫苗的实际应用。
表1 ZIKV疫苗研究进展
Tab. 1 The research advances on preventive vaccines for Zika virus
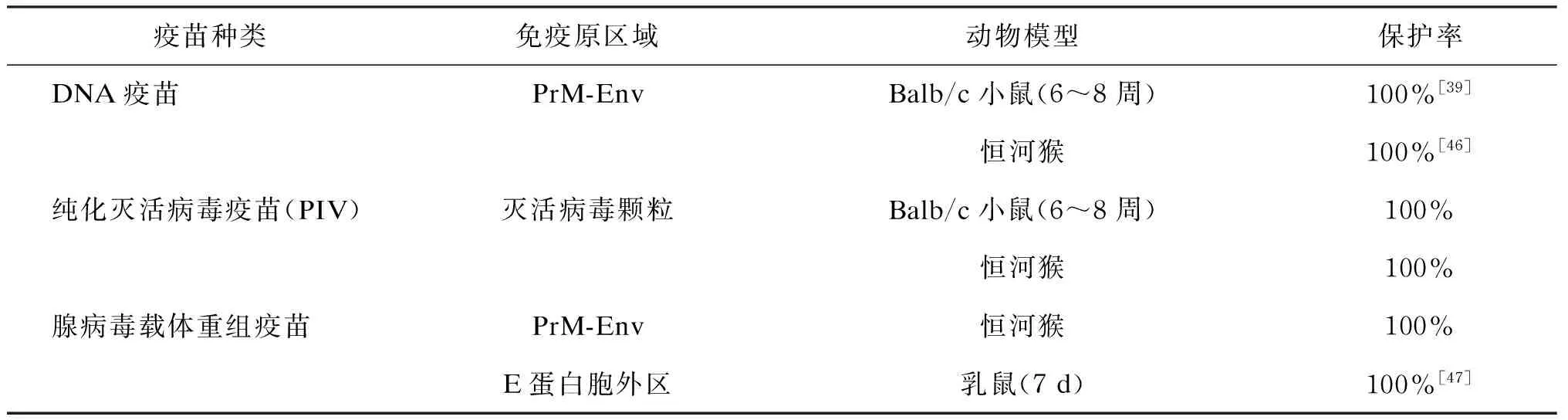
疫苗种类免疫原区域动物模型保护率DNA疫苗PrM-EnvBalb/c小鼠(6~8周)100%[39]恒河猴100%[46]纯化灭活病毒疫苗(PIV)灭活病毒颗粒Balb/c小鼠(6~8周)100%恒河猴100%腺病毒载体重组疫苗PrM-Env恒河猴100%E蛋白胞外区乳鼠(7d)100%[47]
与DENV类似,ZIKV感染者体内会出现抗体依赖性增强(antibody-dependent enhancement,ADE)现象[51-53],这为ZIKV预防性疫苗的安全应用增加了难度,需更充足的时间和更谨慎的安全性评估[54]。同时,还要加强对ZIKV结构蛋白的研究,让其具有免疫原性的抗原表位成为设计抗病毒免疫策略的根本依据[55-56]。有报道发现ZIKV能通过性接触方式传播[57],也有关于ZIKV可穿过胎盘屏障的研究[58]。ZIKV的这些特点提示疫苗研发还需重点关注接种时期,在性成熟前的青少年时期接种可在多个层次更好地阻断ZIKV播散及消除其带来的危害,具有更多的实际应用价值。
预防性疫苗是战胜流行性传染病的最有效方法,随着对ZIKV抗原结构及其相关免疫机制的认识越来越深入,通过不断推动ZIKV相关疫苗研发,预防性疫苗的成功应用指日可待。
4 ZIKV治疗药物研究:形势紧迫
目前还没有针对ZIKV感染的特异性治疗药物上市。对ZIKV感染患者,主要进行基础对症治疗,包括休息、使用退热药和镇痛药等[59]。随着ZIKV感染所致并发症的发现越来越多,其治疗药物的需求越来越紧迫。早在全球关注ZIKV流行之初,美国Ekins及其同事就呼吁进行抗ZIKV感染的药物研发工作[60]。为加快这项工作的进展,美国科学家发起了OpenZika项目,旨在发现能结合ZIKV蛋白晶体结构的具有药物潜能的化合物,并在全球范围内共享[61]。2016年10月, Xu等对约6 000种已批准或还处于临床试验阶段的药物和有药理活性的小分子化合物进行了高通量筛选[62]。结果显示,恩利卡生(Emricasan)作为天冬氨酸特异性半胱氨酸蛋白酶(cysteinyl aspartate specific protease,caspase)广谱抑制剂,能有效保护大脑皮质神经祖细胞抵抗ZIKV感染;B类驱虫剂氯硝柳胺和其他10种细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)抑制剂均能抑制ZIKV复制。此外,他们还发现神经保护性复合物与抗病毒药物联用能进一步增强对神经祖细胞和星形胶质细胞的保护,避免其发生ZIKV诱导的细胞死亡。类似Xu等进行的小分子药物高通量筛选能快速发现ZIKV潜在治疗药物,且成本较低,容易实现。但小分子药物存在较大的安全性风险,尤其是对孕妇群体。目前,在多角度、全方位证据指向孕妇感染ZIKV会导致胎儿小头畸形甚至流产等不良后果的情况下,孕妇感染者的治疗药物需求尤为迫切,ZIKV的治疗药物研发应重视在孕妇和备孕者等特殊群体中的应用[63-64]。当然,如果能直接对美国食品药品管理局(Food and Drug Administration,FDA)已批准的孕妇用药进行抗ZIKV疗效筛选可保证孕妇群体的安全性,筛选结果的可行性也能得到保障。
干扰素诱导的跨膜蛋白(interferon-induced transmembrane protein,IFITM)具有广泛的抗病毒作用,对与ZIKV相近的WNV和DENV均有抑制作用[65-67]。美国Savidis等据此研究了IFITM对ZIKV的影响,发现IFITM1和IFITM3均能在早期阶段抑制ZIKV复制,为研发IFITM生物制剂治疗ZIKV感染提供了思路[68]。
针对ZIKV治疗,各国科学家也在积极探索安全性较高的抗体蛋白药物。黄病毒E蛋白在病毒进入宿主细胞过程中发挥关键作用,成为中和性抗体药物的重要靶点。美国Sapparapu等[69]从有ZIKV感染史的人体中分离出能抑制非洲、亚洲和美洲3类ZIKV毒株活性的人类中和性抗体ZIKV-117,抗原表位作图揭示其可识别E蛋白二聚体连接处表位进而中和ZIKV。他们还通过小鼠模型证实了ZIKV-117对ZIKV感染的体内治疗效应并能保护孕鼠,降低孕鼠胎盘及胎鼠的ZIKV感染。Stettler等[52]发现了针对ZIKV E蛋白DⅢ(domain Ⅲ)抗原表位的单克隆抗体ZKA64,对A129小鼠感染ZIKV有较好的治疗效果,不管是感染前或感染后1 d应用均能有效避免ZIKV所致体重减轻及死亡,与Zhao等[70]认为的应将ZIKV E蛋白DⅢ作为中和性抗体药物研发关键表位的观点一致。C10是另一具有ZIKV交叉中和活性的抗DENV人类中和性抗体,可能通过阻止融合过程中E蛋白发生结构重组而抑制ZIKV,也是ZIKV感染的潜在抗体治疗药物[71]。此外,黄病毒E蛋白还包含一个疏水性的融合环(fusion loop)片段,介导病毒与宿主细胞融合,这段序列在黄病毒属中高度保守。基于这段保守序列,Deng等研发了单克隆抗体2A10G6,对DENV、YFV和ZIKV均有较好的中和效果,空斑减少中和试验(plaque reduction neutralization test,PRNT)显示,2A10G6对ZIKV的50%中和效价为249 μg/mL[72-73]。2A10G6也有希望成为抗黄病毒属的广谱治疗性抗体药物。Z23和Z3L1是来源于ZIKV感染者的人类中和性抗体,研究发现它们分别通过与E蛋白DⅠ、DⅡ和DⅢ表位结合发挥中和作用,体内外实验证实这两种抗体对ZIKV均有很好的中和效果[74]。一部分抗体蛋白药物在小鼠模型中显示了高效的治疗作用,但需在此基础上建立灵长类动物模型并进行验证。ZIKV的抗体蛋白药物有高效治疗作用,但潜在的ADE及不可忽视的高成本可能会限制其广泛应用,尤其是在发展中国家。抗体无法穿过睾血屏障,曾有报道显示埃博拉病毒(Ebola virus,EBOV)感染者使用抗体治愈出院后仍以性接触方式传播,将EBOV传给其性伴侣[75]。最近,Nature及Cell发表的两项研究均显示ZIKV感染会引发睾丸损伤,最终导致雄性不育[76-77]。因此,抗体药物可能无法清除睾丸内的ZIKV及遏制睾丸损伤,从而无法有效阻断ZIKV的性接触传播。除抗体外,多肽药物也属于蛋白药物,具有较好的安全性,且相对抗体而言成本更低,适合发展中国家。同时,多肽药物能穿过胎盘及睾血屏障,可应用于孕期胎儿及男性睾丸内病毒的清除,用途更加广泛,在预防ZIKV性传播中具有更显著的优势。
中药制剂在治疗ZIKV感染中也取得了满意效果。我国首例输入性ZIKV感染病例于2016年2月6日入住赣县人民医院感染疾病科,该院对患者主要采用中药制剂喜炎平注射液进行抗病毒治疗,以及布洛芬、氯霉素滴眼液等对症治疗,患者于2月14日痊愈出院[78]。这充分表明中西医结合治疗ZIKV感染具有一定的优势。
自ZIKV发现到近年暴发流行,间隔几十年的研究空白导致人们面对其引发的公共卫生事件时措手不及。应谨记,后代的健康将取决于我们今天的选择,针对ZIKV防治相关研究的脚步不能停止。
5 结语
随着ZIKV持续传播流行,纵观既往美洲地区DENV流行经验及近期ZIKV传播趋势,ZIKV可能还会在越来越多的地区发生流行,甚至达到全球范围[79]。2015年迄今,ZIKV的相关研究在多方面取得了重大成果,包括ZIKV基因序列测定和蛋白结构分析、建立能协助研究ZIKV是否引发小头症及其机制的体内外模型、研发针对ZIKV的预防性疫苗及发现或发明抗ZIKV感染的药物或化合物。近两年,尽管ZIKV的相关研究进展越来越多,但其防治药物的临床应用尚未实现,特别是针对孕妇群体。ZIKV属于众多黄病毒中的一员,其近期暴发流行的影响相比于整个黄病毒属曾带来的伤害仍是冰山一角。为了能更好地应对类似ZIKV或DENV的其他黄病毒流行,科学家们应投入研究抗黄病毒的广谱性防治药物。目前,流行地区急需针对ZIKV的防治措施。相信通过各国研究者的全力合作,一定能成功遏制此次ZIKV流行。
[1] Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil [J]. Emerg Infect Dis, 2015, 21(10): 1885-1886.
[2] Ministério da saúde. Confirmação do Zika Vírus no Brasil [EB/OL]. [2015-05-14].http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/17701-confirmacao-dozika-virus-no-brasil.
[3] Ministério da Saúde. Ministério da Saúde confirma relacão entre vírus Zika e microcefalia [EB/OL]. [2015-11-28]. http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/21014-ministerio-da-saude-confirma-relacaoentre-virus-zika-e-microcefalia.
[4] Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity [J]. Trans R Soc Trop Med Hyg, 1952, 46(5): 509-520.
[5] Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria [J]. Trans R Soc Trop Med Hyg, 1954, 48(2): 139-145.
[6] Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia [J]. N Engl J Med, 2009, 360(24): 2536-2543.
[7] Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French Polynesia, South Pacific, 2013 [J]. Emerg Infect Dis, 2014, 20(6): 1085-1086.
[8] Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, Vera L, Olivares B, Vilches M, Fernández J. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014 [J]. Arch Virol, 2016, 161(3): 665-668.
[9] Kwong JC, Druce JD, Leder K. Zika virus infection acquired during brief travel to Indonesia [J]. Am J Trop Med Hyg, 2013, 89(3): 516-517.
[10] Leung GH, Baird RW, Druce J, Anstey NM. Zika virus infection in Australia following a monkey bite in Indonesia [J]. Southeast Asian J Trop Med Public Health, 2015, 46(3): 460-464.
[11] Zika virus outbreaks in the Americas [J]. Wkly Epidemiol Rec, 2015, 90(45): 609-610.
[12] World Health Organization. Zika virus microcephaly and Guillain-Barré syndrome [EB/OL]. [2016-03-17]. http://apps.who.int/iris/bitstream/10665/204633/1/zikasitrep_17Mar2016_eng.pdf.
[13] World Health Organization. Zika situation report [EB/OL]. [2016-11-17]. http://www.who.int/emergencies/zika-virus/situation-report/17-november-2016/en/.
[14] National Environment Agency. Zika cases & clusters: Number of Zika cases [EB/OL]. [2017-02-23]. http://www.nea.gov.sg/public-health/vector-control/overview/zika-cases-clusters.
[15] Deng YQ, Zhao H, Li XF, Zhang NN, Liu ZY, Jiang T, Gu DY, Shi L, He JA, Wang HJ, Sun ZZ, Ye Q, Xie DY, Cao WC, Qin CF. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China [J]. Sci China Life Sci, 2016, 59(4): 428-430.
[16] Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT; Brazilian Medical Genetics Society-Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015 [J]. MMWR Morb Mortal Wkly Rep, 2016, 65(3): 59-62.
[17] Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia [J]. Lancet, 2016, 387(10023): 1051-1052.
[18] Malkki H. CNS infections: Zika virus infection could trigger Guillain-Barré syndrome [J]. Nat Rev Neurol, 2016, 12(4): 187.
[19] World Health Organization. Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations [EB/OL]. [2016-02-01]. http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en.
[20] Ashwal S, Michelson D, Plawner L, Dobyns WB. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society [J]. Neurology, 2009, 73(11): 887-897.
[21] Woods CG, Bond J, Enard W. Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings [J]. Am J Hum Genet, 2005, 76(5): 717-728.
[22] Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy [J]. Lancet, 1982, 2(8302): 781-784.
[23] Takano T, Morimoto M, Bamba N, Takeuchi Y, Ohno M. Frontal-dominant white matter lesions following congenital rubella and cytomegalovirus infection [J]. J Perinat Med, 2006, 34(3): 254-255.
[24] Weller TH, Hanshaw JB. Virologic and clinical observations on cytomegalic inclusion disease [J]. N Engl J Med, 1962, 266: 1233-1244.
[25] Secretaria de Vigilancia em Saúde-Ministério da Saúde. Monitoramento dos casos de dengue, febre de Chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 48, 2015 [J/OL]. Botetim Epidemiol, 2015, 46(44). http://portalarquivos.saude.gov.br/images/pdf/2016/janeiro/07/2015-svs-be-pncd-se48.pdf.
[26] Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL. Zika virus infects human cortical neural progenitors and attenuates their growth [J]. Cell Stem Cell, 2016, 18(5): 587-590.
[27] Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage [J]. PLoS Negl Trop Dis, 2012, 6(2): e1477.
[28] Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses [J]. Arch Virol, 2007, 152(4): 687-696.
[29] Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of Zika virus from a French Polynesia outbreak in 2013 [J]. Genome Announc, 2014, 2(3). pii: e00500-14. doi: 10.1128/genomeA.00500-14.
[30] Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A mouse model of Zika virus pathogenesis [J]. Cell Host Microbe, 2016, 19(5): 720-730.
[31] Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids [J]. Science, 2016, 352(6287): 816-818.
[32] Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study [J]. Lancet, 2016, 387(10033): 2125-2132.
[33] Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a novel murine model to study Zika virus [J]. Am J Trop Med Hyg, 2016, 94(6): 1362-1369.
[34] Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice [J]. Cell Stem Cell, 2016, 19(1): 120-126.
[35] Goodfellow FT, Tesla B, Simchick G, Zhao Q, Hodge T, Brindley MA, Stice SL. Zika virus induced mortality and microcephaly in chicken embryos [J]. Stem Cells Dev, 2016, 25(22): 1691-1697.
[36] Lucchese G, Kanduc D. Zika virus and autoimmunity: From microcephaly to Guillain-Barré syndrome, and beyond [J]. Autoimmun Rev, 2016, 15(8): 801-808.
[37] Maurice J. WHO meeting thrashes out R&D strategy against Zika [J]. Lancet, 2016, 387(10024): 1147.
[38] Beck AS, Barrett AD. Current status and future prospects of yellow fever vaccines [J]. Expert Rev Vaccines, 2015, 14(11): 1479-1492.
[39] Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain [J]. Expert Rev Vaccines, 2011, 10(3): 355-364.
[40] Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection [J]. Nat Rev Microbiol, 2016, 14(1): 45-54.
[41] Pierson TC, Graham BS. Zika virus: immunity and vaccine development [J]. Cell, 2016, 167(3): 625-631.
[42] Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Vaccine protection against Zika virus from Brazil [J]. Nature, 2016, 536(7617): 474-478.
[43] Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, Wu SJ, Sun P, Kochel T, Raviprakash K, Hayes CG, Porter KR. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial [J]. Vaccine, 2011, 29(5): 960-968.
[44] Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, Fay M, Koup RA, Roederer M, Bailer RT, Gomez PL, Mascola JR, Chang GJ, Nabel GJ, Graham BS. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial [J]. J Infect Dis, 2007, 196(12): 1732-1740.
[45] Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model [J]. Sci Transl Med, 2016, 8(330): 330ra36.
[46] Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, Sadoff JC, Eckels KH. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys [J]. J Infect Dis, 1996, 174(6): 1176-1184.
[47] Lyons A, Kanesa-thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, Burge R, Towle AC, Wilson P, Tauber E, Vaughn DW. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis [J]. Vaccine, 2007, 25(17): 3445-3453.
[48] Kunz C. TBE vaccination and the Austrian experience [J]. Vaccine, 2003, 21(Suppl 1): S50-S55.
[49] Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JP, Zanotto PM, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys [J]. Science, 2016, 353(6304): 1129-1132.
[50] Kim E, Erdos G, Huang S, Kenniston T, Falo LD Jr, Gambotto A. Preventative vaccines for Zika virus outbreak: preliminary evaluation [J]. EBioMedicine, 2016, 13: 315-320. doi: 10.1016/j.ebiom.2016.09.028.
[51] Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus [J]. Nat Immunol, 2016, 17(9): 1102-1108.
[52] Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection [J]. Science, 2016, 353(6301): 823-826.
[53] Halstead SB. Dengue antibody-dependent enhancement: knowns and unknowns [J]. Microbiol Spectr, 2014, 2(6). doi: 10.1128/microbiolspec.AID-0022-2014.
[54] Fagbami AH, Halstead SB, Marchette NJ, Larsen K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids [J]. Cytobios, 1987, 49(196): 49-55.
[55] Kanduc D. The self/nonself issue: A confrontation between proteomes [J]. Self Nonself, 2010, 1(3): 255-258.
[56] Kanduc D. Peptide cross-reactivity: the original sin of vaccines [J]. Front Biosci (Schol Ed), 2012, 4:1393-1401.
[57] McCarthy M. Zika virus was transmitted by sexual contact in Texas, health officials report [J]. BMJ, 2016, 352: i720.
[58] Adibi JJ, Marques ET Jr, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta [J]. Lancet, 2016, 387(10027): 1587-1590.
[59] Musso D, Gubler DJ. Zika virus [J]. Clin Microbiol Rev, 2016, 29(3): 487-524.
[60] Ekins S, Mietchen D, Coffee M, Stratton TP, Freundlich JS, Freitas-Junior L, Muratov E, Siqueira-Neto J, Williams AJ, Andrade C. Open drug discovery for the Zika virus [J]. F1000Res, 2016, 5: 150.
[61] Ekins S, Perryman AL, Horta Andrade C. OpenZika: An IBM World Community Grid Project to accelerate Zika virus drug discovery [J]. PLoS Negl Trop Dis, 2016, 10(10): e0005023.
[62] Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming GL, Zheng W, Song H, Tang H. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen [J]. Nat Med, 2016, 22(10): 1101-1107.
[63] Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation [J]. Nat Rev Immunol, 2010, 10(5): 301-316.
[64] Wang S, Liu M, Zeng D, Qiu W, Ma P, Yu Y, Chang H, Sun Z. Increasing stability of antibody via antibody engineering: stability engineering on an anti-hVEGF [J]. Proteins, 2014, 82(10): 2620-2630.
[65] Perreira JM, Chin CR, Feeley EM, Brass AL. IFITMs restrict the replication of multiple pathogenic viruses [J]. J Mol Biol, 2013, 425(24): 4937-4955.
[66] Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig E, Adams DJ, Xavier RJ, Farzan M, Elledge SJ. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus [J]. Cell, 2009, 139(7): 1243-1254.
[67] Huang IC, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, Brass AL, Ahmed AA, Chi X, Dong L, Longobardi LE, Boltz D, Kuhn JH, Elledge SJ, Bavari S, Denison MR, Choe H, Farzan M. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus [J]. PLoS Pathog, 2011, 7(1): e1001258.
[68] Savidis G, Perreira JM, Portmann JM, Meraner P, Guo Z, Green S, Brass AL. The IFITMs inhibit Zika virus replication [J]. Cell Rep, 2016, 15(11): 2323-2330.
[69] Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice [J]. Nature, 2016, 540(7633): 443-447.
[70] Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. Structural basis of Zika virus-specific antibody protection [J]. Cell, 2016, 166(4): 1016-1027.
[71] Zhang S, Kostyuchenko VA, Ng TS, Lim XN, Ooi JS, Lambert S, Tan TY, Widman DG, Shi J, Baric RS, Lok SM. Neutralization mechanism of a highly potent antibody against Zika virus [J]. Nat Commun, 2016, 7: 13679.
[72] Deng YQ, Dai JX, Ji GH, Jiang T, Wang HJ, Yang HO, Tan WL, Liu R, Yu M, Ge BX, Zhu QY, Qin ED, Guo YJ, Qin CF. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein [J]. PLoS One, 2011, 6(1): e16059.
[73] Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H, Yan J, Shi Y, Qin CF, Qi J, Gao GF. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody [J]. Cell Host Microbe, 2016, 19(5): 696-704.
[74] Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, Li S, Song J, Liu J, He J, Yuan H, Xiong Y, Liao Y, Li J, Yang J, Tong Z, Griffin BD, Bi Y, Liang M, Xu X, Qin C, Cheng G, Zhang X, Wang P, Qiu X, Kobinger G, Shi Y, Yan J, Gao GF. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus [J]. Sci Transl Med, 2016, 8(369): 369ra179.
[75] Vetter P, Fischer WA 2nd, Schibler M, Jacobs M, Bausch DG, Kaiser L. Ebola virus shedding and transmission: Review of current evidence [J]. J Infect Dis, 2016, 214(Suppl 3): S177-S184.
[76] Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice [J]. Nature, 2016, 540(7633):438-442.
[77] Ma W, Li S, Ma S, Jia L, Zhang F, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. Zika virus causes testis damage and leads to male infertility in mice [J]. Cell, 2016, 167(6): 1511-1524.
[78] Deng Y, Zeng L, Bao W, Xu P, Zhong G. Experience of integrated traditional Chinese and Western medicine in first case of imported Zika virus disease in China [J]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2016, 28(2): 106-109.
[79] Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? [J]. Lancet, 2015, 386(9990): 243-244.
. LU Lu, E-mail: lul@fudan.edu.cn
Zika virus causes microcephaly? Prophylaxis and treatment are more urgent as the causal relationship is sure
WANG Xiaohuan1,2, ZOU Peng1, LI Yuan1, LU Lu1,2
1.ShanghaiPublicHealthClinicalCenterAffiliatedtoFudanUniversity,Shanghai201508,China; 2.KeyLaboratoryofMedicalMolecularVirology,MinistriesofEducationandHealth,SchoolofBasicMedicalSciences,FudanUniversity,Shanghai200032,China
Since early 2015, Zika virus has caused severe epidemic outbreaks, which started from Brazil involving dozens of regions and countries successively, and contemporaneously growing infants with microcephaly have made the whole global alert against Zika virus. A variety of potential neurological disorders caused by Zika virus infection are under exploring worldwide. The need of treatments for infectors is more and more urgent because of increasing evidences indicating that Zika virus is able to impair the brain development of the embryo in cellular level and animal model. This review will summarize recent research achievements concerning epidemiology and advances among causal relationship with microcephaly, potential preventive vaccines and therapeutic drugs of Zika virus.
Zika virus; Microcephaly; Epidemiology; Preventive vaccine; Therapeutic drug
上海市公共卫生临床中心院级科研课题(2016-27)
陆路
2016-12-23)

