酵母生产类胡萝卜素的研究进展
钮亭亭,孙茜萍,吴 涛
(1.上海体育学院,上海 200438;2.浙江工业大学 海洋学院,浙江 杭州 310014)
酵母生产类胡萝卜素的研究进展
钮亭亭1,孙茜萍2,吴 涛2
(1.上海体育学院,上海 200438;2.浙江工业大学 海洋学院,浙江 杭州 310014)
类胡萝卜素是医药、化学、食品和饲料产业中具有重要价值的产品,具有着色、抗氧化和防癌等功能.动物不能合成类胡萝卜素,因此必须从食物中进行摄取.作为潜在色素物质的来源,红酵母和法夫酵母是微生物法生产类胡萝卜素的重要菌种.阐述了红酵母和法夫酵母生物合成类胡萝卜素的途径、影响发酵产量的因素及廉价培养基的应用,进一步介绍了诱变育种方法及代谢工程技术在产类胡萝卜素酵母菌株改良上的应用.
类胡萝卜素;红酵母;法夫酵母;廉价培养基
类胡萝卜素对动物和人类具有重要意义,包括增强免疫、作为维生素A前体和淬灭氧自由基的组分.食用类胡萝卜素有益于预防多种疾病,如动脉硬化、白内障、多硬化和癌症.动物体内不能自发合成类胡萝卜素,必须从含类胡萝卜素的饲料中摄取.相比从蔬菜中提取或者化学合成类胡萝卜素,微生物发酵生产类胡萝卜素只需要低成本天然底物作为碳源,具备明显的经济优势.红酵母和法夫酵母[1]具有合成各种天然类胡萝卜素(β-胡萝卜素、红酵母烯、红酵母红素和虾青素等)的能力,比藻类和霉菌更适于大规模发酵生产.另外以代谢工程方法构建的酿酒酵母也表现出产类胡萝卜素的良好优势.
1 红酵母、法夫酵母的类胡萝卜素合成途径
红酵母(Rhodotorula)和法夫酵母(Phaffia)是产类胡萝卜素的常见酵母种属.由红酵母生产的类胡萝卜素种类主要为β-胡萝卜素、红酵母烯(3′,4′-双脱氢-β-ψ-胡萝卜素)和红酵母红素(3′,4′-双脱氢-β-ψ-胡萝卜素-16′-甲酸),三种主要的类胡萝卜素占总量的相对比例不确定,这与菌株及培养条件相关.Rhodotorulaglutinis和Rhodotorulagraminis生产的γ-胡萝卜素量(β-γ-胡萝卜素)占类胡萝卜素总量11%~15%,而法夫酵母主要生产虾青素(3,3-脱氢-β-β-胡萝卜素-4,4-二酮).
1964年,Simpson[2]及Goodwin[3]重新审视了酵母合成类胡萝卜素的一般途径:1) 由HMG-COA合酶催化乙酰辅酶A向3-羟基-3-甲基戊二酸单酰辅酶A(HMG-COA)转化,HMG-COA再转化为一个C6化合物甲羟戊酸(MVA),MVA通过MVA激酶磷酸化、脱羧等一系列反应进一步转化为异戊二烯基焦磷酸(IPP);2) IPP与依次加入的三个IPP分子异构化得到二甲基烯丙基焦磷酸(DMAPP),上述反应产物由异戊烯基转移酶催化得到C20化合物香叶基焦磷酸(GGPP),两分子GGPP缩合得到八氢番茄红素,脱氢后得到番茄红素;3) 番茄红素异构化同时发生在八氢番茄红素脱氢形成第一或第二个双键过程中,番茄红素作为环状结构类胡萝卜素前体,经一系列代谢反应形成β-胡萝卜素、γ-胡萝卜素、红酵母烯、红酵母红素和虾青素.
2 影响酵母发酵生产类胡萝卜素的因素
在工业规模下利用微生物生产类胡萝卜素必须寻求工艺成本低、产率高以及环境友好的技术.然而,类胡萝卜素的生物合成受诸多因素影响,例如光照、温度、通气和金属离子都有可能对类胡萝卜素发酵产量产生影响.
光照被认为是微生物生产类胡萝卜素的一个重要因素.微生物需要防止自身由于光照而受到损伤,类胡萝卜素生物合成机制就是一个光保护机制.类胡萝卜素生产受到白光的积极影响.Moliné等[4]研究红酵母菌株中类胡萝卜素和麦角固醇以及细胞紫外光抗性之间的关系,结果表明超着色菌株存活率增大(250%).他们还指出,红酵母红素的高产量可以提高紫外光下红酵母的存活率.另一方面,Yen等[5]计算了在具有两个LED(发光二极管)灯的间歇反应器中β-胡萝卜素的生产效率,结果β-胡萝卜素浓度达24.6 μg/g,而无光照条件下β-胡萝卜素浓度只有14.69 μg/g.
温度是酵母生产类胡萝卜素过程中需要考虑到的另一个因素.温度会影响细胞的生长以及代谢产物的生产,主要通过改变生物合成途径包括类胡萝卜素合成途径来发挥作用.温度的影响效果取决于微生物的种类以及产品产量.根据Hayman等[6]的研究,温度影响了涉及类胡萝卜素生产的酶浓度的调节水平.
通气影响类胡萝卜素生产的种类和总产量.这是因为类胡萝卜素生物合成是一个需氧过程,氧气影响着基质同化的速率、生物量的增长以及类胡萝卜素的生物合成.通气的影响取决于微生物的种类.Saenge等[7]研究了通气量对细胞生长、脂质产量、类胡萝卜素产量及甘油消耗量的影响,当通气速率从0 vvm增加到2 vvm时,生物量和脂质产量均达到最高,分别为8.17, 4.32 g/L.
金属离子(钡、铁、镁、钙、锌和钴)也已经被证明是R.Glutinis生产类胡萝卜素的促进剂[8].除此以外,Buzzini等[9]报道了在R.Graminis中某些微量元素表现出对类胡萝卜素的结构具有选择性的影响,比如Zn2+对β胡萝卜素和γ-胡萝卜素生产具有促进作用,而对红酵母烯和红酵母红素生产具有抑制作用.
3 廉价培养基的应用
绝大多数酵母的类胡萝卜素在菌种对数生长后期积累,在稳定期持续增加.在含有多种精细碳源(如葡萄糖、木糖、纤维二糖、蔗糖、甘油和山梨糖醇)的合成培养基中培养时,酵母菌能合成类胡萝卜素.以天然底物(如葡萄汁、未发酵葡萄汁、枣汁、芥末废弃物水解液提取物、半纤维素水解液、水解绿豆废渣、甘蔗汁、甘蔗、玉米糖浆、玉米水解液、乳清等)为碳源的类胡萝卜素合成研究已成为近些年的热点.工农业原产地的原料和副产物被认为是廉价微生物发酵生产过程中所需碳水化合物的可替代来源,这也同时减轻了因废弃物造成的环境问题.鸡毛和甜马铃薯已作为氮源和碳源[10]来生产类胡萝卜素,同时解决了鸡毛和甜马铃薯废弃物环保处理的能耗问题,最大限度地减轻了这些废弃物对环境的压力.表1列出了最新的工农业废渣在类胡萝卜素发酵生产中的应用实例.用工农业废弃物生产类胡萝卜素,其生产能力取决于碳源和氮源的种类、矿物质和其他成分的配比.这些营养物质的种类和配比对确定发酵培养基制定方法、提高微生物的类胡萝卜素生物合成能力非常重要.
表1 酵母以工农业废弃物作为基质 发酵生产类胡萝卜素的研究
Table 1 Researches of using agro-industrial wastes as substrates to yeasts carotenoid production
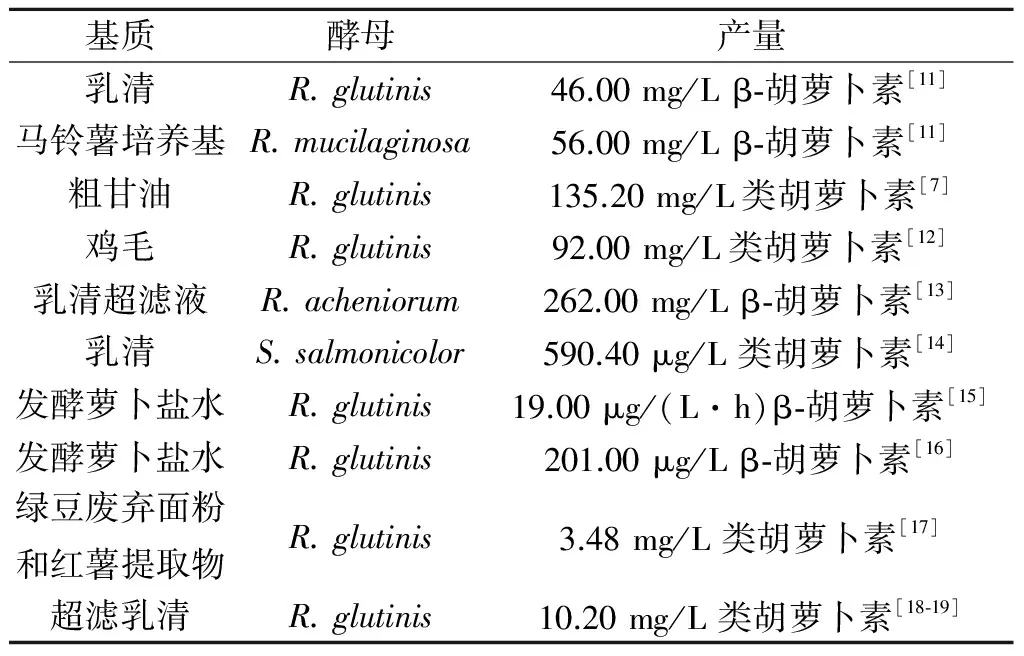
基质酵母产量乳清R.glutinis46.00mg/Lβ⁃胡萝卜素[11]马铃薯培养基R.mucilaginosa56.00mg/Lβ⁃胡萝卜素[11]粗甘油R.glutinis135.20mg/L类胡萝卜素[7]鸡毛R.glutinis92.00mg/L类胡萝卜素[12]乳清超滤液R.acheniorum262.00mg/Lβ⁃胡萝卜素[13]乳清S.salmonicolor590.40μg/L类胡萝卜素[14]发酵萝卜盐水R.glutinis19.00μg/(L·h)β⁃胡萝卜素[15]发酵萝卜盐水R.glutinis201.00μg/Lβ⁃胡萝卜素[16]绿豆废弃面粉和红薯提取物R.glutinis3.48mg/L类胡萝卜素[17]超滤乳清R.glutinis10.20mg/L类胡萝卜素[18⁃19]
4 产类胡萝卜素酵母菌选育策略
4.1 诱 变
Vijayalaksmi等[20]以紫外线诱变R.gracilis菌株,其类胡萝卜素合成能力较原始菌株提高了约1.8倍.粉色酵母株R.glutinis经紫外诱变得到黄色诱变株32,其类胡萝卜素产量比野生株高出24倍.Frengova等[21]发现NTG诱变菌R.rubra56-13也表现出了更高的类胡萝卜素和β-胡萝卜素生产能力.Wang等[22]用高静水压(300 MPa)重复5次处理后得到诱变株,其β-胡萝卜素产量增加了57.89%.
暗红色酵母菌株P.rhodozyma经甲基磺酸乙酯处理后其β-类胡萝卜素产量是之前的5倍[23].NTG诱变XanthophyllomycesDendrourhous后分离得到两种产类胡萝卜素强化菌株X.DendrourhousJH1和JH2[24].虾青素高产突变株JH1产虾青素约为野生型的15倍多.产β-胡萝卜素突变株JH2的β-胡萝卜素产量比野生型增产约4倍.An等[25]发现抗霉素突变株和亚硝基胍衍生突变株产虾青素能力比原始菌株高出很多.Schroeder等[26]还发现游离氧自由基能够通过激活基因诱发P.rhodozyma体内类胡萝卜素的合成.Fleno等[27]用EMS及紫外线处理得到高产类胡萝卜素和虾青素的突变株.
4.2 产类胡萝卜素酵母的代谢工程
代谢工程通过具体的生化反应或利用重组DNA技术引进新的基因来改进细胞性能和提高目标产物的产量[28].非类胡萝卜素生物合成微生物的改造工程是类胡萝卜素生产非常有用的工具,如酿酒酵母被认为是一种安全的酵母并具有容易进行基因操作等优点.虽然自然条件下酿酒酵母不产生类胡萝卜素,但它产生香叶基焦磷酸,如果此酵母集成了源于法夫酵母的两个主要的类胡萝卜素合成酶的基因(即编码番茄红素合成酶(crtYB)和番茄红素脱氢酶(CTRI)的基因)就可以生产类胡萝卜素[29].Yamano等[30]早期尝试把细菌基因导入酿酒酵母中生产β-胡萝卜素,虽然在工程上取得了成功,但是生产水平低下,产量仅103 μg/g.毕赤酵母也是进行类胡萝卜素生产研究的非类胡萝卜素生物合成酵母,主要优势在于它可以在甲醇中生长.Araya-Garay等[31]设计并构建了两个含有编码番茄红素和β胡萝卜素的基因的质粒并转入毕赤酵母中,结果表明得到的重组菌株番茄红素和β胡萝卜素产量分别达到1.141,339 μg/g.
5 结 论
类胡萝卜素在人类健康领域发挥着重要作用.化学合成类胡萝卜素虽能满足市场的部分需求,但其生产过程不够环保高效,产品结构和天然类胡萝卜素也有差异.目前,对微生物生产类胡萝卜素已经进行了广泛研究,微生物发酵生产类胡萝卜素不存在由于季节性和地域性变化而导致的产品和市场问题,从而具有更大的经济效益.目前,红酵母和法夫酵母是类胡萝卜素的潜在来源,而以代谢工程方法改造酿酒酵母生产类胡萝卜素也具有良好前景.生产成本高是大规模发酵生产类胡萝卜素的最大限制因素,采用工农业废弃物等廉价底物可以降低生产成本,并且有助于减少废弃物本身对环境的污染.通过酵母菌种的基因改造和发酵工艺的改进等各种方法提高产量,将有助于实现微生物法发酵生产类胡萝卜素产品的工业化.
[1] 刘颖.法夫酵母生物法生产虾青素的研究进展[J].发酵科技通讯,2012,41(3):47-52.
[2] SIMPSON K L, NAKAYAMA T O, CHICHESTER C O. Biosynthesis of yeast carotenoids[J].Journal of bacteriology,1965,88(6):1688-1694.
[3] GOODWIN T W. Biosynthesis of carotenoids[M]//The Biochemistry of the Carotenoids. Berlin: Springer Netherlands,1980:33-76.
[4] MOLINÉ M, FLORES M R, LIBKIND D, et al. Photoprotection by carotenoid pigments in the yeastRhodotorulamucilaginosa: the role of torularhodin[J].Photochemical & photobiological sciences official journal of the european photochemistry association & the european society for photobiology,2010,9(8):1145-1151.
[5] YEN H W, ZHANG Z. Enhancement of cell growth rate by light irradiation in the cultivation ofRhodotorulaglutinis[J].Bioresource technology,2011,102(19):9279-9281.
[6] HAYMAN E P, YOKOYAMA H, CHICHESTER C O, et al. Carotenoid biosynthesis inRhodotorulaglutinis[J].Journal of bacteriology,1974,120(3):1339-1343.
[7] SAENGE C, CHEIRSILP B, BOURTOOM T. Potential use of oleaginous red yeastRhodotorulaglutinis, for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids[J].Process biochemistry,2011,46(1):210-218.
[8] KOMEMUSHI S, SAKAKI H, YOKOYAMA H, et al. Effect of barium and other metals on the growth of a D-lactic acid assimilating yeastRhodotorulaglutinisN21[J].Journal antibact antifung agents,1994,22:583-587.
[9] BUZZINI P, MARTINI A, GAETANI M, et al. Optimization of carotenoid production byRhodotorulagraminis, DBVPG 7021 as a function of trace element concentration by means of response surface analysis[J].Enzyme & microbial technology,2005,36(5/6):687-692.
[10] DEMAIN A L, PHAFF H J, KURTZMAN C P. Chapter 3. The industrial and agricultural significance of yeasts[M]//The Yeasts. 4th ed. Amsterdam: Elsevier,1998:13-19.
[11] MAROVA I, CARNECKA M, HALIENOVA A, et al. Use of several waste substrates for carotenoid-rich yeast biomass production[J].Journal of environmental management,2012,95(2):338-342.
[12] TASKIN M, SISMAN T, ERDAL S, et al. Use of waste chicken feathers as peptone for production of carotenoids in submerged culture ofRhodotorulaglutinisMT-5[J].European food research & technology,2011,233(4):657-665.
[13] PANESAR P S, KENNEDY J F. Biotechnological approaches for the value addition of whey[J].Critical reviews in biotechnology,2012,32(4):327-348.
[14] VALDUGA E, TATSCH P, VANZO L T, et al. Assessment of hydrolysis of cheese whey and use of hydrolysate for bioproduction of carotenoids bySporidiobolussalmonicolorCBS 2636[J].Journal of the science of food & agriculture,2009,89(6):1060-1065.
[15] MALISORN C, SUNTORNSUK W. Improved β-carotene production ofRhodotorulaglutinis, in fermented radish brine by continuous cultivation[J].Biochemical engineering journal,2009,43(1):27-32.
[16] MALISORN C, SUNTORNSUK W. Optimization of beta-carotene production byRhodotorulaglutinisDM28 in fermented radish brine[J].Bioresource technology,2008,99(7):2281-2287.
[17] TINOI J, RAKARIYATHAM N, DEMING R L. Simplex optimization of carotenoid production byRhodotorulaglutinisusing hydrolyzed mung bean waste flour as substrate[J].Process biochemistry,2005,40(7):2551-2557.
[18] FRENGOVA G, SIMOVA E, PAVLOVA K, et al. Formation of carotenoids byRhodotorulaglutinisin whey ultrafiltrate[J].Biotechnology & bioengineering,1994,44(8):888-894.
[19] FRENGOVA G I, EMILINA S D, BESHKOVA D M. Carotenoid production by lactoso-negative yeasts co-cultivated with lactic acid bacteria in whey ultrafiltrate[J].Zeitschrift für naturforschung C journal of biosciences,2003,58(8):562-567.
[20] VIJAYALAKSHMI G, SHOBHA B, VANAJAKSHI V, et al. Response surface methodology for optimization of growth parameters for the production of carotenoids by a mutant strain ofRhodotorulagracilis[J].European food research and technology,2001,213(3):234-239.
[21] FRENGOVA G I, SIMOVA E D, BESHKOVA D M. Improvement of carotenoid-synthesizing yeastRhodotorularubraby chemical mutagenesis[J].Zeitschrift für naturforschung C journal of biosciences,2004,59(1/2):99-103.
[22] WANG S L, SUN J S, HAN B Z, et al. Optimization of beta-carotene production byRhodotorulaglutinisusing high hydrostatic pressure and response surface methodology[J].Journal of food science,2007,72(8):325-329.
[23] GIRARD P, FALCONNIER B, BRICOUT J, et al. β-Carotene producing mutants ofPhaffiarhodozyma[J].Applied microbiology and biotechnology,1994,41(2):183-191.
[24] KIM J H, KIM C W, CHANG H I. Screening and characterization of red yeastXanthophyllomycesdendrorhousmutants[J].Journal of microbiology & biotechnology,2004,14:570-575.
[25] AN G H, SCHUMAN D B, JOHNSON E A. Isolation ofPhaffiarhodozymamutants with increased astaxanthin content[J].Applied & environmental microbiology,1989,55(1):116-124.
[26] SCHROEDER W A, JOHNSON E A. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis inPhaffiarhodozyma[J].Journal of biological chemistry,1995,270(31):18374-18379.
[27] FLEN S B O, CHRISTENSEN I, LARSEN R, et al. Astaxanthin-producing yeast cells methods for their preparation and their use: US 5356810 A[P].1994-10-13.
[28] PARK P K, KIM E Y, CHU K H. Chemical disruption of yeast cells for the isolation of carotenoid pigments[J].Separation & purification technology,2007,53(2):148-152.
[29] VERWAAL R, WANG J, MEIJNEN J P, et al. High-level production of beta-carotene inSaccharomycescereviseaeby successive transformation with carotenogenic genes fromXanthophyllomycesdendrorhous[J].Applied & environmental microbiology,2007,73(13):4342-4350.
[30] YAMANO S, ISHII T, NAKAGAWA M, et al. Metabolic engineering for production of beta-carotene and lycopene inSaccharomycescerevisiae[J].Agricultural & biological chemistry,1994,58(6):1112-1114.
[31] ARAYA-GARAY J M, FEIJOO-SIOTA L, ROSA-DOS-SANTOS F, et al. Construction of newPichiapastoris, X-33 strains for production of lycopene and β-carotene[J].Applied microbiology & biotechnology,2012,93(6):2483-2492.
(责任编辑:朱小惠)
The research development of carotenoids production by yeast
NIU Tingting1, SUN Xiping2, WU Tao2
(1. Shanghai University of Sport, Shanghai 200438, China; 2. Ocean College, Zhejiang University of Technology, Hangzhou 310014, China)
Carotenoids represent a valuable product for the pharmaceutical, chemical, food and feed industries for their coloring, antioxidant and possible tumor-inhibiting activity. Animals cannot synthesize carotenoids, and these pigments must therefore be obtained from food. As the potential pigment sources, the generaRhodotorulaandPhaffiawere important strains for carotenoids production by microorganism method. In this review, the carotenoids biosynthesis pathway inRhodotorulaandPhaffiawas interpreted and the influence factors and low-cost media were reviewed. It was further introduced that mutation breeding and metabolic engineering technology was applied in yeast strain improvement for the carotenoid production.
carotenoids;Rhodotorula;Phaffia; low-cost media
2016-12-29
钮亭亭(1985—),女,浙江湖州人,讲师,硕士,研究方向为营养学,E-mail:niutingting@sus.edu.cn.通信作者:吴涛副教授,E-mail:wt_hz@zjut.edu.cn.
TQ92
A
1674-2214(2017)01-0050-04

