qRT-PCR检测结核分枝杆菌毒素-抗毒素系统mazE F的表达
刘 微,赵继利,屈艳琳,谢婉莹,袁 俐
qRT-PCR检测结核分枝杆菌毒素-抗毒素系统mazE F的表达
刘 微,赵继利,屈艳琳,谢婉莹,袁 俐
目的 探讨结核分枝杆菌毒素基因mazF3,6,9及抗毒素基因mazE3,6,9的表达差异。方法 运用实时定量PCR检测结核分枝杆菌单耐药株20株,耐多药株20株和标准株H37Rv毒素基因mazF3,6,9及抗毒素基因mazE3,6,9的表达水平;组间基因表达水平的差异用one-way ANOVA进行统计分析。结果 与对照株相比较,毒素基因mazF6,9在单耐药组(11.151 9±22.317 21;8.430 6±17.978 97)及耐多药组(4.601 6±1.290 18;6.962 7±6.929 48)表达均上调且差异有统计学意义(P<0.01),mazF3基因在单耐药组及耐多药组差异无统计学意义(P>0.05),抗毒素基因mazE3在单耐药(0.360 6±0.125 27)及耐多药组(0.201 6±0.165 42)中表达均下调,差异有统计学意义(P<0.01),mazE6均无统计学意义,mazE9只有在耐多药组(0.398 9±0.376 79)中表达下调,差异有统计学意义,(P<0.01)。结论 毒素基因mazF6,9抗毒素基因mazE3,9可能参与了结核分枝杆菌的耐药形成,具体机制尚待进一步的研究。
结核分枝杆菌;毒素-抗毒素系统;耐药性;耐多药
毒素-抗毒素系统(toxin-antitoxin systems, TAS)最早发现于低拷贝的质粒中,随后的研究发现在某些细菌如结核分枝杆菌的染色体上也存在TA系统[1]。TA系统是由两个相互重叠的基因组成的一个操纵子,其中一个编码TA毒素蛋白,另一个编码抗毒素[2-3]。毒素蛋白以不同的方式影响细胞功能,如DNA复制,蛋白质合成,细胞分裂,肽聚糖生物合成以及核糖体组装等,其中RNA裂解是常见的方式[4-7]。根据抗毒素的作用方式及化学本质TA家族可分成5型[8]。mazEF是II型系统,其中mazE编码不稳定的抗毒素MazE,mazF编码稳定的毒素蛋白MazF,MazF为一种核糖核酸内切酶能切割单链mRNA,MazF切割mRNA具有ACA特异性[9-12]。毒素-抗毒素系统(TAS)能够感应不同的环境条件如氨基酸缺乏,氧化应激,缺氧,被巨噬细胞吞噬及在宿主组织中[13-17]。
结核病(tuberculosis, TB)是一种由结核分枝杆菌(Mycobacteriumtuberculosis, MTB)引起的一种慢性传染病。随着结核分枝杆菌耐药率的增高,结核病的感染率及死亡率日益增高。结核分枝杆菌的毒素-抗毒素系统(TAS)可能促进细菌适应环境变化,休眠及产生耐药[18]。本实验选择用qRT-PCR的方法检测mazEF系统中抗毒素基因mazE3,6,9及毒素基因mazF3,6,9在耐药菌株中的表达,并与对照株H37Rv进行比较,同时观察单耐药及耐多药菌株在不同培养条件下的生长情况。
1 材料与方法
1.1 材料
1.1.1 标本来源 结核分枝杆菌标准株H37Rv及40株结核分枝杆菌临床分离株(菌株为本实验室人员从新疆开放性结核病患者痰液中分离并做药敏鉴定,并由本实验室保存),其中单耐药组20株,耐多药组20株。
1.1.2 主要试剂与仪器 细菌RNA提取试剂盒购自Qiagen公司,逆转录试剂盒购自北京天根生化科技有限公司,荧光定量PCR试剂盒及实时定量PCR仪购自Life Technologies公司。
1.2 方法1.2.1 引物设计及合成 将目的片段发给上海生物工程公司,由该公司设计实时定量引物(表1)并合成。
表1 引物序列
Tab.1 Primer sequences
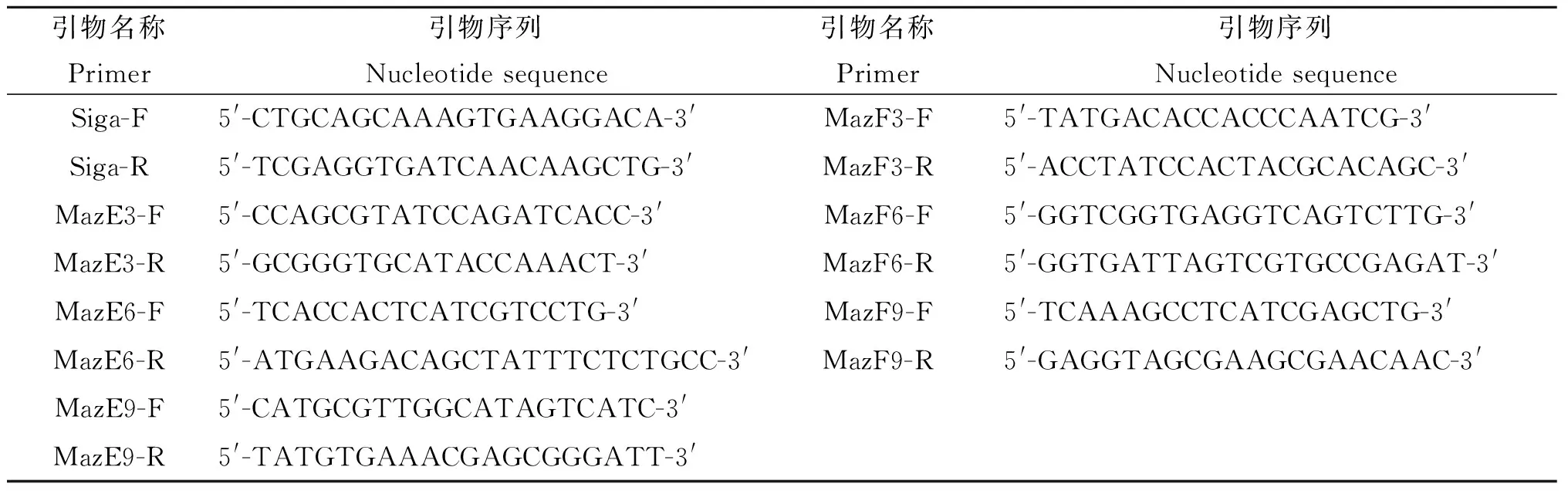
引物名称Primer引物序列Nucleotidesequence引物名称Primer引物序列NucleotidesequenceSiga-F5'-CTGCAGCAAAGTGAAGGACA-3'MazF3-F5'-TATGACACCACCCAATCG-3'Siga-R5'-TCGAGGTGATCAACAAGCTG-3'MazF3-R5'-ACCTATCCACTACGCACAGC-3'MazE3-F5'-CCAGCGTATCCAGATCACC-3'MazF6-F5'-GGTCGGTGAGGTCAGTCTTG-3'MazE3-R5'-GCGGGTGCATACCAAACT-3'MazF6-R5'-GGTGATTAGTCGTGCCGAGAT-3'MazE6-F5'-TCACCACTCATCGTCCTG-3'MazF9-F5'-TCAAAGCCTCATCGAGCTG-3'MazE6-R5'-ATGAAGACAGCTATTTCTCTGCC-3'MazF9-R5'-GAGGTAGCGAAGCGAACAAC-3'MazE9-F5'-CATGCGTTGGCATAGTCATC-3'MazE9-R5'-TATGTGAAACGAGCGGGATT-3'
1.2.2 细菌总RNA提取及逆转录 取在7H9液体培养基(含OADC增菌剂)中生长至对数期的细菌1~3 mL,按Qiagen公司RNA提取试剂盒操作步骤提取RNA,1.2%变性琼脂糖电泳检测其完整性。取5 μL的RNA加入2 μL oligo(dT),2 μL super pure dntps,70 ℃加热5 min后迅速在冰上冷却,然后加入4 μL 5×First-strand buffer,0.5 μL Rnasin,1 μL lM-Mlv混匀,42 ℃ 50 min,95 ℃ 5 min终止反应逆转录为cDNA,最后无酶水定容至20 μL,-20℃保存备用。以cDNA 为模板进行特异性扩增,反应条件(表2)。以结核分枝杆菌H37Rv标准株为模板扩增出mazE3 6 9 mazF3 6 9 长度分别为321 bp; 249 bp; 231 bp; 312 bp; 345 bp;357 bp(图1)。
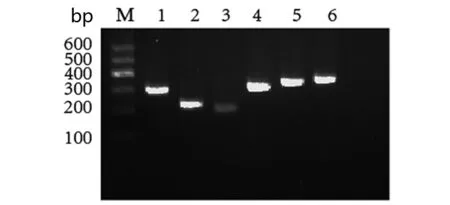
M:DNA marker;1 to 6 gene of mazE3,6,9 and mazF3,6,9 in turnM为DNA maker,1到6泳道分别为抗毒素基因mazE3,6,9和毒素基因mazF3,6,9图1 基因mazE3 6 和mazF3 6 9 扩增片段。Fig.1 PCR amplification of mazE3,6,9 and mazF3,6,9 genes
表2 PCR反应条件
Tab.2 PCR Reaction conditions
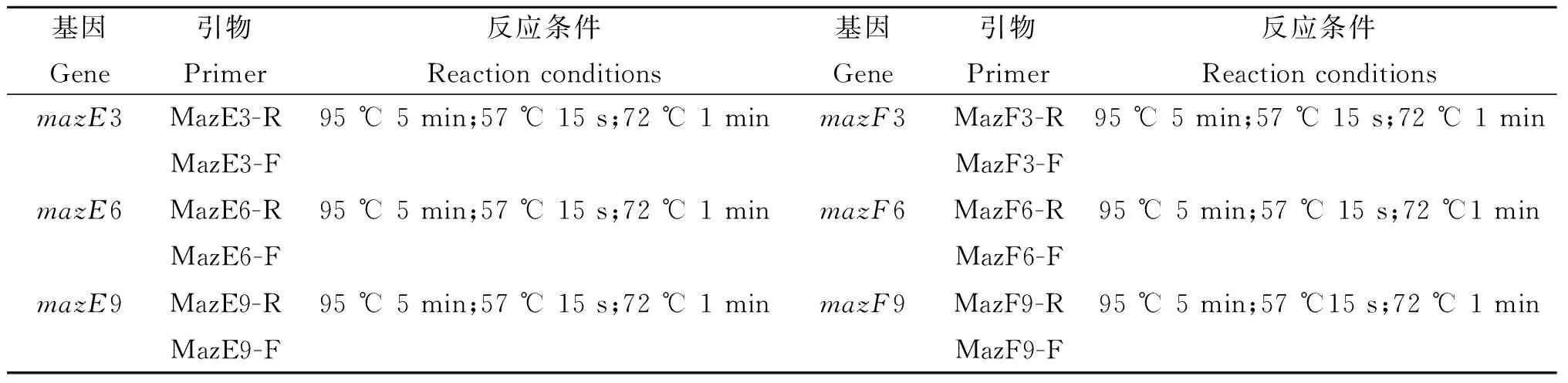
基因Gene引物Primer反应条件Reactionconditions基因Gene引物Primer反应条件ReactionconditionsmazE3MazE3-R95℃5min;57℃15s;72℃1minmazF3MazF3-R95℃5min;57℃15s;72℃1minMazE3-FMazF3-FmazE6MazE6-R95℃5min;57℃15s;72℃1minmazF6MazF6-R95℃5min;57℃15s;72℃1minMazE6-FMazF6-FmazE9MazE9-R95℃5min;57℃15s;72℃1minmazF9MazF9-R95℃5min;57℃15s;72℃1minMazE9-FMazF9-F
1.2.3 qRT-PCR检测mazE3,6,9 和mazF3,6,9mRNA的表达水平 以Siga为阳性对照,反应体系共20 μL LSYBR Select Master Mix 10 μL,上下游引物各0.5 μL,模板2 μL,无酶水7 μL ,50 ℃ 2 min激活SYBR,95 ℃预变性2 min,95 ℃ 15 s,60 ℃15 s ,72 ℃ 1 min 40个循环。实时定量PCR仪器自动绘制溶解曲线。
2 结 果
2.1 毒素基因mazF3,6,9在单耐药、耐多药株及标准株H37Rv中的表达(图2),结果显示,mazF6,9基因在单耐药及耐多药菌组中的表达均高于标准株H37Rv,有统计学意义,而mazF3无论在单耐药还是耐多药组中的表达与标准株相比均无统计学意义。

注:**P<0.01Measuring toxin transcript levels by quantitative RT-PCR (qRT-PCR). For RT-PCR analysis, mRNA was extracted from different groups, significant differences were observed for the different groups (**P<0.01).图2 qPT-PCR检测毒素基因mazF3,6,9在各组的表达量Fig.2 Measuring toxin genes transcription levels by quantitative RT-PCR (qRT-PCR)
2.2 抗毒素基因mazE3,6,9在单耐药,耐多药及标准株H37Rv中的表达(图3),结果显示抗毒素基因mazE3在单耐药及耐多药菌株中表达都低于标准株H37Rv,mazE9只有在耐多药菌株中的表达才低表达,而mazE6的表达量与标准株H37Rv相比无统计学意义。
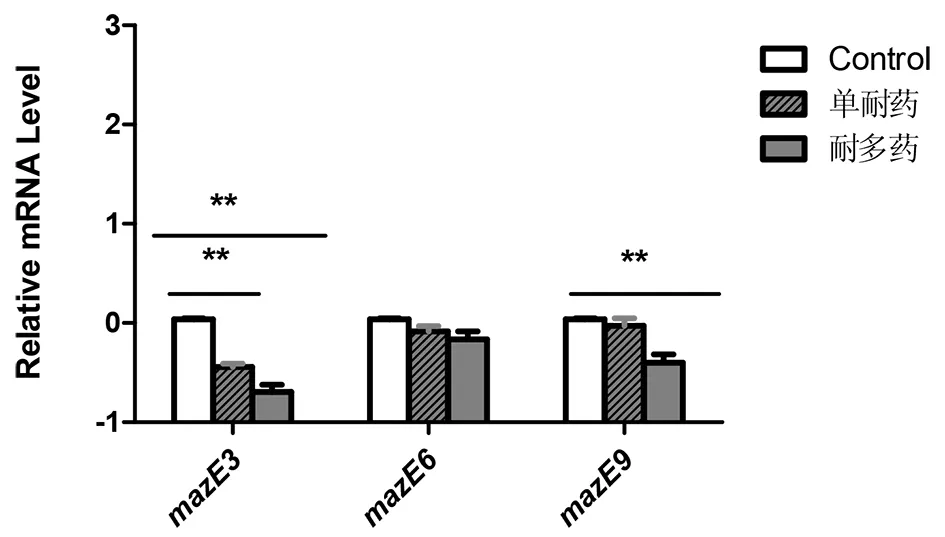
注:**P<0.01Measuring antitoxin transcript levels by quantitative RT-PCR (qRT-PCR). For RT-PCR analysis, mRNA was extracted from different groups, significant differences were observed for the different groups (**P<0.01).图3 qPT-PCR检测抗毒素基因mazE3,6,9在各组的表达量Fig.3 Measuring antitoxin genes transcription levels by quantitative RT-PCR (qRT-PCR)
2.3 结核分枝杆菌在不同培养条件下的生长情况
分别从单耐药及耐多药菌株中取出3株菌株及H37Rv共7株菌,在7H9液体培养基中37 ℃,摇床中培养7 d,调整浊度为0.06MCF后分别接种到7H9液体培养基,PBS(低营养)及缺氧的试管中,37 ℃摇床中培养,分别于培养的第2,4,6,8,10 d检测浊度,然后进行统计学分析。
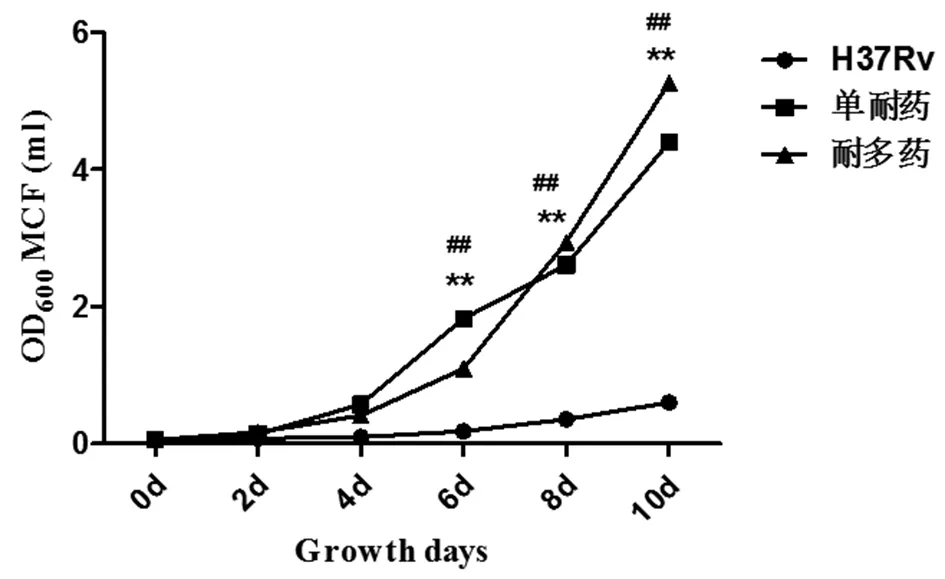
2.3.1 单耐药菌株,耐多药菌株及标准株H37Rv在7H9液体培养基中第2,4,6,8,10 d的生长浊度(图4),结果显示,单耐药组在培养的第2,4,6 d浊度不同但是无统计学意义(P>0.05),第8,10 d有统计学差异(P<0.01),耐多药组在培养第2,4 d浊度不同但是差异无统计学意义(P>0.05),第6,8,10 d差异有统计学意义(P<0.01)。

注:*单耐药菌株与H37Rv相比,#耐多药菌株与H37Rv相比,**表示P<0.01*mono-resistance strains compared with H37Rv, #multidrug resistance strains compared with H37Rv, **P<0.01图4 不同培养时间点结核分枝杆菌在7H9液体培养基中的生长浊度Fig.4 The counts of bacteria at different culture time points under the condition of 7H9 liquid culture
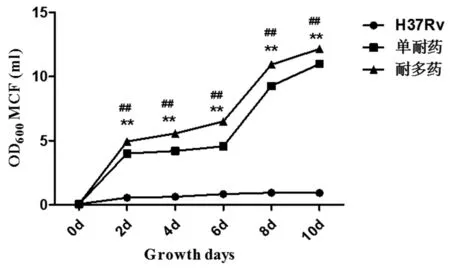
2.3.2 单耐药菌株,耐多药菌株及标准株H37Rv在PBS(低营养)培养基中第2,4,6,8,10 d的生长浊度(图5),结果显示,单耐药组及耐多药组在培养的第2,4 d生长浊度不同但无统计学意义(P>0.05),在培养的第6,8,10 d浊度有差异,差异有统计学意义(P<0.01)。
注:*单耐药菌株与H37Rv相比, #耐多药菌株与H37Rv相比,**表示P<0.01*mono-resistance strains compared with H37Rv, #multidrug resistance strains compared with H37Rv, **P<0.01图5 不同培养时间点结核分枝杆菌在PBS液体培养基中的生长浊度Fig.5 The counts of bacteria at different culture time points under the condition of PBS liquid culture
2.3.3 单耐药菌株,耐多药菌株及标准株H37Rv在低氧条件下培养第2,4,6,8,10 d的生长浊度(图6),结果显示单耐药,耐多药在培养的第2 d开始浊度就有差异,差异有统计学意义(P<0.01)。
注:*单耐药菌株与H37Rv相比,#耐多药菌株与H37Rv相比,**表示P<0.01*mono-resistance strains compared with H37Rv, #multidrug resistance strains compared with H37Rv,**P<0.01图6 不同培养时间点结核分枝杆菌在缺氧条件下的生长浊度Fig.6 The counts of bacteria at different culture time points under the condition of anoxia culture
3 讨 论
我国是全球第二大结核病高负担国家,也是全球27个耐多药结核病严重的国家之一,耐药结核病报告发病人数始终位居法定报告甲乙类传染病前列。结核分枝杆菌耐药机理尚不完全清楚,与许多机制有关[19]。
尽管毒素-抗毒素(TA)系统的功能尚未完全阐明,但是一些研究表明TA系统能够对外界压力产生应答[20,9]。在环境胁迫条件下不稳定的抗毒素降解,毒素发挥作用,介导细菌的耐药,持留形成或死亡的发生[8]。在大量的结核分枝杆菌TA系统中,已有30个被证明具有一定的功能[20],TA系统在该菌可能遭遇多种压力如低氧[21]、营养缺乏[22]、被巨噬细胞吞噬[16]和抗生素毒性[14]等,可引起细菌生长抑制、持留状态及耐药,提高对抗生素及不良环境的适应能力,对于引起结核病的迁延及反复感染起着重要作用。现在研究最多的TA系统是mazEF家族。
本实验选择了单耐药及耐多药菌株作为研究对象,根据结果看毒素mazF3,6,9以及抗毒素mazE3,6,9表达并不相同,毒素基因MazF3 6在单耐药及耐多药组与H37RV相比均高表达,差异有统计学意义,而MazF9在两实验组中差异均无统计学意义。抗毒素mazE3在单耐药及耐多药组中都低表达,与对照株H37Rv相比差异有统计学意义,而mazE6无论在单耐药还是耐多药组中,差异都无统计学意义(图1.2),这是否与不同药物的作用机制不同有关还不清楚,MazEF家族中的毒素抗毒素是否会相互作用,这都需要进一步的研究。不同条件下培养比浊结果表明无论是单耐药组还是耐多药组对于低氧和低营养的耐受都比对照株H37Rv好。在单耐药及耐多药菌株中mazF6,9都高表达,而mazF作为一种限制性内切酶切割单链ACA,毒素持续过表达也会导致细菌的死亡[10,23-24]。从不同条件培养的生长曲线来看,毒素高表达的耐药株反而能更好的适应不利生存环境而没有死亡,是否是因为毒素表达量没有达到导致菌体死亡的浓度还需要进一步研究。如果毒素高表达在一定范围内只是提高了菌体对不利环境的耐受性,只有更高的表达才能诱导菌体的死亡,那么我们是否可以找到这个临界值诱导耐药菌中的MazF表达达到杀菌的浓度?从mRNA到最终的功能蛋白,这其中还要经过翻译,蛋白修饰等过程,这些因素都将影响蛋白质的功能,因此,联合蛋白质水平进行研究结核菌的耐药性也是十分必要的。
[1] Chang JN,Ning DG. Identification of a pair ofToxin-antitoxin(TA) gene in the chromosome of cyanobacteria synechocystis sp. PCC6803. Microbiology China, 2009, 36(1):31-36 (in Chinese)
常家宁, 宁德刚. 蓝细菌PCC6803染色体上的一对毒素-抗毒素基因的鉴定[J]. 微生物学通报, 2009, 36(1):31-36.
[2] Gerdes K, Christensen SK, Lubnerolesen A. Prokaryotic toxin-antitoxin stress response loci[J]. Nat Rev Microbiol, 2005, 3(5):371-382.
[3] Yamaguchi Y, Inouye M. Regulation of growth and death inEscherichiacoliby toxin-antitoxin systems[J]. Nat Rev Microbiol, 2011, 9(11):779-790.
[4] Winther KS, Gerdes K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA[J]. Proc Nat Acad Sci, 2011, 108(18):7403-7407.
[5] Mutschler H, Gebhardt M, Shoeman R L, et al. A novel mechanism of programmed cell death in bacteria by Toxin-antitoxin systems corrupts peptidoglycan synthesis[J]. PLoS Biol, 2011, 9(3):e1001033-e1001033.
[6] Tan Q, Awano N, Inouye M. YeeV is anEscherichiacoliToxin that Inhibits Cell Division by Targeting the Cytoskeleton Proteins, FtsZ and MreB[J]. Mol Microbiol, 2011, 79(1):109-118.
[7] Prysak M H, Mozdzierz C J, Cook A M, et al. Bacterial toxin YafQ is an endoribonuclease that associates with the ribosome and blocks translation elongation through sequence-specific and frame-dependent mRNA cleavage[J]. Mol Microbiol, 2009, 71(5):1071-1087.
[8] Schuster C F, RalphBertram. Toxin-antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate[J]. FEMS Microbiol Lett, 2013, 340(2):73-85.
[9] Aizenman E, Engelbergkulka H, Glaser G. AnEscherichiacolichromosomal "addiction module" regulated by guanosine [corrected] 3′,5′-bispyrophosphate: a model for programmed bacterial cell death[J]. Proc Nat Acad Sci, 1996, 93(12):6059-6063.
[10] Zhang Y, Zhang J, Hoeflich KP, et al. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis inEscherichiacoli[J]. Mol Cell, 2003, 12(4):913-923.
[11] Suzuki M, Mao L, Inouye M. Single protein production (SPP) system inEscherichiacoli[J]. Nat Protoc, 2007, 2(7):1802-1810.
[12] Suzuki M, Zhang J, Liu M, et al. Single protein production in living cells facilitated by an mRNA interferase[J]. Mol Cell, 2005, 18(2):253-261.
[13] Ramage HR, Connolly LE, Cox JS. Comprehensive functional analysis ofMycobacteriumtuberculosistoxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution[J]. PLoS Genet, 2009, 5(12):1000767.
[14] Singh R, Barry CE, Boshoff HI. The three RelE homologs ofMycobacteriumtuberculosishave individual, drug-specific effects on bacterial antibiotic tolerance[J]. J Bacteriol, 2010, 192(5):1279-1291.
[15] Keren I, Minami S, Rubin E, et al. Characterization and transcriptome analysis ofMycobacteriumtuberculosispersisters.[J]. Mbio, 2011, 2(3):00100-00111.
[16] Korch SB, Contreras HClark-Curtiss JE. ThreeMycobacteriumtuberculosisRel toxin-antitoxin modules inhibit mycobacterial growth and are expressed in infected human macrophages[J]. J Bacteriol, 2009, 191(5):1618-1630.
[17] Ramirez MV, Dawson CC, Crew R, et al. MazF6 toxin ofMycobacteriumtuberculosis, demonstrates antitoxin specificity and is coupled to regulation of cell growth by a Soj-like protein[J]. BMC Microbiol, 2013, 13(1):986-991.
[18] Han JS, Lee JJ, Anandan T, et al. Characterization of a chromosomal toxin-antitoxin, Rv1102c-Rv1103c system inMycobacteriumtuberculosis[J]. Biochem Biophys Res Commun, 2010, 400(3):293-298.
[19] Xu Y, Zhang Z, Sun Z. Drug resistance toMycobacteriumtuberculosis: from the traditional Chinese view to modern systems biology[J]. Crit Rev Mycrobio, 2015,41(3):399-410.
[20] Buts L, Lah J, Dao-Thi MH, et al. Toxin-antitoxin modules as bacterial metabolic stress managers[J]. Trends Biochem Sci, 2005, 30(12):672-679.
[21] Rustad TR, Harrell MI, Liao R, et al. The enduring hypoxic response ofMycobacteriumtuberculosis[J]. PloS One, 2008, 3(1) e1502.
[22] Albrethsen J, Agner J, Piersma SR, et al. Proteomic profiling ofMycobacteriumtuberculosisidentifies nutrient-starvation-responsive toxin-antitoxin systems [J]. Mol Cell Proteomics, 2013, 12(5):1180-1191.
[23] Zhang Y, Zhang J, Hara H, et al. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase[J]. J Biol Chem, 2005, 280(5):3143-3150.
[24] Erental A, Sharon I, Engelbergkulka H. Two programmed cell death systems in,Escherichiacoli: an apoptotic-Like death is inhibited by the mazEF-mediated death pathway[J]. PLoS Biol, 2012, 10(3):490-493.
Detection on expression levels of mazE F toxin-antitoxin system inMycobacteriumtuberculosisby qRT-PCR
LIU Wei,ZHAO Ji-li,QU Yan-lin,XIE Wan-ying,YUAN Li
(DepartmentofImmunology,SchoolofMedicineSheheziUniversity,Shehezi832000,China)
We investigate the different expression of toxin genemazF3,6,9 and antitoxin genemazE3,6,9 in the drug-resistanceMycobacteriumtuberculosis,we used quantitative real-time polymerase chin reaction method to detect the expression level of toxin genemazF3,6,9 and antitoxin genemazE3,6,9 inM.tuberculosis(20 mono-resistance strains, 20 multidrug resistance strains and standard strain H37Rv).The differences of gene expression levels between groups were analyzed by one-way ANOVA. Contrasting with control group, toxin genesmazF6,9 were up-regulated expression levels both in mono-resistance (11.1519±22.31721;8.4306±17.97897) and multidrug resistance (4.6016±1.29018;6.9627±6.92948), had statistical significance (P<0.01),mazF3 expression levels had statistical significance neither in mono-resistance nor in multidrug resistance (P>0.05); antitoxin genesmazE3 was in down-expression level, and had statistical significance both in mono-resistance (0.3606±0.12527) and multidrug resistance (0.2016±0.16542) (P<0.01),mazE6 had no statistical significance (P>0.05)either in mono-resistance or multi drug resistance,mazE9 only in multidrug resistance(0.3989±0.37679) was in down-expression level, and has statistical significance (P<0.001). The toxin genemazF6,9 and antitoxin genemazE3,9 may participate in the drug-resistance formation ofM.tuberculosis.
Mycobacteriumtuberculosis; toxin-antitoxin system; drug resistance; multidrug resistance
Yuan Li,Email:yuanli832000@sina.com
10.3969/j.issn.1002-2694.2017.02.010
国家自然科学基金(No.81341079,81560264)资助
袁 俐, yuanli832000@sina.com
石河子大学医学院,免疫学教研室,石河子 832000
R378
A
1002-2694(2017)02-0143-05
2016-09-26 编辑:王晓欢
Supported by the National Natural Science Foundation of China(No:81341079 and 81560264)

