Prevalence and genotypes of Chlamydiapsittaci in pigeons in Jilin Province, Northeastern China
YAO Qiu-xia, ZHANG Xiao-xuan, CHEN Kai, MA Jian-gang, ZHENG Wen-Bing, XU Xiao-qin, ZHU Xing-quan
(1. College of Veterinary Medicine, Yangzhou University, Yangzhou 225009 China; 2. State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute,Chinese Academy of Agricultural Sciences, Lanzhou 730046 China; 3. College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118 China)
Prevalence and genotypes ofChlamydiapsittaciin pigeons in Jilin Province, Northeastern China
YAO Qiu-xia1,2, ZHANG Xiao-xuan2,3, CHEN Kai2, MA Jian-gang2,3, ZHENG Wen-Bing2,3, XU Xiao-qin1, ZHU Xing-quan2
(1.CollegeofVeterinaryMedicine,YangzhouUniversity,Yangzhou225009China; 2.StateKeyLaboratoryofVeterinaryEtiologicalBiology,LanzhouVeterinaryResearchInstitute,ChineseAcademyofAgriculturalSciences,Lanzhou730046China; 3.CollegeofAnimalScienceandTechnology,JilinAgriculturalUniversity,Changchun130118China)
Chlamydiapsittaciis a causative agent of psittacosis, which can infect a wide range of hosts including birds and humans. However, information regardingC.psittaciinfection in pigeons is scarce. In the present study, a total of 399 fecal samples from pigeons were collected from Jilin Province, northeastern China, between March and May 2015, and examined by nested PCR amplification of outer membrane protein A (ompA) gene. The overallChlamydiosisprevalence was 5.01% (21/399), with 3.19% in Changchun City and 9.40% in Jilin City. Furthermore, breed was the major risk factor associated withChlamydiainfection in pigeon, boiler pigeons had a prevalence of 7.49%, whereas noC.psittaciwas detected in racing pigeons. Sequence analysis of theompAgene revealed that all the identified isolates representedC.psittacigenotype B. Our results firstly indicated the presence of zoonoticC.psittaciin boiler pigeons in Jilin Province, northeastern China, and effective measures should be implemented to reduce the risk ofC.psittacitransmission from pigeons to humans.
Chlamydiapsittaci; pigeon; genotypes; Jilin Province Supported by the China Agricultural Science and Technology Innovation Program (ASTIP) (No. CAAS-ASTIP-2014-LVRI-03) and the Fundamental Research Funds of Chinese Academy of Agricultural Sciences (No. Y2016JC05).
Correspondence authors:Xu Xiao-qin, Email: xuxq@yzu.edu.cn; Zhu Xing-quan, Email: xingquanzhu1@hotmail.com
Chlamydiais a kind of parasitic gram-negative bacteria that can infect a range of hosts worldwide. Chlamydiosis (also called psittacosis) caused byC.psittacihas been reported in humans, birds and mammals[1-2]. More than 400 avian species have been identified as reservoir hosts forC.psittaci[3]. Hosts acquireC.psittaciinfection mainly through inhalation of infected excretions and discharges[4-5], showing the symptoms of clinical (gastrointestinal disease and encephalitis) and/or subclinical, and even death[4].
C.psittaciis classified into nine genotypes, namely A to F, E/B, M56 and WC, based on sequences of the outer membrane protein A (ompA) gene[6]. Interestingly, they seem to be animal-specific[7]. For example, genotype A was commonly found inPsittaciformefas(cockatoos, parakeets, lories), C inAnseriformes(mainly ducks and geese), E/B in ducks, D in turkeys and B inColumbiformes(doves and pigeons)[8]. However, occasionally, A, C, D, E, E/B have also been identified in pigeons[9-11].
In 1940, pigeon was firstly identified as the reservoir host ofC.psittaci[12]. Thereafter, a large number of studies concerningC.psittaciinfection in pigeons have been recorded[9-11,13], but limited information about theC.psittacigenotypes in pigeons in China is available[14-15]. The aims of the present study were to estimate theC.psittaciprevalence in pigeons in Jilin Province and characterize their genotypes.
Materials and methodsThe study site
The investigation was carried out in two cities in Jilin Province, northeastern China. Changchun (n=282, 43°05′-45°15′N, 124°18′-127°05′E) is the capital of Jilin Province, and it is one of the central cities in Northeast China. Jilin City (n=117, 42°31′-44°40′N, 125°40′-127°56′E) is also located in Jilin Province. The climate of Jilin Province is northerly continental monsoon type, and the average annual temperature is -10 ℃ to -23 ℃, moreover, the annual precipitation is 400-600 mm.
Collection and preparation of samples
A total of 399 fecal samples were collected from 282 pigeons in Changchun City, and from 117 pigeons in Jilin City, northeastern China, between March and May 2015. Each of these samples were collected into a separate sterile glove after the fecal defecation onto the ground, immediately, and then were sent to the laboratory, and stored at 4 ℃ until further analysis. Information about breed, age and geographic origin of pigeons were acquired, and listed in Tables 1 and 2.
Tab.1C.prevalencein pigeons in different farms in Jilin Province, northeastern China
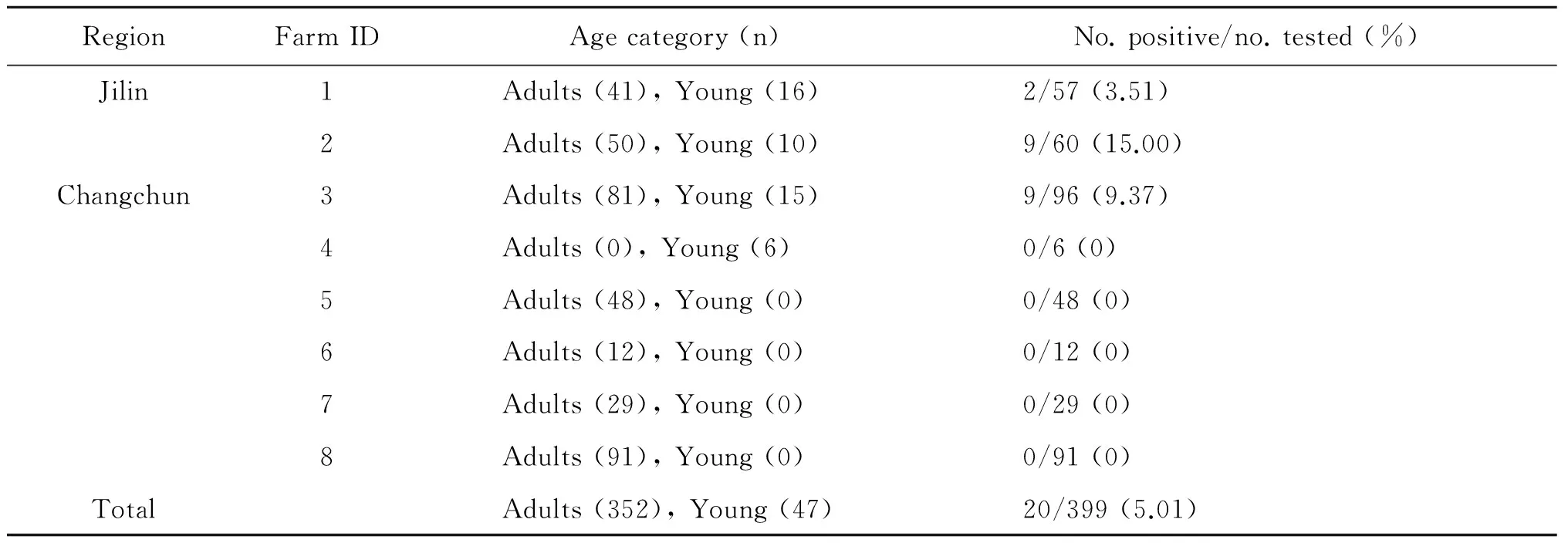
RegionFarmIDAgecategory(n)No.positive/no.tested(%)Jilin1Adults(41),Young(16)2/57(3.51)2Adults(50),Young(10)9/60(15.00)Changchun3Adults(81),Young(15)9/96(9.37)4Adults(0),Young(6)0/6(0)5Adults(48),Young(0)0/48(0)6Adults(12),Young(0)0/12(0)7Adults(29),Young(0)0/29(0)8Adults(91),Young(0)0/91(0)TotalAdults(352),Young(47)20/399(5.01)
Tab.2 Factors associated with prevalence of chlamydiosis pigeons in Jilin Province, northeastern China

FactorCategoryNo.testedNo.positivePrevalence(%)(95%CI)OR(95%CI)P-valueRegionChangchun28293.19(1.14-5.24)Reference0.0097Jilin117119.40(4.11-14.69)3.15(1.27-7.81)BreedRacingpigeon13200(-)Reference0.0013Boilerpigeon267207.49(4.33-10.65)-AgeAdult352174.83(2.59-7.07)Reference0.6500Young4736.38(0.00-13.37)1.34(0.38-4.77)Total399205.01(2.87-7.15)
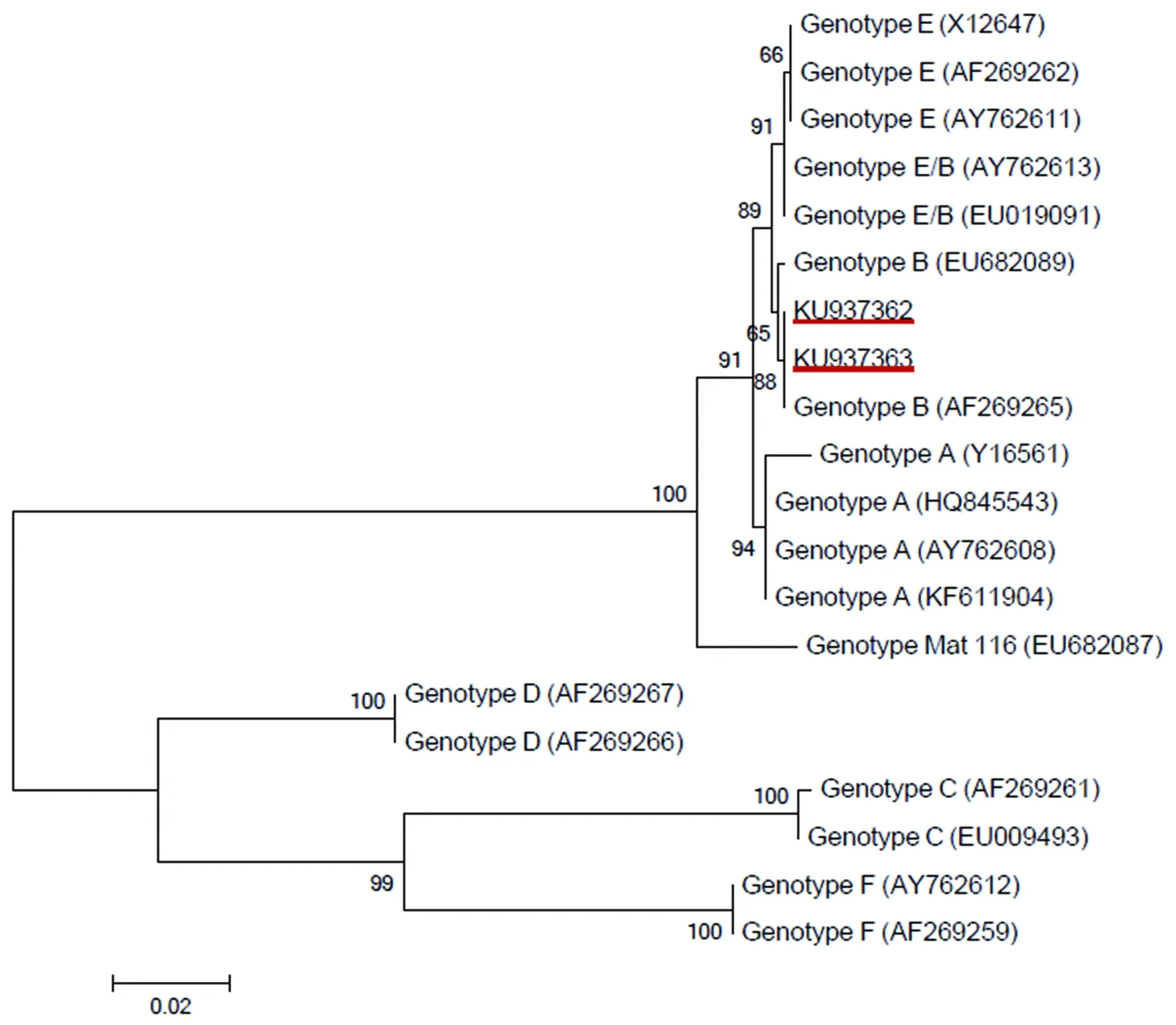
Fig.1 Phylogenetic analyses of C.psittaci based on the 1 019 bp sequence of the ompA gene. The numbers at nodes indicate bootstrap values. The C. psittaci isolates identified in this study are underlined.
DNA testing and sequencing
The Stool DNA kit (Omega, USA) was used to extract the genomic DNA from feces. All the operations were performed according to the manufacturer's recommendations. All the obtained DNA samples were stored at -20 ℃ until PCR analysis. TheompAgene was used to determine theC.psittacispecies/genotypes by semi-nested PCR[16-17]. The PCR reaction was performed under the conditions of 5 min at 95 ℃ for initial denature, followed by 40 cycles of 20 sec at 95 ℃, 1 min at 55 ℃, and 1 min at 72 ℃, and terminated at 72 ℃ for 10 min. The PCR products were electrophoresed in 1% agarose gels containing 0.5 μg/mL GoldViewTM(Solarbio, China) and were observed under UV light.
To determine the genotypes ofC.psittaci, a 1 000 bp fragment of theompAgene was amplified using a pair of primers, namely FOMPF1/ FOMPF2, according to previous studies[18-19]. The positive PCR products were sent to Genscript Company (Nanjing, China) for sequencing. All the obtained sequences were then aligned with reference sequences ofChlamydiaavailable in GenBank using the software of Clustal 2.0 and BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Neighbor-joining (NJ) method (Kimura 2-parameter model) with 1 000 bootstrap replicates was used to analyze the evolutionary relationships using the Mega 6.0 software.
Results and discussion
In the present study, of 399 pigeons, 20(5.01%) samples were detectedChlamydia-positive, with 9 (3.19%) in Changchun and 11 (9.04%) in Jilin. Prevalence ofChlamydiain different farm groups ranged from 0% to 15%. Boiler pigeons has a 7.49% prevalenceChlamydia, whereas no racing pigeon was detected positive forChlamydia. In addition,Chlamydiaprevalence in young and adult pigeons was 6.38% and 4.83%, respectively. Sequences analysis of theompAgene indicated that all 20Chlamydiaisolates wereC.psittacigenotype B. The representative sequences from the present study were deposited in the GenBank database with accession nos of KU937362 and KU937363.
The overallChlamydiaprevalence (5.01%, 20/399) in this study was lower compared with the 19.4%-95.6% seroprevalence rates in pigeons in Europe[10], 10% seroprevalence in racing pigeons in Beijing (37/370)[15], and 31.09% seroprevalence in pigeons in north-western China[14]. Furthermore, it is also lower than that in pigeons in Belgium (6.3%)[11], Poland (7.6%)[20], but higher than that in pigeons in Switzerland (3.2%, 3.3%)[21-22]. Feeding and living conditions, different detection methods, socioeconomic and ecological conditions may contribute to these differences[14].
Transmission ofC.psittacioccurs mainly through the respiratory tract[5], so higher breeding density in Jilin City is possibly one of the most important reasons why pigeons in Jilin City (9.40%) has a significantly higherC.psittaciprevalence than that from Changchun (3.19%). Moreover, the different raising conditions and individual health status may also be related to the difference. Probably due to good animal husbandry practice in racing pigeon industry, no racing pigeon was testedChlamydia-positive, but higher prevalence was detected in boiler pigeons, the difference was significant statistically (P=0.001 3).Moreover, no significant difference was found among different age groups (P=0.65), which was different to a report in pet birds in northwest China[19].
Many gene loci, such as the inclusion membrane protein A gene (IncA)[23], ribosomal RNA genes (16S-23S), and theompAgene[24-25], have been used to detectChlamydiainfection previously. In the present study, the genotypes ofChlamydiawere determined by nested PCR amplification of theompAgene, one of the most commonly used loci for identifying theChlamydiagenotypes[10]. Although genotypes A, B, C, D, E and E/B have been recorded in pigeons previously[26], only genotype B was identified in this study. The results further confirmed that genotype B was the most prevalentChlamydiagenotype in pigeons[27-28]. In addition to pigeons, genotype B was also reported in a range of other animals and humans around the world[8,15], which indicates that pigeons may be an important resource for human infection. Moreover, it is also reported in adult chickens, ducks and pigeons in northeastern China[14], which suggests that transmission ofC.psittacigenotype B might occur in these areas, which raises public health concern. Interestingly, the genotype B identified in this study was also found in pigeons in USA[8], Sweden[29], Poland[20]and several European countries[6,28], indicating a worldwide distribution.
Conclusion
The present study revealed the occurrence ofChlamydiainfection in pigeons in Jilin Province for the first time. DNA sequence analysis indicates that all the isolates representC.psittacigenotype B. Moreover, this study provides important base-data for designing and executing strategies and measures for controllingC.psittaciinfection in pigeons and humans in the examined areas.
[1] Szeredi L, Bacsadi A. Detection ofChlamydophila(Chlamydia)abortusandToxoplasmagondiiin smears from cases of ovine and caprine abortion by the streptavidin-biotin method [J]. Comp Pathol, 2002, 127: 257-263.
[2] Pantchev A, Sting R, Bauerfeind R, et al. Detection of allChlamydophilaandChlamydiaspp. of veterinary interest using species-specific real-time PCR assays [J]. Comp Immunol Microbiol Inf Dis, 2010, 33: 473-484. DOI: 10.1016/j.cimid.2009.08.002
[3] Kaleta EF, Taday EM. Avian host range ofChlamydophilaspp. based on isolation, antigen detection and serology [J]. Avian Pathol, 2003, 32: 435-461.
[4] Beeckman, DSA, Vanrompay, DCG. ZoonoticChlamydophilafrom a clinical perspective [J]. Clin Microbiol Infect, 2009, 15: 11-17. DOI: 10.1111/j.1469-0691.2008.02669
[5] Stewardson AJ, Grayson ML. Psittacosis [J]. Infect Dis Clin North Am, 2010, 24: 7-25.
[6] Geens T, Desplanques A, Van Loock M, et al. Sequencing of theChlamydophilapsittaciompAgene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method [J].J Clin Microbiol,2005, 43: 2456-2461.
[7] Sachse K, Laroucau K, Vorimore F, et al. DNA microarray-based genotyping ofChlamydophilapsittacistrains from culture and clinical samples [J]. Vet Microbiol, 2009, 135: 22-30. DOI: 10.1016/j.vetmic.2008.09.041
[8] Van Lent S, Piet JR, Beeckman D, et al. Full genome sequences of all nineChlamydiapsittacigenotype reference strains [J]. J Bacteriol, 2012, 194: 6930-6931. DOI: 10.1128/JB.01828-12
[9] Heddema ER, ter Sluis S, Buys JA, et al. Prevalence ofChlamydophilapsittaciin fecal droppings from feral pigeons in Amsterdam, The Netherlands [J].Appl Environ Microb, 2006,72: 4423-4425.
[10] Magnino S,Haag-Wackernagel D,Geigenfeind I,et al.Chlamydialinfections in feral pigeons in Europe: review of data and focus on public health implications [J]. Vet Microbiol, 2009, 135: 54-67. DOI: 10.1016/j.vetmic.2008.09.045
[11] Dickx V, Beeckman DSA, Dossche L, et al.Chlamydophilapsittaciin homing and feral pigeons and zoonotic transmission [J].J Med Microbiol,2010, 59: 1348-1353. DOI: 10.1099/jmm.0.023499-0
[12] Pinkerton H, Swank RL. Recovery of virus morphologically identical with psittacosis from thiamin-deficient pigeons [J]. Exp Biol and Med, 1940, 45: 704-706.
[13] Geigenfeind I, Haag-Wackernagel D. Detection ofChlamydophilapsittacifrom feral pigeons in environmental samples: problems with currently available techniques [J].Integr Zool, 2010,5: 63-69. DOI: 10.1111/j.1749-4877.2010.00187
[14] Cong W, Huang SY, Zhang XY, et al. Seroprevalence ofChlamydiapsittaciinfection in market-sold adult chickens, ducks and pigeons in north-western China [J]. J Med Microbiol, 2013, 62: 1211-1214. DOI: 10.1099/jmm.0.059287-0
[15] Ling Y, Chen H, Chen X, et al. Epidemiology ofChlamydiapsittaciinfection in racing pigeons and pigeon fanciers in Beijing, China [J]. Zoonoses Public Hlth, 2015, 62: 401-406. DOI: 10.1111/zph.12161
[16] Buxton D, Rae A, Maley SW, et al. Pathogenesis ofChlamydiapsittaciinfection in sheep: detection of the organism in a serial study of the lymph node [J]. J Comp Pathol, 1996, 114: 221-230.
[17] De Lima VY, Langoni H, Da Silva AV, et al.ChlamydophilapsittaciandToxoplasmagondiiinfection in pigeons (Columba livia) from São Paulo State, Brazil [J].Vet Parasitol,2011, 175: 9-14.DOI: 10.1016/j.vetpar.2010.10.006
[18] Herrmann B, Persson H, Jensen JK, et al.Chlamydophilapsittaciin fulmars, the Faroe Islands [J]. Emerg Infect Dis,2006, 12: 330-332.
[19] Zhang NZ, Zhang XX, Zhou DH, et al. Seroprevalence and genotype ofChlamydiain pet parrots in China [J]. Epidemiol Infect, 2015, 14: 55-61. DOI: 10.1017/S0950268814000363
[20] Stenzel T, Pestka D, Choszcz D. The prevalence and genetic characterization ofChlamydiapsittacifrom domestic and feral pigeons in Poland and the correlation between infection rate and incidence of pigeon circovirus [J]. Poultry Sci, 2014, 93: 3009-3016. DOI: 10.3382/ps.2014-04219
[21] Zweifel D, Hoop R, Sachse K, et al. Prevalence ofChlamydophilapsittaciin wild birds—potential risk for domestic poultry, pet birds, and public health [J]. Eur J Wildlife Res, 2009, 55: 575-581.
[22] Geigenfeind I, Vanrompay D, Haag-Wackernagel D. Prevalence ofChlamydiapsittaciin the feral pigeon population of Basel, Switzerland [J]. J Med Microbiol,2012, 61: 261-265. DOI: 10.1099/jmm.0.034025-0
[23] Ménard A, Clerc M, Subtil A, et al. Development of a real-time PCR for the detection ofChlamydiapsittaci[J]. J Med Microbiol, 2006, 55: 471-473.
[24] Smith KA, Bradley KK, Stobierski MG,et al. Compendium of measures to controlChlamydophilapsittaci(formerlyChlamydiapsittaci) infection among humans (psittacosis) and pet birds [J]. J Am Vet Med Assoc, 2005, 226: 532-539.
[25] Binet R, Maurelli AT. Frequency of development and associated physiological cost of azithromycin resistance inChlamydiapsittaci6BC andC.trachomatisL2[J]. Antimicrob Agents Chemother,2007, 51: 4267-4275.
[26] Heddema ER, Van Hannen EJ, Duim B, et al. Genotyping ofChlamydophilapsittaciin human samples [J].Emerg Infect Dis, 2006,12: 1989-1990.
[27] Dolz G, Solórzano-Morales, Angelova L, et al.Chlamydiapsittacigenotype B in a pigeon (Columba livia) inhabiting a public place in San José, Costa Rica [J].Open Vet J,2013, 3: 135-139.
[28] Madani SA, Peighambari SM. PCR-based diagnosis, molecular characterization and detection of atypical strains of avianChlamydiapsittaciin companion and wild birds [J]. Avian Pathol, 2013, 42: 38-44. DOI: 10.1080/03079457.2012.757288
[29] Rehn M, Ringberg H, Runehagen A, et al. Unusual increase of psittacosis in southern Sweden linked to wild bird exposure [J]. Euro Surveillance, 2013, 18: 13-20.
Received:2016-10-14 Editor:LIN Dan
吉林省鸽子鹦鹉热衣原体的分子流行病学调查和基因型分布研究
姚秋霞1,2,张晓轩2,3,陈 凯2,马剑刚2,3,郑文斌2,3,许小琴1,朱兴全2
目的 2015年3-5月调查吉林省长春市和吉林市肉鸽与信鸽中鹦鹉热衣原体的流行情况及基因型分布。方法本研究共采集鸽子粪便样本399份,其中长春市样本282份,吉林市样本117份。利用PCR技术进行鹦鹉热衣原体ompA基因扩增、测序以及基因型分析。结果 本实验结果显示:鹦鹉热衣原体的感染率为5.01%(21/399),其中吉林市鹦鹉热衣原体的感染率(9.40%)明显高于长春市的感染率(3.19%)。此外,品种也是与衣原体感染相关的主要风险因素, 肉鸽的感染率为7.49%,而信鸽的感染率为0。ompA基因的序列分析显示,这些鹦鹉热衣原体都属于B型。结论 综上所述,我国吉林省肉鸽具有较高的B型鹦鹉热衣原体流行,给人类的健康带来了潜在的威胁。
鹦鹉热衣原体;鸽子;基因型;吉林省
R374
A
1002-2694(2017)02-0104-06
许小琴,Email: xuxq@yzu.edu.cn; 朱兴全, Email: xingquanzhu1@hotmail.com
1.扬州大学兽医学院,扬州 225009; 2.中国农业科学院兰州兽医研究所,家畜疫病病原生物学国家重点实验室,兰州 730046; 3.吉林农业大学动物科学与技术学院,长春 130118
中国农业科技创新工程项目(ASTIP) (No.CAAS-ASTIP-2014-LVRI-03)及中国农业科学院基本科研业务费专项(No.Y2016JC05)资助

