PI3K/AKT激动剂和抑制剂对巨噬细胞炎症反应的影响*
王 静 徐 萍# 杨志文 徐 凯 赖跃兴
上海交通大学附属第一人民医院松江分院消化内科1(201600) 药剂科2
PI3K/AKT激动剂和抑制剂对巨噬细胞炎症反应的影响*
王 静1徐 萍1#杨志文2徐 凯1赖跃兴1
上海交通大学附属第一人民医院松江分院消化内科1(201600) 药剂科2
背景:近年发现磷脂酰肌醇3激酶/丝氨酸-苏氨酸激酶(PI3K/AKT)在重度急性胰腺炎(SAP)的发病中发挥重要作用,但机制尚未明确。目的:探讨PI3K/AKT激动剂胰岛素样生长因子-Ⅰ(IGF-Ⅰ)和抑制剂wortmannin对巨噬细胞株RAW264.7 Toll样受体4(TLR4)信号通路的影响,阐明PI3K/AKT参与调节SAP炎症反应的作用机制。方法:分别以不同浓度脂多糖(LPS)、IGF-Ⅰ、wortmannin处理RAW264.7细胞,采用CCK-8实验检测细胞活性。RAW264.7细胞分为空白对照组(不予处理)、LPS组(LPS 1 μg/mL)、IGF-Ⅰ组(IGF-Ⅰ 100 ng/mL+LPS 1 μg/mL)、wortmannin组(wortmannin 100 nmol/L+LPS 1 μg/mL)和IGF-Ⅰ+wortmannin组(wortmannin 100 nmol/L+IGF-Ⅰ 100 ng/mL+LPS 1 μg/mL),采用ELISA法检测肿瘤坏死因子-α(TNF-α)、白细胞介素-6(IL-6)蛋白表达,采用real-time PCR检测TLR4、髓样分化因子88(MyD88)、AKT、PI3K、p38丝裂原活化蛋白激酶(p38MAPK)、核因子-κB(NF-κB)mRNA表达。结果:RAW264.7细胞经不同浓度LPS、IGF-Ⅰ、wortmannin处理后,各浓度组间细胞活性无明显差异(P>0.05)。LPS组、IGF-Ⅰ组、wortmannin组、IGF-Ⅰ+wortmannin组TNF-α、IL-6表达水平均较空白对照组显著升高(P<0.05);wortmannin组TNF-α、IL-6表达水平较LPS组和IGF-Ⅰ组显著降低(P<0.05);IGF-Ⅰ+wortmannin组 TNF-α、IL-6表达水平较IGF-Ⅰ组显著降低(P<0.05)。LPS组AKT、PI3K、TLR4及其下游分子MyD88、p38MAPK、NF-κB mRNA表达均显著高于空白对照组(P<0.05);IGF-Ⅰ组上述指标较LPS组进一步升高,差异有统计学意义(P<0.05);wortmannin组上述指标较LPS组和IGF-Ⅰ组显著降低(P<0.05);IGF-Ⅰ+wortmannin组上述指标显著高于wortmannin组(P<0.05),但较IGF-Ⅰ组显著降低(P<0.05)。结论:PI3K/AKT可能通过调节巨噬细胞中的TLR4及其下游分子影响促炎细胞因子表达,从而参与SAP炎症反应的发生。
胰腺炎; 磷酸肌醇3-激酶类; 蛋白质丝氨酸苏氨酸激酶; Toll样受体4; 胰岛素样生长因子 Ⅰ; Wortmannin; 肿瘤坏死因子α; 白细胞介素6
重度急性胰腺炎(severe acute pancreatitis, SAP)是病死率较高的消化系统疾病,其发病机制尚未完全阐明。本课题组前期研究[1]发现磷脂酰肌醇3激酶/丝氨酸-苏氨酸激酶(PI3K/AKT)参与调控SAP 大鼠胰腺组织促炎细胞因子的释放,可加重胰腺组织损伤;而PI3K/AKT抑制剂wortmannin可减轻胰腺组织损伤,提高SAP大鼠生存率。然而,PI3K/AKT参与SAP发病的具体机制尚未明确。已有研究[2]证实Toll样受体4(Toll-like receptor 4, TLR4)信号通路是促炎细胞因子释放的主要通路,刺激TLR4可激活核因子-κB(NF-κB)、p38丝裂原活化蛋白激酶(p38MAPK)信号通路,导致炎性因子释放。故本研究通过探讨PI3K/AKT激动剂胰岛素样生长因子-Ⅰ (insulin-like growth factor-Ⅰ, IGF-Ⅰ)和抑制剂wortmannin对小鼠巨噬细胞TLR4信号通路的影响,旨在阐明PI3K/AKT参与调节SAP炎症反应的作用机制。
材料与方法
一、细胞株、主要试剂和仪器
小鼠巨噬细胞株RAW264.7购自中国科学院上海细胞库。CCK-8试剂盒(日本同仁公司);脂多糖(LPS)、wortmannin(美国Sigma公司);IGF-Ⅰ、肿瘤坏死因子-α(TNF-α) ELISA试剂盒(美国Abcam公司);白细胞介素-6(IL-6) ELISA试剂盒(南京建成生物工程研究所);TRIzol试剂(美国Invitrogen公司);cDNA第一链合成试剂盒(美国Promega公司); PCR扩增试剂盒[生工生物工程(上海)股份有限公司];SYBR®PrimeScriptTMreal-time RT-PCR试剂盒(日本TaKaRa公司)。
二、方法
1. 细胞培养:RAW264.7细胞在37 ℃、5% CO2条件下,以含10%胎牛血清的RPMI1640培养基培养。
2. CCK-8实验检测LPS、IGF-Ⅰ和wortmannin对RAW264.7细胞活性的影响:取对数生长期RAW264.7细胞,以1×103~5×103/孔接种于96孔板培养过夜,分别以LPS、IGF-Ⅰ、wortmannin处理细胞,每组设6个浓度组,LPS、IGF-Ⅰ、wortmannin浓度分别为0、0.1、0.3、1、3、10 μg/mL;0、1、10、30、100、300 ng/mL;0、3、10、20、100、300 nmol/L。加入100 μL无血清DMEM培养基和10 μL CCK-8试剂,37 ℃培养1 h,于酶标仪450 nm波长处测定吸光度(A)值。
3. RAW264.7细胞分组处理:取对数生长期RAW264.7细胞,以5×103/mL接种于6孔板,设置空白对照组、LPS组、IGF-Ⅰ组、wortmannin组和IGF-Ⅰ+wortmannin组。空白对照组不予处理;LPS组以LPS(1 μg/mL)处理细胞;IGF-Ⅰ组以IGF-Ⅰ(100 ng/mL)预处理1 h后加入LPS(1 μg/mL)处理细胞;wortmannin组以wortmannin(100 nmol/L)预处理30 min后加入LPS(1 μg/mL)处理细胞;IGF-Ⅰ+wortmannin组以wortmannin(100 nmol/L)预处理30 min,以IGF-Ⅰ(100 ng/mL)预处理1 h,再以LPS(1 μg/mL)处理细胞。每孔细胞悬液终体积为1 mL,培养6 h后收集细胞。
4. ELISA法检测促炎细胞因子TNF-α、IL-6表达:取空白对照组、LPS组、IGF-Ⅰ组、wortmannin组和IGF-Ⅰ+wortmannin组细胞,检测TNF-α、IL-6表达,具体步骤参照相应ELISA试剂盒说明书进行。
5. Real-time PCR检测TLR4、髓样分化因子88(MyD88)、AKT、PI3K、p38MAPK、NF-κB mRNA表达:取空白对照组、LPS组、IGF-Ⅰ组、wortmannin组和IGF-Ⅰ+wortmannin组细胞,弃上清液,PBS漂洗,加入1 mL TRIzol试剂抽提细胞总RNA。采用cDNA第一链合成试剂盒合成cDNA,以之为模板,采用ABI 7300型定量PCR仪行real-time PCR扩增,检测TLR4、MyD88、AKT、PI3K、p38MAPK、NF-κB mRNA表达。引物由生工生物工程(上海)股份有限公司合成,以GAPDH作为内参照。TLR4引物上游:5’-CTA TGA ACA AAG GGT CTA TCA G-3’,下游:5’-AAG AAC AGC AAC CAC TAA AG-3’;MyD88引物上游:5’-CAC TCG CAG TTT GTT GGA TG-3’,下游:5’-TGT AAA GGC TTC TCG GAC TC-3’;AKT引物上游:5’-GGG CAC ATC AAG ATA ACG-3’,下游:5’-TGG TCC TGG TTG TAG AAG-3’;PI3K引物上游:5’-ATG CCA GAA AGG AGA ATG-3’,下游:5’-TGT TGG ACT CAG CAA TAC-3’;p38MAPK引物上游:5’-GTG TTC ACA CCC GCA AGG TC-3’,下游:5’-CGG TCA GCT TCT GGC ACT TC-3’;NF-κB引物上游:5’-CCC GAA ACT CAA CTT CTG-3’,下游:5’-ATC TGC CCT GAT GGT AAC-3’;GAPDH引物上游:5’-ATC ACT GCC ACC CAG AAG-3’,下游:5’-TCC ACG ACG GAC ACA TTG-3’。PCR反应条件:95 ℃ 10 min;95 ℃ 15 s,60 ℃ 45 s,40个循环。以2-△△Ct法分析目的基因相对表达量。
三、统计学分析
结 果
一、不同浓度LPS、IGF-Ⅰ、wortmannin对细胞活性的影响
LPS、IGF-Ⅰ、wortmannin各浓度组间RAW264.7细胞活性无明显差异(P>0.05)(图1)。最终选择以 1 μg/mL LPS、100 ng/mL IGF-Ⅰ和100 nmol/L wortmannin进行后续实验。
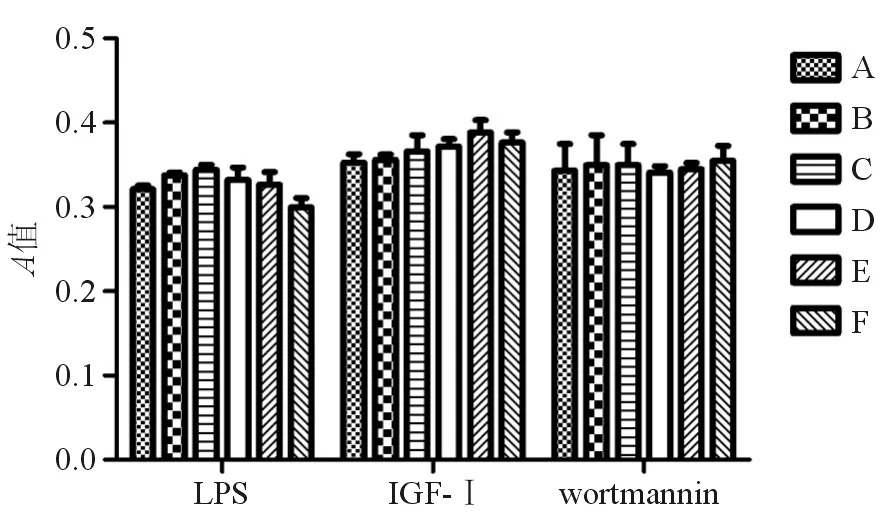
A-F:LPS浓度分别为0、0.1、0.3、1、3、10 μg/mL;IGF-Ⅰ浓度分别为0、1、10、30、100、300 ng/mL;wortmannin浓度分别为0、3、10、20、100、300 nmol/L
图1 不同浓度LPS、IGF-Ⅰ 和wortmannin对RAW264.7细胞 活性的影响
二、促炎细胞因子TNF-α、IL-6表达变化
LPS组RAW264.7细胞TNF-α、IL-6表达水平较空白对照组显著升高(P<0.05);IGF-Ⅰ组TNF-α、IL-6表达水平与空白对照组和LPS组相比均升高,与空白对照组间差异显著(P<0.05),与LPS组间差异则无统计学意义(P>0.05);Wortmannin组TNF-α、IL-6表达水平与空白对照组相比显著升高(P<0.05),与LPS组和IGF-Ⅰ组相比显著降低(P<0.05);IGF-Ⅰ+wortmannin组 TNF-α、IL-6表达水平与空白对照组相比显著升高(P<0.05),与LPS组相比有所降低,但差异无统计学意义(P>0.05),与IGF-Ⅰ组相比显著降低(P<0.05),与wortmannin组相比有所升高,但差异无统计学意义(P<0.05)(表1、图2)。
三、TLR4、MyD88、AKT、PI3K、p38MAPK、NF-κB mRNA表达
LPS组TLR4、MyD88、AKT、PI3K、p38MAPK、NF-κB mRNA表达水平均显著高于空白对照组(P<0.05)。IGF-Ⅰ 组上述指标均显著高于空白对照组和LPS组(P<0.05)。Wortmannin组上述指标显著高于空白对照组(P<0.05),与LPS组、IGF-Ⅰ组相比则显著降低(P<0.05)。IGF-Ⅰ+wortmannin组上述指标显著高于wortmannin组(P<0.05),但较IGF-Ⅰ组显著降低(P<0.05)(表2)。


组 别TNF⁃αIL⁃6空白对照组325.45±15.4653.64±3.06LPS组545.53±59.35137.80±12.30IGF⁃Ⅰ组570.29±51.24148.72±16.11wortmannin组490.20±25.97120.47±6.34IGF⁃Ⅰ+wortmannin组519.51±32.30131.92±9.58
讨 论
PI3K是生长因子超家族信号转导过程中的重要分子,可被多种细胞因子和理化因素激活。AKT主要负责由PI3K始动的生物信息传递。PI3K/AKT作为细胞内主要信号通路,在细胞代谢、细胞周期调控、细胞增殖、凋亡等多种生物学过程中发挥重要作用[3]。研究[4]表明PI3K/AKT通路可引起促炎细胞因子释放,参与SAP发病。本课题组前期研究[1]结果显示,SAP大鼠胰腺组织中磷酸化AKT表达增高,促炎细胞因子表达增加,予PI3K抑制剂wortmannin预处理后,AKT活性受抑,促炎细胞因子表达降低,胰腺组织病理学改变明显缓解,SAP大鼠生存率升高。本研究结果显示PI3K/AKT激动剂IGF-Ⅰ可促进LPS诱导巨噬细胞释放促炎细胞因子TNF-α、IL-6,而wortmannin作用于巨噬细胞后可抑制LPS引起的促炎细胞因子分泌,并可拮抗IGF-Ⅰ对促炎细胞因子的上调作用,与前期动物实验研究结果一致。
PI3K/AKT活化可导致细胞产生大量促炎细胞因子,此过程在炎症反应中发挥重要作用[5]。目前尚不明确PI3K/AKT促进促炎细胞因子产生的具体机制。TLR4信号通路是促炎细胞因子释放的主要通路, 活化的TLR4可通过MyD88等接头蛋白激活其下游NF-κB、 p38MAPK,产生大量炎性因子。研究[6-8]证实,SAP动物模型的胰腺、肠道以及肺炎症损伤均涉及TLR4信号通路激活。TLR4信号通路激活是SAP引发全身性炎症反应和脏器功能衰竭的重要病理生理学机制。本研究应用LPS刺激巨噬细胞激活TLR4信号通路,并分别以PI3K/AKT激动剂、抑制剂以及两者同时应用干预LPS引起的炎症反应,结果显示TLR4、 MyD88、p38MAPK、NF-κB表达随PI3K/AKT激动剂和抑制剂的干预而发生变化,提示PI3K/AKT与TLR4信号通路存在关联。国外一项研究[9]发现,尼古丁可减轻脓毒血症小鼠的炎症反应,此作用可能是通过影响PI3K/AKT信号通路、进而调节TLR4表达实现的,进一步提示PI3K/AKT与TLR4炎症信号通路密切相关。本研究结果显示IGF-Ⅰ可进一步激活LPS引起的炎症反应,活化TLR4信号通路,使TLR4、MyD88、p38MAPK、NF-κB表达上调,促炎细胞因子TNF-α、IL-6表达升高,而wortmannin可拮抗IGF-Ⅰ的作用;单独应用wortmannin作用于LPS刺激的巨噬细胞则可抑制LPS引起的TLR4信号通路活化,下调TLR4、MyD88、p38MAPK、NF-κB表达,并使促炎细胞因子表达降低。上述研究结果提示PI3K/AKT可能通过调节TLR4及其下游分子影响促炎细胞因子表达,从而参与SAP炎症反应的发生。

图2 各组RAW264.7细胞TNF-α、IL-6表达变化

组 别TLR4MyD88AKTPI3Kp38MAPKNF⁃κB空白对照组1.00±0.071.00±0.031.00±0.081.00±0.060.06±0.011.01±0.18LPS组7.06±0.793.26±0.084.05±0.314.89±0.150.25±0.033.92±0.27IGF⁃Ⅰ组9.11±0.365.97±0.565.13±0.346.04±0.590.35±0.015.28±0.31wortmannin组1.77±0.161.74±0.411.27±0.231.32±0.200.08±0.011.52±0.09IGF⁃Ⅰ+wortmannin组7.91±0.663.80±0.344.71±0.344.94±0.210.25±0.083.98±0.62
综上所述,本研究从细胞水平证实PI3K/AKT可通过影响TLR4信号通路调控p38MAPK、NF-κB活性,从而调节促炎细胞因子表达,参与SAP炎症反应。后期研究还需完善相关体内实验,进一步验证SAP中PI3K/AKT与TLR4信号通路的关系,为控制SAP炎症反应提供理论依据。
1 Xu P, Wang J, Yang ZW, et al. Regulatory roles of the PI3K/AKT signaling pathway in rats with severe acute pancreatitis[J]. PLoS One, 2013, 8 (11): e81767.
2 Zhao W, Ma G, Chen X. Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-κB pathway[J]. Vascul Pharmacol, 2014, 63 (3): 162-172.
3 Cantley LC. The phosphoinositide 3-kinase pathway[J]. Science, 2002, 296 (5573): 1655-1657.
4 康新,王立志,王屹刚,等. 重症急性胰腺炎肺损伤时磷脂酰肌醇3激酶/蛋白激酶B信号转导通路的表达[J]. 中华医学杂志, 2010, 90 (11): 732-737.
5 Zhao M, Zhou A, Xu L, et al. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroin-flammation in hippocampal neurons[J]. Neuroscience, 2014, 269: 93-101.
6 Li Y, Zhou ZG, Zhang J, et al. Microcirculatory detection of Toll-like receptor 4 in rat pancreas and intestine[J]. Clin Hemorheol Microcirc, 2006, 34 (1-2): 213-219.
7 Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice[J]. Gut, 2009, 58 (6): 813-819.
8 Sawa H, Ueda T, Takeyama Y, et al. Role of toll-like receptor 4 in the pathophysiology of severe acute pancreatitis in mice[J]. Surg Today, 2007, 37 (10): 867-873.
9 Kim TH, Kim SJ, Lee SM. Stimulation of the α7 nicotinic acetylcholine receptor protects against sepsis by inhibiting Toll-like receptor via phosphoinositide 3-kinase activation[J]. J Infect Dis, 2014, 209 (10): 1668-1677.
(2016-07-01收稿;2016-07-20修回)
Effect of Agonist and Inhibitor of PI3K/AKT on Inflammatory Response in Macrophages
WANGJing1,XUPing1,YANGZhiwen2,XUKai1,LAIYuexing1.1
DepartmentofGastroenterology,2DepartmentofPharmacy,SongjiangHospitalAffiliatedtotheFirstPeople’sHospital,ShanghaiJiaotongUniversity,Shanghai(201600)
XU Ping, Email: sjzxxp@yeah.net
Pancreatitis; Phosphatidylinositol 3-Kinases; Protein-Serine-Threonine Kinases; Toll-Like Receptor 4; Insulin-Like Growth Factor Ⅰ; Wortmannin; Tumor Necrosis Factor-alpha; Interleukin-6
10.3969/j.issn.1008-7125.2017.02.004
上海市松江区卫计委医学领先专业项目(201358)
#本文通信作者,Email: sjzxxp@yeah.net
Background: Phosphoinositide 3-kinase/serine-threonine kinase (PI3K/AKT) has been found playing an important role in the pathogenesis of severe acute pancreatitis (SAP) in recent years, but the underlying mechanism has not been clarified. Aims: To investigate the role of PI3K/AKT in regulating the inflammatory response in SAP by evaluating the effect of insulin-like growth factor-Ⅰ (IGF-Ⅰ) and wortmannin, the agonist and inhibitor of PI3K/AKT on Toll-like receptor 4 (TLR4) signaling pathway in macrophage cell line RAW264.7. Methods: RAW264.7 cells were treated with different concentrations of lipopolysaccharide (LPS), IGF-Ⅰ and wortmannin, respectively, and cell viability was determined by CCK-8 assay. RAW264.7 cells were divided into blank control group (no treatment), LPS group (LPS 1 μg/mL), IGF-Ⅰ group (IGF-Ⅰ 100 ng/mL+LPS 1 μg/mL), wortmannin group (wortmannin 100 nmol/L+LPS 1 μg/mL) and IGF-Ⅰ+wortmannin group (wortmannin 100 nmol/L+IGF-Ⅰ 100 ng/mL+LPS 1 μg/mL). Protein expressions of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were detected by ELISA; mRNA expressions of TLR4, myeloid differentiation factor 88 (MyD88), AKT, PI3K, p38 mitogen-activated protein kinase (p38MAPK) and nuclear factor-κB (NF-κB) were determined by real-time PCR. Results: After treated with LPS, IGF-Ⅰ and wortmannin, respectively, no differences in cell viability of RAW264.7 cells were found between different concentrations groups (P>0.05). Protein expressions of TNF-α and IL-6 in LPS, IGF-Ⅰ, wortmannin and IGF-Ⅰ+wortmannin groups were significantly higher than those in blank control group (P<0.05). Protein expressions of TNF-α and IL-6 in wortmannin group were significantly lower than those in LPS and IGF-Ⅰ groups (P<0.05), and those in IGF-Ⅰ+wortmannin group were significantly lower than those in IGF-Ⅰ group (P<0.05). In LPS group, mRNA expressions of AKT and PI3K as well as TLR4 and its downstream molecules MyD88, p38MAPK and NF-κB were significantly higher than those in blank control group (P<0.05). Expressions of all above-mentioned mRNAs in IGF-Ⅰ group were further increased and significantly higher than those in LPS group (P<0.05). Expressions of all above-mentioned mRNAs in wortmannin group were significantly lower than those in LPS and IGF-Ⅰ groups (P<0.05), and those in IGF-Ⅰ+wortmannin group were significantly higher than those in wortmannin group (P<0.05), but significantly lower than those in IGF-Ⅰ group (P<0.05). Conclusions: PI3K/AKT might regulate TLR4 signaling pathway and its downstream molecules in macrophages, thereby affects the expressions of inflammatory cytokines and being involved in the pathogenesis of inflammatory response in SAP.

