Characterization and catabolic genes analyses of oil-degrading Pseudomonas aeruginosa 1217
YANG Zhi, CHEN Ji-xiang, LI Wen-xin, HU Wei, SUN Shang-chen, LI Yan-lin, WANG Yong-gang
(1. Coll. of Energy & Power Engin., Lanzhou Uni. of Technol., Lanzhou 730050; 2. Schl. of Petrochem. Engin., Lanzhou Uni. of Technol., Lanzhou 730050; 3. Lanzhou Lan Shi Eqpt. Engin. Res. Inst. Co. Ltd., Lanzhou 730314)
Oil pollution has caused serious environmental problems around the world[1-2]. Crude oil contains thousands of individual hydrocarbons and related compounds. Alkanes, aromatics, asphaltenes and resins are the main components and considered as the major biological organic contaminants, some of which have toxic, mutagenic, and carcinogenic effects[3-5]. Many remediation technologies have been used for removal of oil residues[6-7].Bioremediation technology with oil-degradation microorganisms is proven to be one of the most safe, efficient and cost-effective methods[5-8]. Oil-degrading microorganisms are ubiquitous in natural environments and degradation of petroleum by microorganisms is a very common phenomenon[9-11]. More than 200 genera of bacteria, cyanobacteria, fungi, and algae are known to degrade or transform hydrocarbons, using them for energy and carbon[12]. Bacterial genera such asPseudomonas,Achromobacter,Acinetobacter,Alcanivorax,Arthrobacter,Rhodococcus,Bacillus,Nocardia,Mycobacterium,Marinobacter,Microbacterium,Gordonia,Novosphingobium,Corynebacteriumare very important microorganisms[13-15]. ThePseudomonasspecies are distributed widely throughout environments, including soil, water, sewage, and activated sludge, and a large number ofPseudomonasspecies are known as effective degraders of alkanes and aromatic hydrocarbons[16-17].PseudomonasmandeliiandPseudomonasveroniiwere isolated from NP-contaminated soil[18].PseudomonasputidaE41 isolated from oil contaminated soil showed high efficiency of biodegradation of ethylbenzene with the optimum conditions. Benzene, toluene and ethylbenzene were degraded when these compounds were provided together[19].PseudomonasaeruginosaDQ8 was capable of degrading diesel, crude oil,n-alkanes and polycyclic aromatic hydrocarbons (PAHs) in petroleum. The strain oxidizedn-alkanes via a terminal oxidation pathway and degraded fluorene via two pathways[20]. The alkane-degradation pathway of the best-characterizedPseudomonasputidaGPo1 (formerlyPseudomonasoleovoransGPo1) is encoded by the OCT plasmid[21-22]. These alkane degradation genes are clustered in two operons[22].Pseudomonasspecies have also been used for genetic engineering[1].In this study, one effective oil-degradingPseudomonasaeruginosastrain 1217 was isolated from the oil contaminated soil. The growth characteristics and oil degradation capacities of the strain were investigated. The oil-degrading related genes were also analyzed. All findings benefit this strain in the remediation of oil contaminated environments and the degradation of oil pollutants.
1 Materials and methods
1.1 Materials
1.1.1 Chemicals Crude oil was obtained from PetroChina Lanzhou Petrochemical Company.n-heptane,n-decane,n-dodecane,n-tetradecane,n-hexadecane,n-octadecane,n-docosane,n-tetracosane,n-octacosane, benzene, toluene, xylene, pyrocatechol, naphthalene and pyrene (>95% purity) were purchased from Heowns (Tianjin, China).n-Hexane was from Tianjin Biaoshiqi Science and Technology Development Co., Ltd (Tianjin, China). All other reagents were of analytical reagent grade.
1.1.2 Media(g/L) Luria-Bertani medium (LB) was as following: tryptone 10.0, yeast extract 5.0, NaCl 10.0,agar 20.0; Basal salt medium (BSM) was as following: NH4NO33.0, K2HPO41.5, KH2PO41.5, NaC1 0.5, MgSO4·7H2O 0.1, CaCl20.01, FeC120.01.
1.2 Methods
1.2.1 Enrichment and isolation of the oil-degrading bacteria Samples were collected from the oil contaminated soil in an oil field in Gansu, China. Ten gram of the soil sample was inoculated into a 250 mL flask with 100 mL normal saline, the solution was shaken at 150 r/min for 3-4 hours at 30 ℃, then stand for 30 minutes. 1 mL of the solution was inoculated into 100 mL of BSM containing 500 mg/L of crude oil. The mixture was cultured at 30 ℃ with shaking of 150 r/min for 3 days. 1 mL of the culture was then inoculated into 100 mL of BSM containing
1 000 mg/L of crude oil and cultured for another 3 days under the same condition. The concentrations of crude oil in the BSM were increased from 1 000 mg/L, 2 000 mg/L and 3 000 mg/L. The final culture was diluted and plated onto LB agar plates. The colonies grown on the plates with different morphology were selected and tested for their oil utilizing capabilities. One strain 1217 which exhibited a fast growth rate and oil degradation activity was selected for further analysis and stored as liquid cultures containing 15% glycerol at -80 ℃.
1.2.2 Bacterial identification Bacterial identificationThe selected strain was characterized by morphological and biochemical tests and 16S rDNA sequence analysis. Carbohydrates were tested as described by Leifson[23]. Additional enzyme activities and biochemical features were determined by using API kits (API 20E) at 37 ℃ as recommended by the manufacturer (bioMe′rieux). The total genomic DNA was extracted by using Ezup type of bacterial genomic DNA Extraction Kit (Sangon Biotech (Shanghai) Co., Ltd., China). The 16S rDNA gene of strain 1217 was amplified using the bacterial universal primers of 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGCTT-3′). The cycling conditions of the PCR reaction consisted of an initial denaturation at 94 ℃ for 5 min, 30 cycles of denaturation at 94 ℃ for 50 s, annealing at 56 ℃ for 1 min, and extension at 72 ℃ for 2 min, with a final extension at 72 ℃ for 10 min. The product of the amplification was sent to Sangon Biotech (Shanghai) Co., Ltd. for sequencing. The result was analyzed and compared by using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/). A phylogenic tree was constructed with MEGA 4.0 by using the neighbor joining method with 1 000 replications.
1.2.3 Growth characteristics under different environment conditions To analyze the effect of culture conditions (temperature, initial pH and salinity) on the bacterial growth, the bacterial cells were cultured in LB broth with shaking at 150 r/min at a range of 5-65 ℃ with initial pH range of 2-10 and NaCl concentrations of 0-9% (m/v) respectively for 24 h, the cell growth was monitored using a UV-2102PC spectrophotometer at 600 nm (Unico Instrument Co., Ltd., Shanghai, China).
1.2.4 Growth characteristics in hydrocarbons The bacterial cells were inoculated in 5 mL of LB liquid and cultured at 30 ℃ for 24 h, 1 mL of the culture was centrifuged at 5 000 r/min for 10 min and washed twice with normal saline. The washed cells were resuspended with 1 mL of normal saline and incubated into BSM containing different amounts ofn-dodecane,n-hexadecane,n-octadecane, benzene, toluene, xylene, naphthalene, pyrene and pyrocatechol as sole carbon sources, respectively, the mixtures were then cultured at 30 ℃ with shaking at 150 r/min for 3 days. Plate count method was used to monitor cell growth in differentn-alkanes and aromatic hydrocarbons.
1.2.5 Oil-degradation analysis at different temperatures The strain was inoculated in a flask that contains BSM medium (100 mL) amended with 3 000 mg/L of crude oil as the sole carbon source, and cultured at 30 ℃ with shaking at 150 r/min for 7 days. The cell cultures were extracted with petroleum ether. For degradation analysis at low temperature, the washed cells were resuspended with 1 mL of normal saline and incubated into BSM containing different amounts ofn-heptane,n-decane,n-dodecane,n-tetradecane,n-hexadecane,n-octadecane,n-docosane,n-tetracosane andn-octacosane as sole carbon sources. The mixture was then cultured at 10 ℃ with shaking at 150 r/min for 7 days. The residue oil was extracted withn-hexane. The BSM medium containing crude oil was used as control. The residue oil of experimental and control groups were dissolved with 100 mL ofn-hexane. The residue components of crude oil were analyzed by the gas chromatography-mass spectrometry (GC-MS) technique. The GC-MS system (TRACE DSQ, Thermo Fisher Scientific, USA) was equipped with a fused DB-5 MS capillary column (30 m×0.25 mm×0.25 μm). The program of GC-MS was listed as below. The temperature was increased to 50 ℃ and kept for 3 minutes, then to 120 ℃ with a constant rate of 15 ℃/min, followed by an increase to 260 ℃ with a rate of 8 ℃/min and maintained for 25 minutes. Helium was used as the carrier gas with a constant flow rate of 1 mL/min. The split ratio was 10∶ 1, and the temperature of the injector, the connector and the ion source was 250 ℃, 260 ℃ and 250 ℃, respectively. The ionization mode was set as EI+,70 ev. The scanning range was 35-650 amu. All experiments were performed in triplicates. The oil-degrading efficiency was calculated as follows:
WhereM1is the initial mass of crude oil,M2is the residue mass of crude oil in sample.
1.2.6 Biosurfactant production and analysis The bacterial wells were inoculated in BSM containing 3 000 mg/L of crude oil, and incubated at 30 ℃ with shaking at 150 r/min for 3 days. The cultures were centrifuged at 8 000 r/min for 10 min, the supernates were collected and the surface tension was detected with a BZY-201 Tensiometer (Shanghai Fangrui instrument Co., Ltd., China).
1.2.7 The hydrocarbon-degrading gene detection Bacterial genome and plasmid DNA preparation, DNA ligation, transformation and agarose gel electrophoresis were carried out by standard protocols described before[24]. The oil-degrading related genes including alkane monooxygenases, toluate dioxygenase, toluene dioxygenase, biphenyl dioxygenase and oxidoreductase genes of the strain 1217 were amplified using the specific primers (Table 1). The cycling conditions of the PCR reaction consists of an initial denaturation at 94 ℃ for 5 min, 30 cycles of denaturation at 94 ℃ for 1 min, annealing at 56-62 ℃ for 1 min, and extension at 72 ℃ for 2 min, with a final extension at 72 ℃ for 10 min.
The complete genes of alkane monooxygenase and ring-hydroxylating dioxygenase were further amplified. The PCR reaction consists of an initial denaturation at 94 ℃ for 5 min, 30 cycles of denaturation at 94 ℃ for 1 min, annealing at 56 ℃ for 1 min, and extension at 72 ℃ for 2 min, with a final extension at 72 ℃ for 10 min. The target genes were cloned to pUCm-T Vector (Sangon Biotech (Shanghai) Co., Ltd., China) and analyzed.
2 Results and discussion
2.1 Isolation and identification of the oil-degrading bacterium
An efficient oil-degrading bacterial strain 1217 was isolated from oil contaminated soil. The bacterium formed brown and green, round, moist and glossy colonies on LB medium. The cells were rod-shaped and Gram-negative. The physiological and biochemical characteristics were similar with those ofP.aeruginosa(Table 2). The 16S rDNA sequence (Genbank accession number: KT378057) was 100% identical to that ofPseudomonasaeruginosaPAO1 and the phylogenetic tree analysis supported a strong relationship between strain 1217 and members of genusPseudomonas(Fig. 1). The strain was designated asPseudomonasaeruginosa1217.

Table 1 Primers for PCR detection of oil-degrading related genes

Table 2 Physiological biochemical characteristics of the oil-degrading bacterium
Note:"+": positive; "-": negative; Nd: Not done

Fig.1 Phylogenetic tree based on the 16S rDNA of P. aeruginosa 1217 Numbers in parentheses represent the sequences′ accession number in GenBank. The numbers at each branch point are the percentage supported by bootstrap. Bar: 0.01 substitutions per nucleotide position
2.2 Growth characteristics of Pseudomonas aeruginosa 1217 under different conditions
Pseudomonasaeruginosa1217 cells had a wide range of adaptability to different environmental conditions. The bacterium could grow at a temperature range from 5 ℃ to 65 ℃, and the optimal cell growth was obtained at 35 ℃. The initial pH for cell growth was from 2 to 10, with optimal growth at pH 9. The cell could grow with salinities from 0% to 9% of NaCl (m/v), but the optimal growth occurred with zero of the NaCl concentration (Fig. 2).

Fig.2 Effect of culture conditions on the growth of P. aeruginosa 1217A: Temperature; B: Initial pH; C: NaCl concentration
2.3 Growth characteristics in different hydrocarbons
The strain were incubated into BSM containing different amounts of hydrocarbons as sole carbon sources and cultured at 30 ℃ with shaking at 150 r/min. It was found that the inoculated cells grew well in the medium containing different amounts of toluene, xylene, naphthalene,n-hexadecane, benzene,n-dodecane,n-octodecane and pyrocatechol respectively, except for pyrene. The cell counts inn-hexadecane, benzene,n-dodecane,n-octodecane and pyrocatechol groups increased more than four times than that of the control groups. The cell counts in toluene, xylene, naphthalene groups increased more than 10 times, compared with that of the control group (Table 3), which suggests that the strain has a very wide degrading-range of hydrocarbons, especially for some aromatic hydrocarbons.
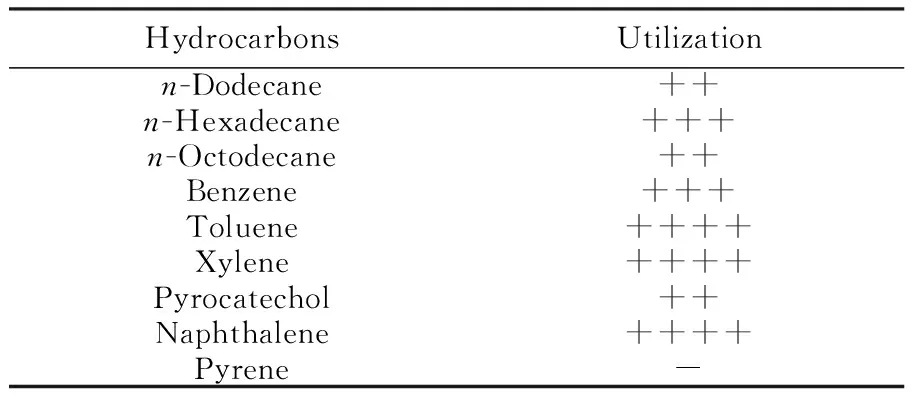
Table 3 Hydrocarbon utilization of P. aeruginosa 1217
Note:"-": the experimental group′s colonies less than 2 times the control group′s; "+": the experimental group′s colonies between 2 and 4 times the control group′s; "++": the experimental group′s colonies between 4 and 6 times the control group′s; "+++": the experimental group′s colonies between 6 and 10 times the control group′s; "++++": the experimental group′s colonies more than 10 times the control group′s
2.4 Degrading efficiency of hydrocarbons in different temperatures
The bacterial cells were incubated into BSM containing oil or different amounts ofn-alkanes as sole carbon sources and cultured at 30 ℃ and 10 ℃ with shaking at 150 r/min. The strain could grow and utilize crude oil as sole carbon in BSM medium
with a degradation rate of 21.57% for 7 days at 30 ℃ (Fig.3), it could degrade different chainn-alkanes. The medium-chain alkanes such asn-hexadecane,n-heptadecane,n-octadecane and longer chain alkanes (≥C20) such asn-eicosane,n-heneicosane,n-heptacosane andn-hexatriacontane were completely degraded, except forn-pentadecane with a degrading rate of 86.95%. The bacterium could partly degrade some branched alkanes. The degrading efficiencies for 2, 6, 10-trimethyl pentadecane, 9-hexylheptadecane and 1, 3, 5-trimethyl-2-octadecyl cyclohexane were 38.44%, 82.29% and 26.90%, respectively. When it was cultured at 10 ℃, The different chainn-alkanes were partly degraded with the degrading rate of 15.15%, and the degrading rates forn-decane,n-dodecane,n-tetradecane,n-hexadecane,n-octadecane,n-docasane,n-tetracosane,n-octacosane were 55.21%, 21.35%,16.00%,13.88%,10.86%,12.56%,14.51%,18.27% respectively (Fig.4).
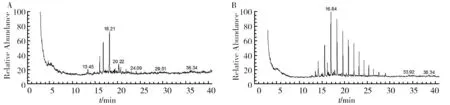
Fig.3 The GC-MS analysis of the degraded oil by P. aeruginosa 1217A: The control group; B: P. aeruginosa 1217,same figure 4

Fig.4 The GC-MS analysis of the degraded alkanes mixture by P. aeruginosa 1217 in low temperature
2.5 Biosurfactant production
The bacterial cells were inoculated in BSM medium containing crude oil and cultured at 30 ℃ for 3 days. The surface tension of the cell culture solution was determined to be 35.14 mN/m, compared with 72.2 mN/m of the BSM liquid medium.
2.6 The hydrocarbon-degrading genes detection and sequence analysis
The oil-degrading genes such as alkane monooxygenases (alkB1 andalkB2), toluate dioxygenase, toluene dioxygenase, biphenyl dioxygenase and oxidoreductase genes were detected from the genomic DNA ofPseudomonasaeruginosa1217 by PCR amplification with the specific primers (Fig.5). The alkane monooxygenase (alkB1) and ring-hydroxylating dioxygenase genes were further cloned and analyzed. The similarities of the alkane monooxygenase and ring-hydroxylating dioxygenase genes ofPseudomonasaeruginosa1217 withP.aeruginosaPAO1 were 99.91% and 99.22% (GenBank accession number of alkane monooxygenase gene was KT378058, and ring-hydroxylating dioxygenase gene was KT378059). The phylogenetic trees based onthe cloned alkane monooxygenase and ring-hydroxylating dioxygenase genes showed strong relationship betweenPseudomonasaeruginosa1217 and
the genusPseudomonas(Fig.6).
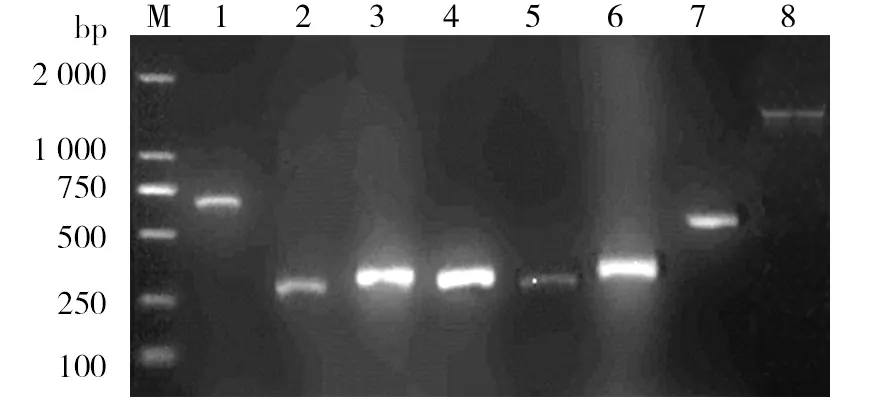
Fig.5 PCR detection of the oil-hydrocarbon- degrading genes of P. aeruginosa 1217M: DNA Marker; 1-3: alkB1 like gene, alkB1 & alkB2; 4: toluate dioxygenase (xylX); 5: toluene1.2-dioxygenase; 6: biphenyl ioxygenase; 7: oxidoreductase; 8: ring hydroxylating dioxygenase
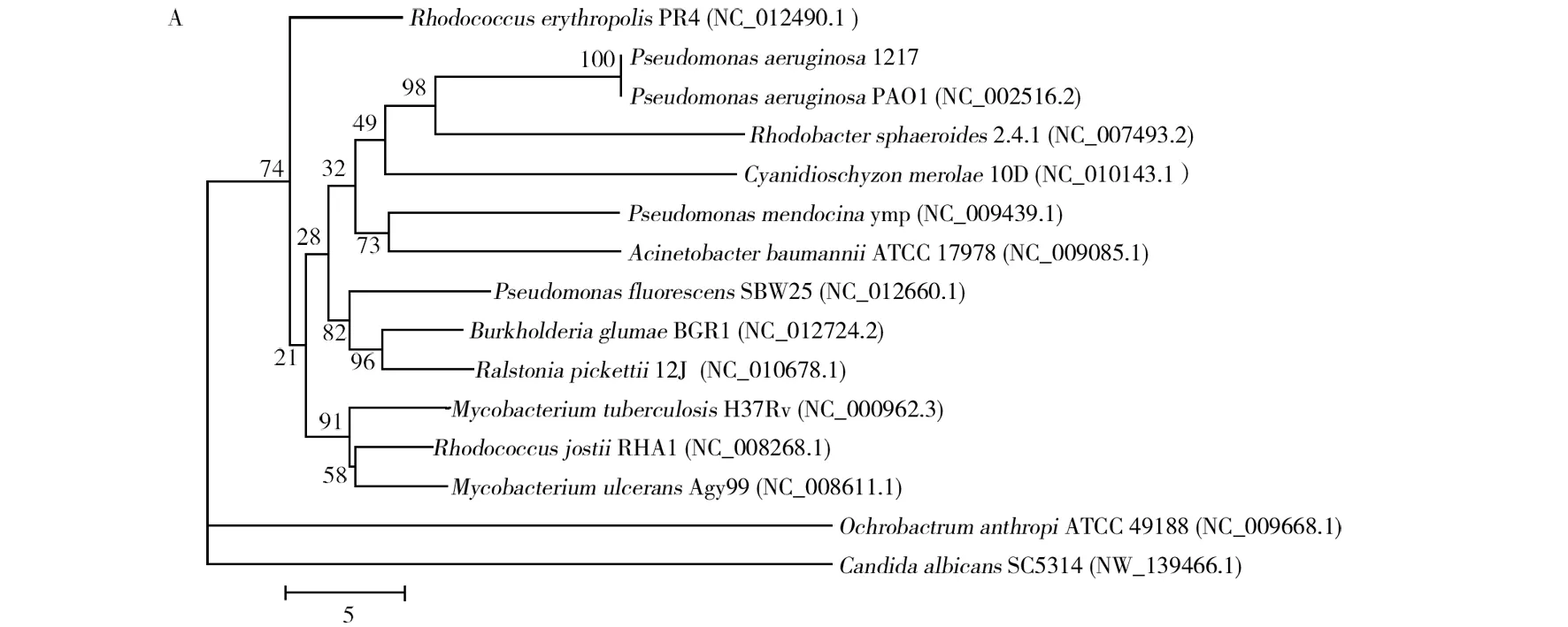
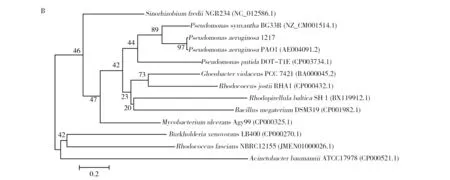
Fig.6 Phylogenetic trees based on the alkane monooxygenase and ring-hydroxylating dioxygenase of P. aeruginosa 1217 Numbers in parentheses represent the sequences′ accession number in GenBank; The numbers at each branch point are the percentage supported by bootstrap; Bar: 5 & 0.2 substitutions per nucleotide position; A: alkane monooxygenase; B: ring-hydroxylating dioxygenase
3 Discussion
Pseudomonasspecies exists widely in natural environments[16]. The strain 1217 was isolated from the oil contaminated desert soil in northwest China. The physiological biochemical and 16S rDNA sequence analyses confirmed that it belonged toPseudomonasaeruginosa. The bacterium grew well in medium containing different amounts of hydrocarbons such as toluene, xylene, naphthalene,n-hexadecane, benzene,n-dodecane,n-octodecane and pyrocatechol as sole carbon sources. It could efficiently degrade medium- and longer chain alkanes (from C20 to C37). Some branched alkanes could also be degraded. The biodegradation capacities and substrate hydrocarbons differ among thePseudomonasgenus strains. Some strains are able to grow on most hydrocarbons, and the others were specialized degrading only few substrates[5,17].P.asaeruginosaSJTD-1 isolated from oil contaminated soil could efficiently mineralize medium and long-chainn-alkanes (C12-C30) as its sole carbon source, meaning the most optimal growth onn-hexadecane[25]. Yuste and Smits found thatP.aeruginosaRR1 andP.fluorescensCHAO could degraden-alkanes ranging from C12-C34[26-27].PseudomonasputidaE41 isolated from oil contaminated soil showed its ability to grow on ethylbenzene as the sole carbon and energy source[19].
P.aeruginosa1217 can be used in a variety of environmental condition. It could grow at a range of 5 ℃ to 65 ℃ with pH range from 2 to 10. It also grew with the NaCl concentration of 0% to 9%. When it was cultured at 10 ℃, the different chainn-alkanes were partly degraded, which suggested that the bacterium could be used for the soil bioremediation in severe environments. The bacterium produced biosurfactants during their growth. The biosurfactants have been recognized to enhance biodegradation by increasing available substrate surface area and improving affinity of microbial cells for the substrates[28-30].
Alkanes are the major components of crude oil with an estimated abundance of 20%-50%[31-32]. Most bacteria can break down medium-chainn-alkanes or long-chainn-alkanes[33]. Alkanes are metabolized via a series of oxidation of a terminal methyl group to render alcohols, aldehydes, and fatty acids finally by bacteria[34]. Different alkane hydroxylases have been reported in thePseudomonasgenus.P.putidaGPo1 contain only one alkane hydroxylase[35].P.aeruginosaDQ 8 could oxidizen-alkanes via a terminal oxidation pathway and harbored only onealkB homologue[20].P.aeruginosaPAO1 contains two alkane hydroxylase genes (alkB1 andalkB2) which encode two alkane-1 monooxygenase of 383 and 378 amino acids respectively. The similarity of thealkB1 andalkB2 genes was 70.41 %, both of the genes participated in the oxidation ofn-alkanes[27]. ThealkB1 andalkB2 genes were also found in the genome DNA ofP.aeruginosa1217. The identity ofP.aeruginosa1217alkB1 toP.aeruginosaPAO1 and M18alkB1 was 100%. In addition, anotheralkB like gene fromP.aeruginosaSCV20265 was detected inP.aeruginosa1217. TheP.aeruginosaSCV20265alkB like gene exhibited 25.10% and 24.92% identity with those ofalkB1 andalkB2 inP.aeruginosaPAO1.
The initial steps in aerobic catabolism of aromatic compounds such as benzene, toluene, xylene, naphthalene, pyrocatechol and pyrene are carried out by oxygenases that introduce one (monooxygenases) or two atoms (dioxygenases) of molecular oxygen into the phenyl rings[36-38]. These oxygenases belong to the ring-hydroxylating oxygenase families. Different ring-hydroxylating oxygenases have been reported to play important roles in degradation of naphthalene, ethylbenzene, biphenyl and chlorobiphenyl. For example, monoaromatic compounds are initially oxygenated via dioxygenases or monooxygenases to yield catechols. Napthalene is initially oxygenated by napthalene dioxygenase and further degraded to yield salicylate. Salicylate is metabolised either through gentisic acid or through catechol[39-40]. We detected the ring-hydroxylating dioxygenase, toluate 1, 2-dioxygenase (xylX), toluene 1.2-dioxygenase, biphenyl dioxygenase and oxidoreductase inPseudomonasaeruginosa1217. The similarity of the ring-hydroxylating dioxygenase gene ofPseudomonasaeruginosa1217 with that ofP.aeruginosaPAO1 was 99.22%. These results explained our previous observation that the strain could grow in the medium containing toluene, xylene, naphthalene,n-hexadecane, benzene,n-dodecane,n-octodecane and pyrocatechol as sole carbon sources.
4 Conclusion
In summary, here we reported the isolation, identification and characterization of an effective oil-degrading strain 1217 in oil contaminated soil. The oil-degrading related genes such as ring-hydroxylating dioxygenase, toluate 1, 2-dioxygenase, toluene 1.2-dioxygenase, biphenyl dioxygenase and oxidoreductase were detected. TheP.aeruginosa1217 has a wide degrading-range of hydrocarbons especially for aromatic hydrocarbons and could be recommended for the bioremediation of crude oil contaminated soil in extreme environments.
Reference
[1] Chakraborty R, Coates JD. Anaerobic degradation of monoaromatic hydrocarbons[J]. Applied microbiology and biotechnology, 2004, 64:437-446.
[2] Hamzah A, Phan CW, Abu Bakar NF, et al. Biodegradation of crude oil by constructed bacterial consortia and the constituent single bacteria isolated from Malaysia[J]. Bioremediation Journal, 2013, 17(1):1-10.
[3] Yemashova NA, Murygina VP, Zhukov DV, et al. Biodeterioration of crude oil and oil derived products: a review[J]. Reviews in Environmental Science and Bio/Technology, 2007, 6(4):315-337.
[4] Wasmund K, Burns KA, Kurtboke DI, et al. Novel alkane hydroxylase gene (alkB) diversity in sediments associated with hydrocarbon seeps in the Timor sea, Australia[J]. Applied and Environmental Microbiology, 2009, 75(23):7391-7398.
[5] Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview[J]. Biotechnology Research International, 2011, 12(1):110-116.
[6] Medina-Bellver JI, Marin P, Delgado A, et al. Evidence for in situ crude oil biodegradation after the Prestige oil spill[J]. Environmental Microbiology, 2005, 7(6):773-779.
[7] Kostka JE, Prakash O, Overholt WA, et al. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill[J]. Applied and Environmental Microbiology, 2011, 77(22):7962-7974.
[8] Okoh AI. Biodegradation alternative in the clean up of petroleum hydrocarbon pollutants[J]. Microbiology and Molecular Biology Reviews, 2006, 1(2):38-50.
[9] Labinger JA, Bercaw JE. Understanding and exploiting CH bond activation[J]. Nature, 2002, 417(48):507-514.
[10]Head IM, Jones DM, Roling WFM. Marine microorganisms make a meal of oil[J]. Nature Reviews Microbiology, 2006, 4(3):173-182.
[11]Rojo F. Degradation of alkanes by bacteria[J]. Environmental Microbiology, 2009, 11(10):2477-2490.
[12]Humphrey SR. Chemical dispersants and crude oil-efficacy and toxicity. http://www.theoildrum.com/node/6724, 2010-07-14.
[13]Chaineau CH, Morel J, Dupont J, et al. Comparison of the fuel oil biodegradation potential of hydrocarbon-assimilating microorganisms isolated from a temperate agricultural soil[J]. The Science of the Total Environment, 1999, 227(2-3):237-247.
[14]Mahjoubi M, Jaouani A, Guesmi A, et al. Hydrocarbonoclastic bacteria isolated from petroleum contaminated sites in Tunisia: isolation, identification and characterization of the biotechnological potential[J]. New Biotechnology, 2013, 30(6):723-733.
[15]Wang WP, Zhong RQ, Shan DP, et al. Indigenous oil-degrading bacteria in crude oil-contaminated seawater of the Yellow Sea, China[J]. Applied Microbiology and Biotechnology, 2014, 98(16):7253-7269.
[16]Spiers AJ, Buckling A, Rainey PB. The causes ofPseudomonasdiversity[J]. Microbiology, 2000, 146(4):2345-2350.
[17]Hasanuzzaman M, Umadhay-Briones K M, Zsiros S M, et al. Isolation, identification, and characterization of a novel, oil-degrading bacteriumPseudomonasaeruginosaT1[J]. Current Microbiology, 2004, 49(2):108-114.
[18]Soares A, Guieysse B, Delgado O, et al. Aerobic biodegradation of nonylphenol by cold-adapted bacteria[J]. Biotechnology Letters, 2003, 25(9):731-738.
[19]Kim LH, Lee SS. Isolation and characterization of ethylbenzene degradingPseudomonasputidaE41[J]. Journal of Microbiology, 2011, 49(4):575-584.
[20]Zhang ZZ, Hou ZW, Yang CY, et al. Degradation ofn-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolatedPseudomonasaeruginosaDQ8[J]. Bioresource Technology, 2011, 102(5):4111-4116.
[21]Baptist JN, Gholson RK, Coon MJ. Hydrocarbon oxidation by a bacterial enzyme system: I. Products of octane oxidation[J]. Biochimica Et Biophysica Acta, 1963, 69(2):40-47.
[22]Van Beilen JB, Panke S, Lucchini S, et al. Analysis ofPseudomonasputidaalkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of thealkgenes[J]. Microbiology, 2001, 147(6):1621-1630.
[23]Leifson E. Determination of carbohydrate metabolism of marine bacteria[J]. Journal of bacteriology, 1963, 85(5):1183-1184.
[24]Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual, 3rd edn[M]. New York:Cold Spring Harbor, 1989.
[25]Liu H, Xu J, Liang R, et al. Characterization of the medium- and long-chainn-alkanes degradingPseudomonasaeruginosastrain SJTD-1 and its alkane hydroxylase genes[J]. Plos One, 2014, 9(8):1-14.
[26]Yuste L, Corbella ME, Turiégano MJ, et al. Characterization of bacterial strains able to grow on high molecular mass residues from crude oil processing[J]. FEMS Microbiology Ecology, 2000, 32(1):69-75.
[27]Smits TH, Balada SB, Witholt B, et al. Functional analysis of alkane hydroxylases from gram-negative and gram-positive bacteria[J]. Journal of bacteriology, 2002, 184(6):1733-1742.
[28]Ito S, Inoue S. Sophorolipids from torulopsis bombicola: possible relation to alkane uptake[J]. Applied and Environmental Microbiology, 1982, 43(6):1278-1283.
[29]Rahman KSM, Rahman TJ, Kourkoutas Y, et al. Enhanced bioremediation ofn-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients[J]. Bioresource Technology, 2003, 90(2):159-168.
[30]Bonilla M, Olivaro C, Corona M, et al. Production and characterization of a new bioemulsifier fromPseudomonasputidaML2[J]. Journal of Applied Microbiology, 2005, 98(2):456-463.
[31]Marín MM, Yuste L, Rojo F. Differential expression of the components of the two alkane hydroxylases fromPseudomonasaeruginosa[J]. Journal of Bacteriology, 2003, 185(10):3232-3237.
[32]Kaplan CW, Kitts CL. Bacterial succession in a petroleum land treatment unit[J]. Applied and Environmental Microbiology, 2004, 70(3):1777-1786.
[33]Hao R, Lu A, Wang G. Crude-oil-degrading thermophilic bacterium isolated from an oil field[J]. Canadian Journal of Microbiology, 2004, 50(3):175-182.
[34]Rehm HJ, Reiff I. Mechanisms and occurrence of microbial oxidation of long-chain alkanes[J]. Advances in Biochemical Engineering, 1981, 19:175-215.
[35]Alonso H, Roujeinikova A. Characterization and two-Dimensional crystallization of membrane componentalkB of the medium-chain alkane hydroxylase system fromPseudomonasputidaGPo1[J]. Applied and Environmental Microbiology, 2012, 78(22):7946-7953.
[36]Gibson DT. Mcrobial degradation of aromatic compounds[J]. Science, 1968, 161(3846):1093-1097.
[37]Gibsona DT, Chapmanb PJ. The microbial oxidation of aromatic hydrocarbons[J]. CRC Critical Reviews in Microbiology, 1971, 1(2):199-223.
[38]Fuchs G, Boll M, Heider J. Microbial degradation of aromatic compounds-from one strategy to four[J]. Nature Reviews Microbiology, 2011, 9(11):803-816.
[39]Denome SA, Stanley DC, Olson ES, et al. Metabolism of dibenzothiophene and naphthalene inPseudomonasstrains: complete DNA sequence of an upper naphthalene catabolic pathway[J]. Journal of Bacteriology, 1993, 175(21):6890-6901.
[40]Izmalkova TIU, Sazonova OI, Sokolov SL, et al. Diversity of genetic systems responsible for biodegradation of naphthalene inPseudomonasfluorescensstrains[J]. Mikrobiologiia, 2005, 74(74):70-78.

